


Effective site management stands as a cornerstone of successful clinical trials, especially in regions like Serbia, where local dynamics play a pivotal role. By grasping the intricate web of regulations, healthcare infrastructure, and patient demographics, research teams can significantly enhance their operational efficiency and participant recruitment. This understanding raises a crucial question: how can sponsors and researchers adapt their strategies to not only comply with local standards but also optimize their study outcomes in a competitive landscape? This inquiry is essential for navigating the complexities of clinical research and achieving success.
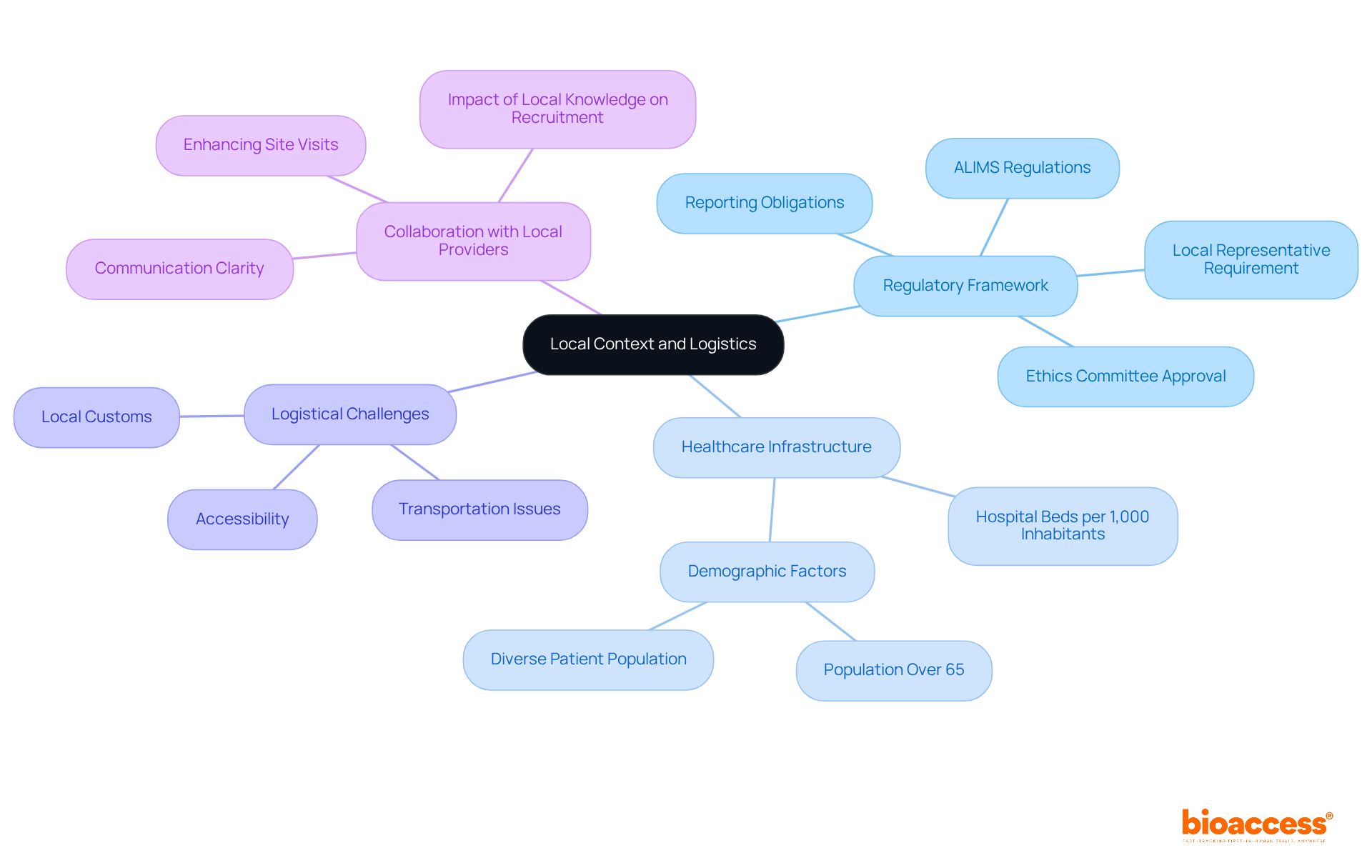
Efficiently overseeing research sites in the region demands a thorough understanding of site management best practices in Serbia as well as logistics. Central to this is the regulatory framework established by the Medicines and Medical Devices Agency (ALIMS), which governs research study applications and approvals. This structure aligns closely with EU standards, necessitating local representation for non-EU sponsors and ensuring adherence to ethical guidelines. It's crucial to note that without a Local Representative, no clinical study authorization can proceed in the country-something that may catch foreign sponsors off guard if they're not familiar with the legal landscape.
The healthcare infrastructure in Serbia, which boasts 5.41 hospital beds per 1,000 inhabitants and a diverse patient population with 21.3% aged over 65, significantly influences site management best practices in Serbia. This demographic presents a valuable recruitment pool, especially for studies focused on age-related conditions. However, in order to adhere to site management best practices in Serbia, logistical challenges such as transportation, accessibility, and local customs must be navigated to enhance operational efficiency.
Moreover, sponsors are required to disclose study results, including both favorable and unfavorable outcomes, to public registries. This practice is vital for maintaining the integrity of medical research. The approval process for ethics committee applications can be completed within 60 days, with some protocols receiving approval in as little as three weeks, illustrating the efficiency of the regulatory landscape.
Collaborating with local logistics providers can significantly enhance the effectiveness of site visits and patient recruitment efforts. For instance, studies that emphasize transparency and clarity in communication have shown up to a 30% increase in participant recruitment compared to those that lack clarity. By leveraging local knowledge and resources, research teams can apply site management best practices in Serbia to streamline processes, proactively address potential obstacles, and ultimately achieve more successful study outcomes.
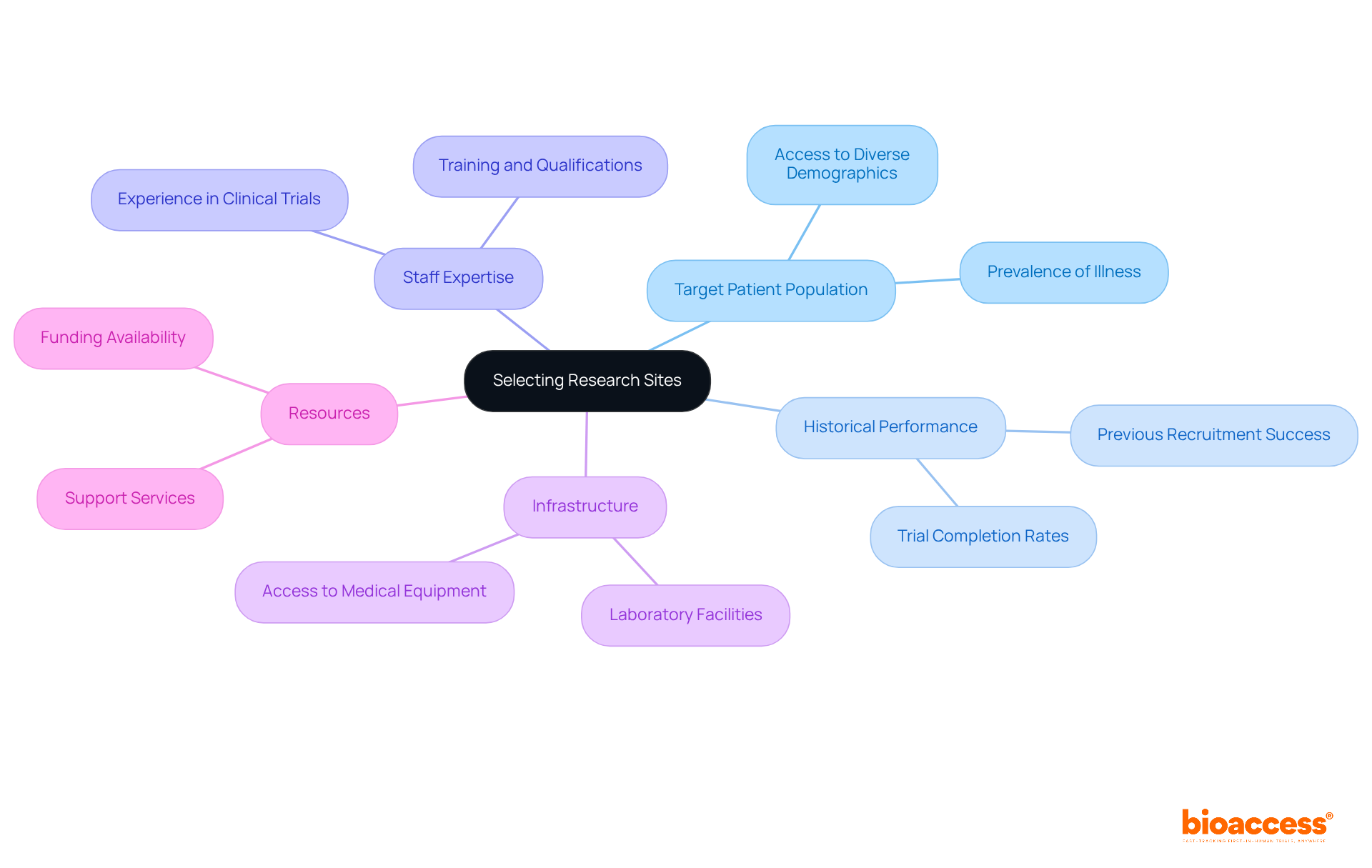
Selecting research locations in Serbia is crucial for aligning with the specific goals of your study. It’s essential to assess key factors such as:
For instance, if your study targets an illness prevalent in a certain area, prioritizing sites within that region can significantly boost patient recruitment and retention rates.
Moreover, evaluating the infrastructure and resources at each site - like laboratory facilities and access to medical equipment - is vital. By strategically choosing locations that align with your study's objectives, you can enhance enrollment rates and ensure efficient execution. This tailored approach not only facilitates prompt patient enrollment but also improves the overall effectiveness of research studies in the region. With a growing public interest in health issues and a skilled workforce, these initiatives are further strengthened.
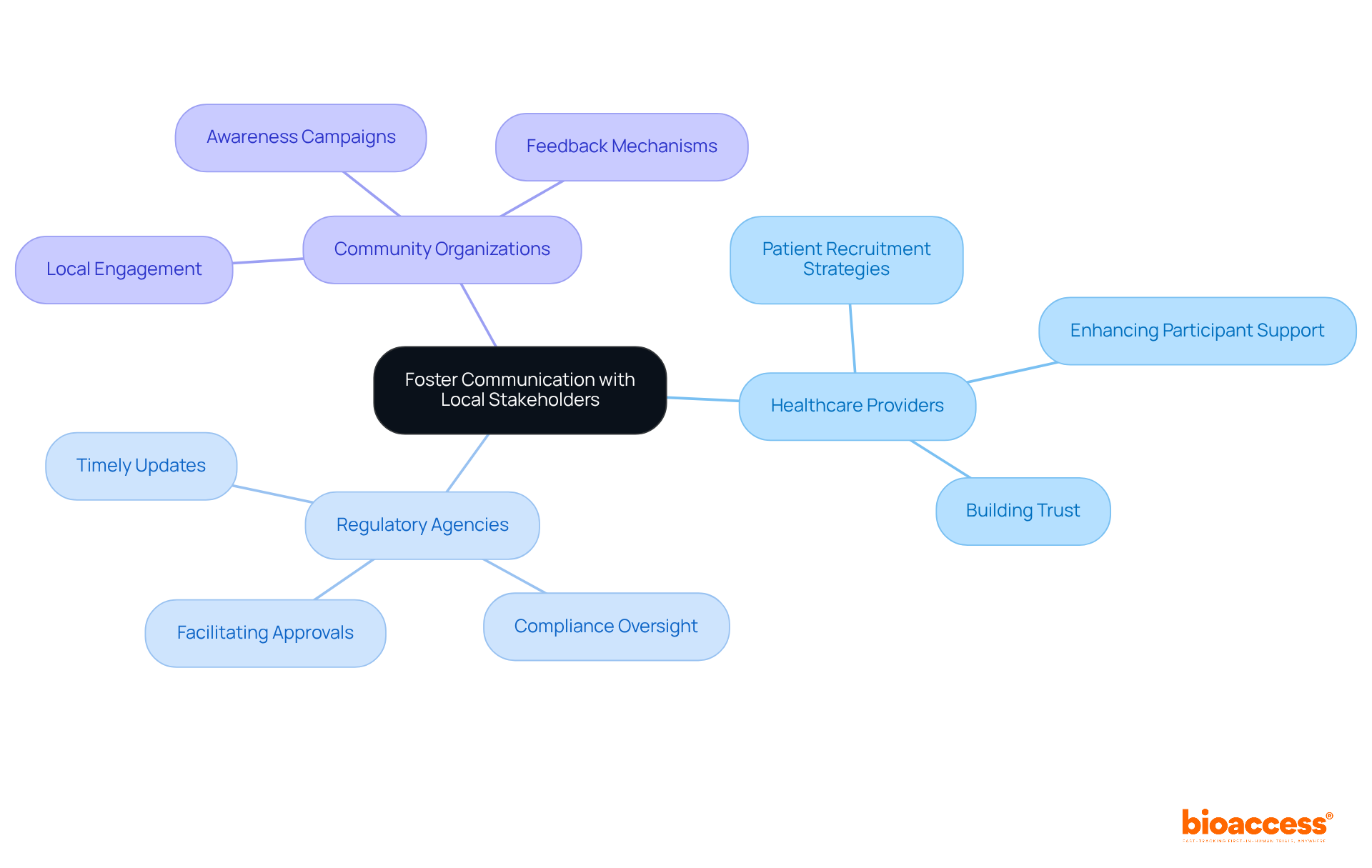
Effective communication with local stakeholders is crucial for the success of clinical studies in Serbia. By involving healthcare providers, regulatory agencies, and community organizations, we foster collaboration and support that are vital for smooth study operations. Regular meetings and updates keep all parties informed about the progress, allowing for timely resolution of any concerns. Engaging local stakeholders in the study design and execution not only boosts support but also facilitates smoother operations.
For example, collaborating with healthcare providers can significantly enhance patient recruitment strategies, ensuring participants receive essential support throughout the study. By prioritizing clear communication, research teams can build trust and create a cooperative atmosphere that enhances overall study success.
Adaptability in planning is essential for navigating the complexities of research studies in Serbia. Researchers must develop flexible timelines that can accommodate unforeseen challenges, such as delays in patient recruitment or regulatory approvals. bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, and setup, significantly simplifying these processes.
By adopting a dynamic scheduling approach, teams can adjust their plans as needed, ensuring that experiments remain on track. Utilizing project management tools not only helps visualize timelines but also identifies potential bottlenecks early on. Furthermore, fostering a culture of flexibility within the research team promotes proactive problem-solving and collaboration.
By maintaining flexibility in scheduling, clinical research teams enhance their ability to respond to challenges and optimize trial outcomes. This ultimately allows them to leverage bioaccess's expertise in accelerating approval processes, ensuring that they stay ahead in the competitive Medtech landscape.
Mastering site management in Serbia is crucial for achieving clinical success. Understanding the local regulatory framework, healthcare infrastructure, and logistical challenges is essential for foreign sponsors and researchers aiming to conduct effective studies in the region. By leveraging local knowledge, fostering communication with stakeholders, and maintaining flexibility in scheduling, research teams can navigate the complexities of clinical trials and enhance their overall effectiveness.
Key insights from this discussion highlight the importance of selecting sites that align with research objectives. It’s vital to consider patient populations and historical performance. Collaborating with local logistics providers and healthcare professionals can significantly improve recruitment and retention rates. Moreover, transparency in communication with stakeholders fosters trust and support, which are vital for the smooth execution of clinical studies.
In a landscape where patient demographics and regulatory requirements play a crucial role, adopting best practices in site management streamlines research processes and contributes to the integrity of clinical trials. Embracing these strategies is essential for researchers looking to make impactful contributions to the healthcare sector in Serbia and beyond. By prioritizing local engagement and adaptability, clinical research teams can position themselves for success in an ever-evolving environment.
What is essential for overseeing research sites in Serbia?
A thorough understanding of site management best practices and logistics, as well as the regulatory framework established by the Medicines and Medical Devices Agency (ALIMS), is essential for overseeing research sites in Serbia.
What role does the Medicines and Medical Devices Agency (ALIMS) play in research studies?
ALIMS governs research study applications and approvals in Serbia, aligning closely with EU standards and requiring local representation for non-EU sponsors.
Why is a Local Representative necessary for clinical studies in Serbia?
A Local Representative is necessary because no clinical study authorization can proceed in Serbia without one, which may surprise foreign sponsors unfamiliar with the legal landscape.
How does Serbia's healthcare infrastructure impact site management practices?
Serbia's healthcare infrastructure, which includes 5.41 hospital beds per 1,000 inhabitants and a diverse patient population, influences site management practices by providing a valuable recruitment pool, especially for studies focused on age-related conditions.
What logistical challenges must be navigated in Serbia for effective site management?
Logistical challenges include transportation, accessibility, and local customs, which must be addressed to enhance operational efficiency.
What are the requirements for disclosing study results in Serbia?
Sponsors are required to disclose study results, including both favorable and unfavorable outcomes, to public registries to maintain the integrity of medical research.
How long does the ethics committee approval process take in Serbia?
The ethics committee approval process can be completed within 60 days, with some protocols receiving approval in as little as three weeks.
How can collaborating with local logistics providers benefit research studies in Serbia?
Collaborating with local logistics providers can enhance the effectiveness of site visits and patient recruitment efforts, leading to a potential increase in participant recruitment by up to 30% when communication is transparent and clear.
What is the significance of transparency and clarity in communication for research studies?
Transparency and clarity in communication are significant because they can lead to a substantial increase in participant recruitment compared to studies that lack these qualities.