


Navigating the complex landscape of clinical trials in Croatia demands a profound understanding of the intertwined ethics and regulatory dual approval process. This framework, governed by the Clinical Trials Act and the Medical Devices Act, is crucial for ensuring the safety and rights of participants while adhering to EU standards. As researchers explore this intricate system, they will discover essential strategies to streamline their applications and enhance trial efficiency. Yet, a pressing challenge persists: how can one effectively balance the rigorous demands of ethical scrutiny with the regulatory requirements to secure timely approvals and achieve successful study outcomes?
In Croatia, the ethics and regulatory dual approval process is primarily shaped by the Clinical Trials Act and the Medical Devices Act, both of which align with EU regulations. The Central Ethics Committee (CEC) plays a pivotal role in overseeing this process, ensuring that studies uphold participants' rights and welfare. Furthermore, the Agency for Medicinal Products and Medical Devices (HALMED) is tasked with regulating medical products. A comprehensive understanding of the ethics and regulatory dual approval process in Croatia is essential for compliance and the successful execution of clinical trials. Key principles include:
All clinical studies are subject to a stringent ethics and regulatory dual approval process to ensure adherence to established ethical standards. The CEC conducts reviews of study protocols, investigator qualifications, and facility adequacy, with meetings held every 2 to 3 weeks to facilitate timely evaluations. Adherence to both national and EU regulations is mandatory for regulatory compliance, ensuring that studies are conducted safely and ethically. The CEC typically provides its opinion within 30 to 90 days from the submission of documentation, with the Ministry of Health conducting a subsequent review.
To support these processes, bioaccess offers a comprehensive suite of clinical trial management services, including:
Recent statistics reveal that from May 2004 to the end of 2008, the CEC reviewed a total of 407 clinical evaluations, with a notable increase in postponed opinions due to the growing demand for thorough documentation. In 2008 alone, 99 experimental applications were reviewed, primarily in oncology, mental and behavioral disorders, and endocrine diseases. This underscores the evolving landscape of clinical trials in Croatia and the critical importance of the ethics and regulatory dual approval process to uphold high moral standards throughout the research.
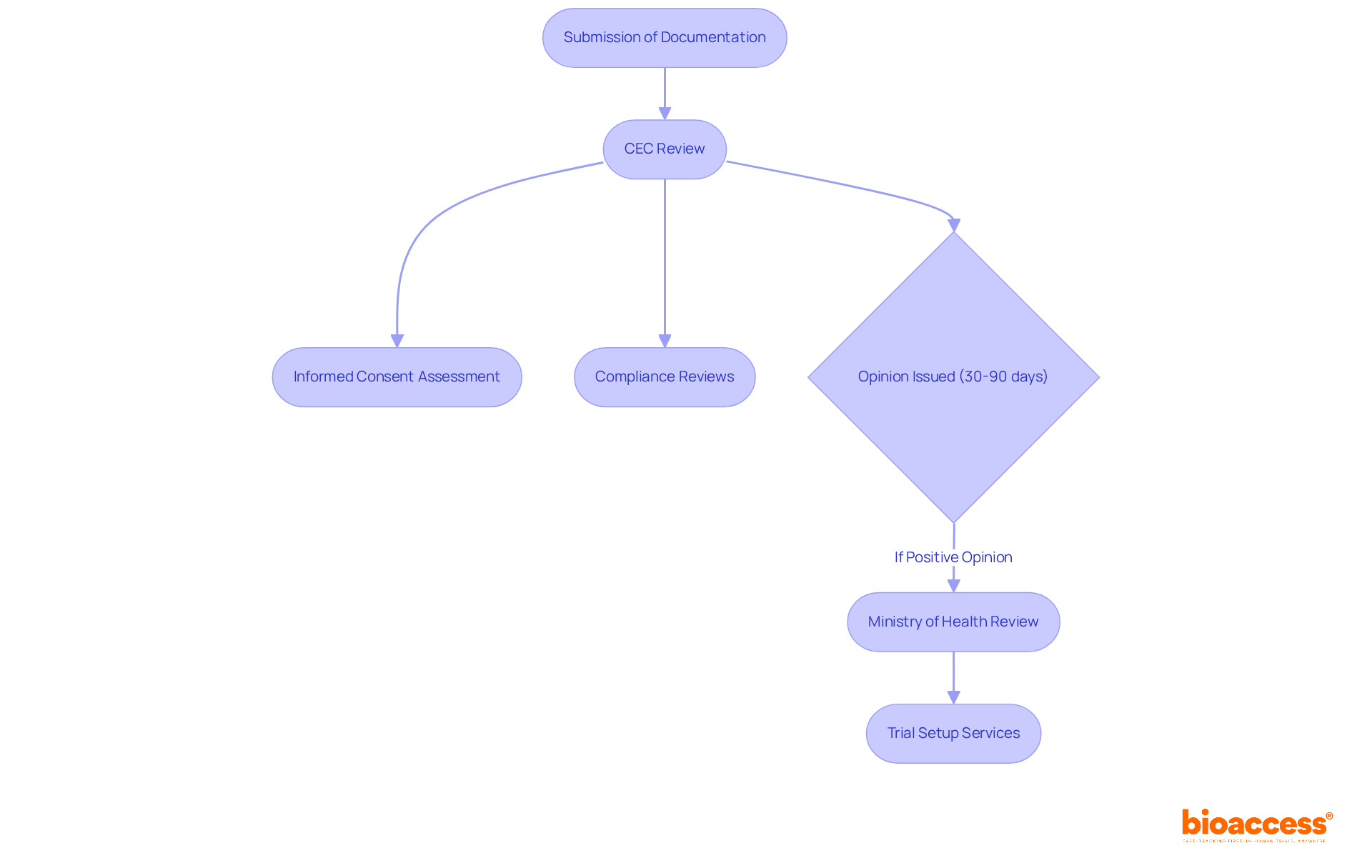
Researchers must navigate the ethics and regulatory dual approval process in Croatia, which is a critical pathway to ensure the integrity of clinical research.
Preparation of Documentation: A comprehensive application is crucial, encompassing the study protocol, informed consent forms, and any necessary supporting documents. Researchers should prioritize clarity and completeness in their submissions to minimize delays and enhance the likelihood of approval.
Submission to the Central Ethics Committee (CEC): The application is submitted to the CEC, which meticulously evaluates the ethical implications of the proposed study. This centralized review model simplifies the procedure and enhances efficiency, making it easier for researchers to navigate the approval landscape.
Review Timeline: The CEC typically provides feedback within 30 to 90 days, depending on the complexity of the study. Notably, recent statistics reveal that 28% of applications may require additional documentation or modifications, potentially extending the review period and impacting the overall timeline.
Addressing Feedback: If modifications are requested, researchers must promptly address these concerns and resubmit their application. Effective communication and thorough documentation preparation are vital at this stage to facilitate a smooth resubmission process.
Consent Notification: Upon confirmation, researchers receive formal notice, enabling them to commence the study. The typical timeline from submission to acceptance is approximately 60 days, contingent on fulfilling all required criteria, underscoring the significance of comprehensive initial submissions.
Understanding these steps is essential for ensuring timely trial initiation and adherence to the ethics and regulatory dual approval process in Croatia. This knowledge ultimately contributes to the success of clinical research in Croatia.

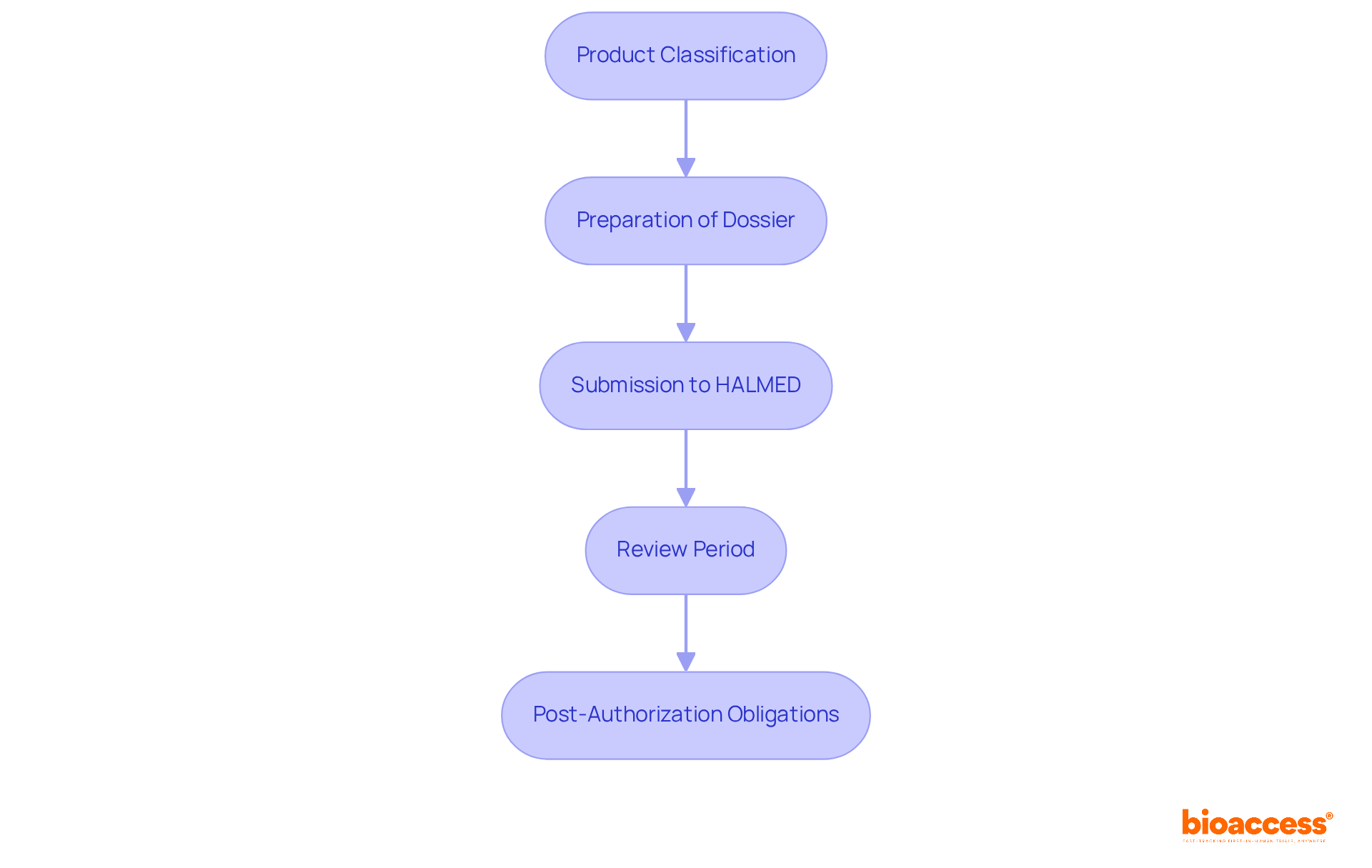
The ethics and regulatory dual approval process in Croatia is a critical pathway for ensuring the safety and efficacy of medical products. Understanding this process is essential for manufacturers aiming to introduce their products to the market successfully.
Product Classification: Initially, determining the classification of the medical product according to EU regulations is vital. This classification dictates the subsequent authorization pathway, making it a foundational step in the process.
Preparation of Dossier: Manufacturers are required to compile a comprehensive technical dossier. This document must encompass detailed product specifications, clinical data, and evidence that demonstrates compliance with established safety and efficacy standards.
Submission to HALMED: Once the dossier is complete, it is submitted to the Agency for Medicinal Products and Medical Devices (HALMED) for a thorough review. This step is crucial for ensuring that all necessary information is presented for evaluation.
Review Period: HALMED typically completes its review of applications within an average of 60 days. However, this timeframe can vary based on the complexity and type of the product being evaluated, highlighting the importance of thorough preparation.
Post-Authorization Obligations: Following approval, manufacturers must comply with post-market surveillance and reporting duties. This ongoing commitment ensures that products continue to meet regulatory standards throughout their lifecycle.
Effectively navigating the ethics and regulatory dual approval process in Croatia is essential for the successful market introduction of medical products. It guarantees that these products meet the necessary safety and efficacy benchmarks, ultimately fostering trust and reliability in the healthcare system.
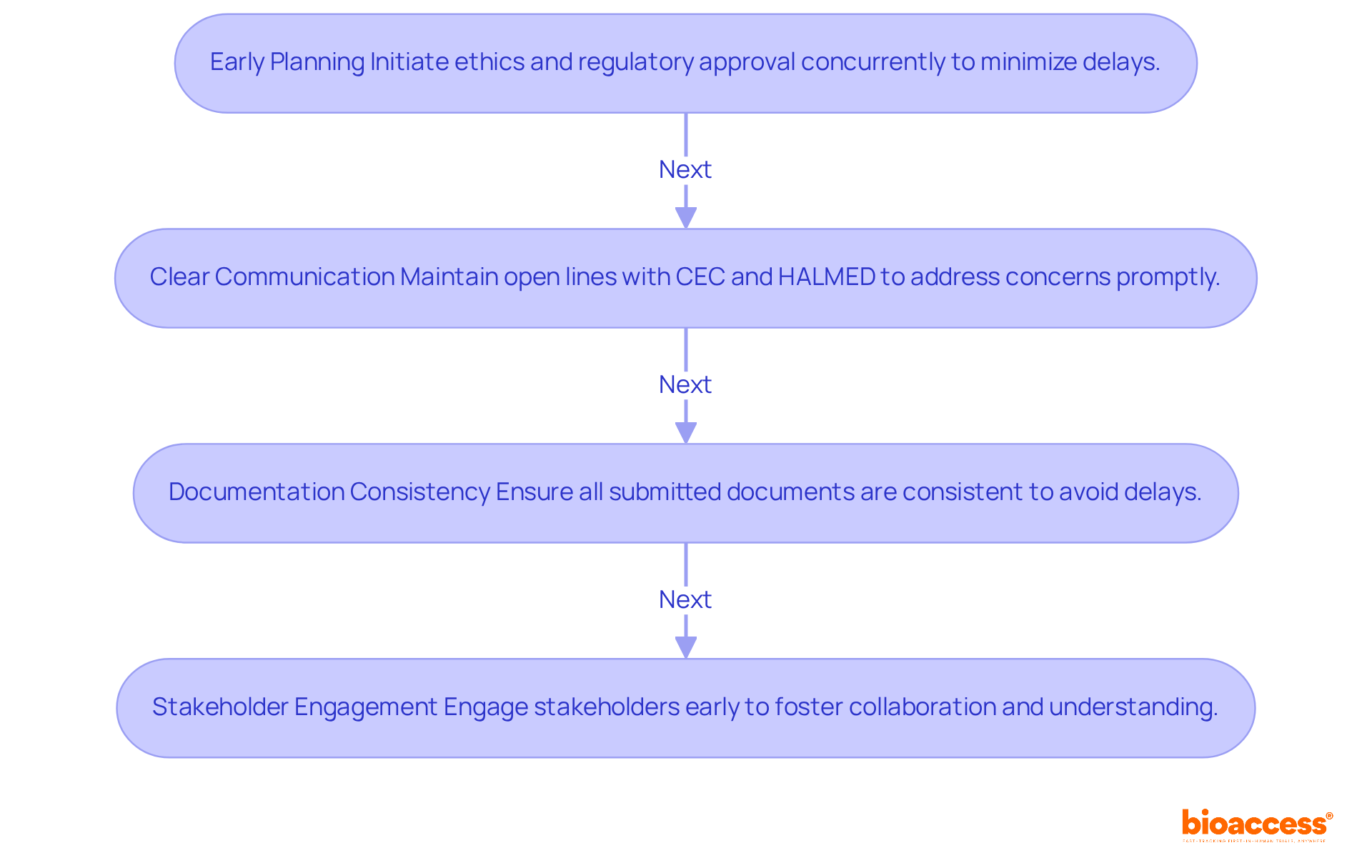
For the success of clinical studies in Croatia, it is essential to incorporate the ethics and regulatory dual approval process in Croatia. To achieve this, researchers must adopt key strategies that enhance efficiency and compliance.
Early planning: Initiating the ethics and regulatory dual approval process in Croatia concurrently is crucial to minimize delays. This approach demands careful coordination of timelines and documentation. Early testing phases and mutual acceptance of ethical clearances have been linked to reduced overall time to site activation. Bioaccess offers comprehensive clinical trial management services, including feasibility studies and site selection, to streamline this process.
Clear Communication: Establishing and maintaining open lines of communication with both the Central Ethics Committee (CEC) and HALMED is vital. Promptly addressing any concerns ensures that all requirements are met efficiently, especially considering that the median time to ethics approval in Croatia is approximately 48 days. Bioaccess's expertise in compliance reviews can facilitate this essential communication.
Documentation Consistency: It is imperative to ensure that all submitted documents are consistent across both ethical and regulatory applications. Discrepancies can lead to significant delays, with the overall median time to site activation potentially reaching up to 234 days. Bioaccess assists in maintaining this consistency through thorough project management and monitoring.
Stakeholder Engagement: Engaging with stakeholders, including ethics committees and regulatory bodies, early in the process fosters collaboration and a mutual understanding of expectations. Navigating the complexities of the ethics and regulatory dual approval process in Croatia is essential. With Bioaccess's expertise in study setup and import permits, researchers can significantly improve their engagement strategies.
By effectively integrating these processes, researchers can enhance the efficiency of their trials and ensure compliance with both ethical and regulatory standards. This ultimately contributes to the competitiveness of Croatia as a clinical research site.
Understanding the ethics and regulatory dual approval process in Croatia is essential for ensuring the integrity and success of clinical trials. Governed by the Clinical Trials Act and the Medical Devices Act, this process underscores the significance of participant welfare and regulatory compliance, ultimately fostering a trustworthy research environment.
Key insights from this article reveal the structured approach necessary for obtaining both ethical and regulatory approvals. From the meticulous preparation of documentation to the pivotal role of the Central Ethics Committee and HALMED, each step is crafted to uphold high ethical standards and ensure the safety and efficacy of medical products. Moreover, strategies such as early planning, clear communication, and stakeholder engagement are vital for navigating this complex landscape efficiently.
For researchers and manufacturers, mastering the dual approval process not only enhances the likelihood of successful trials but also bolsters Croatia's competitiveness as a clinical research hub. By embracing these best practices, compliance with ethical and regulatory standards is assured, leading to advancements in healthcare and improved outcomes for participants. Engaging with this framework is a crucial step toward fostering innovation and maintaining the highest moral standards in clinical research.
What are the main laws governing the ethical and regulatory approval process for clinical trials in Croatia?
The main laws are the Clinical Trials Act and the Medical Devices Act, both of which align with EU regulations.
What role does the Central Ethics Committee (CEC) play in clinical trials in Croatia?
The CEC oversees the ethical review process, ensuring that studies uphold participants' rights and welfare, and evaluates study protocols, investigator qualifications, and facility adequacy.
What is required for informed consent in clinical trials?
Participants must receive thorough information about the trial's purpose, procedures, risks, and benefits before providing consent. This process is rigorously assessed by the CEC.
How often does the CEC meet to review clinical trial submissions?
The CEC holds meetings every 2 to 3 weeks to facilitate timely evaluations of study protocols.
What is the typical timeframe for the CEC to provide its opinion on a clinical trial submission?
The CEC typically provides its opinion within 30 to 90 days from the submission of documentation.
What organization conducts a subsequent review after the CEC's opinion?
The Ministry of Health conducts a subsequent review after the CEC's opinion.
What services does bioaccess offer to support the clinical trial approval process in Croatia?
Bioaccess offers services including feasibility studies, site selection, compliance reviews, trial setup and start-up services, import permits, project management, and monitoring.
What has been the trend in the number of clinical evaluations reviewed by the CEC from 2004 to 2008?
There has been a notable increase in postponed opinions due to growing demand for thorough documentation, with 407 clinical evaluations reviewed from May 2004 to the end of 2008.
In which areas were the majority of experimental applications reviewed in 2008?
The majority of experimental applications reviewed in 2008 were primarily in oncology, mental and behavioral disorders, and endocrine diseases.