


This article outlines the essential criteria and steps for selecting and collaborating with an in vitro diagnostics (IVD) company, emphasizing the critical role of regulatory compliance, technological expertise, and quality assurance in achieving successful clinical trials. Understanding these elements is vital for any organization aiming to enhance their medical research outcomes.
In the current Medtech landscape, the collaboration between clinical researchers and IVD companies is more important than ever. Establishing clear objectives and effective communication channels is paramount. These factors not only foster strong partnerships but also significantly enhance the quality of research outcomes. Have you considered how these elements could impact your own clinical research initiatives?
As we delve deeper into the intricacies of this collaboration, it becomes evident that a strategic approach is necessary. By focusing on these key factors, organizations can navigate the complexities of clinical trials more effectively. The importance of collaboration cannot be overstated; it is the foundation upon which successful research is built. What steps will you take to ensure your partnerships are effective and productive?
Navigating the intricate landscape of in vitro diagnostics (IVD) is crucial for the success of clinical trials, as these diagnostics offer vital insights that influence treatment decisions. With rapid advancements in technology and a burgeoning market, understanding how to select and collaborate with the right IVD company can significantly enhance research outcomes.
However, this process presents its own set of challenges—how can one ensure that the chosen partner aligns with regulatory standards, possesses the necessary technological capabilities, and maintains quality assurance while fostering effective communication?
This guide explores the essential considerations and strategies for establishing fruitful partnerships in the dynamic realm of in vitro diagnostics.
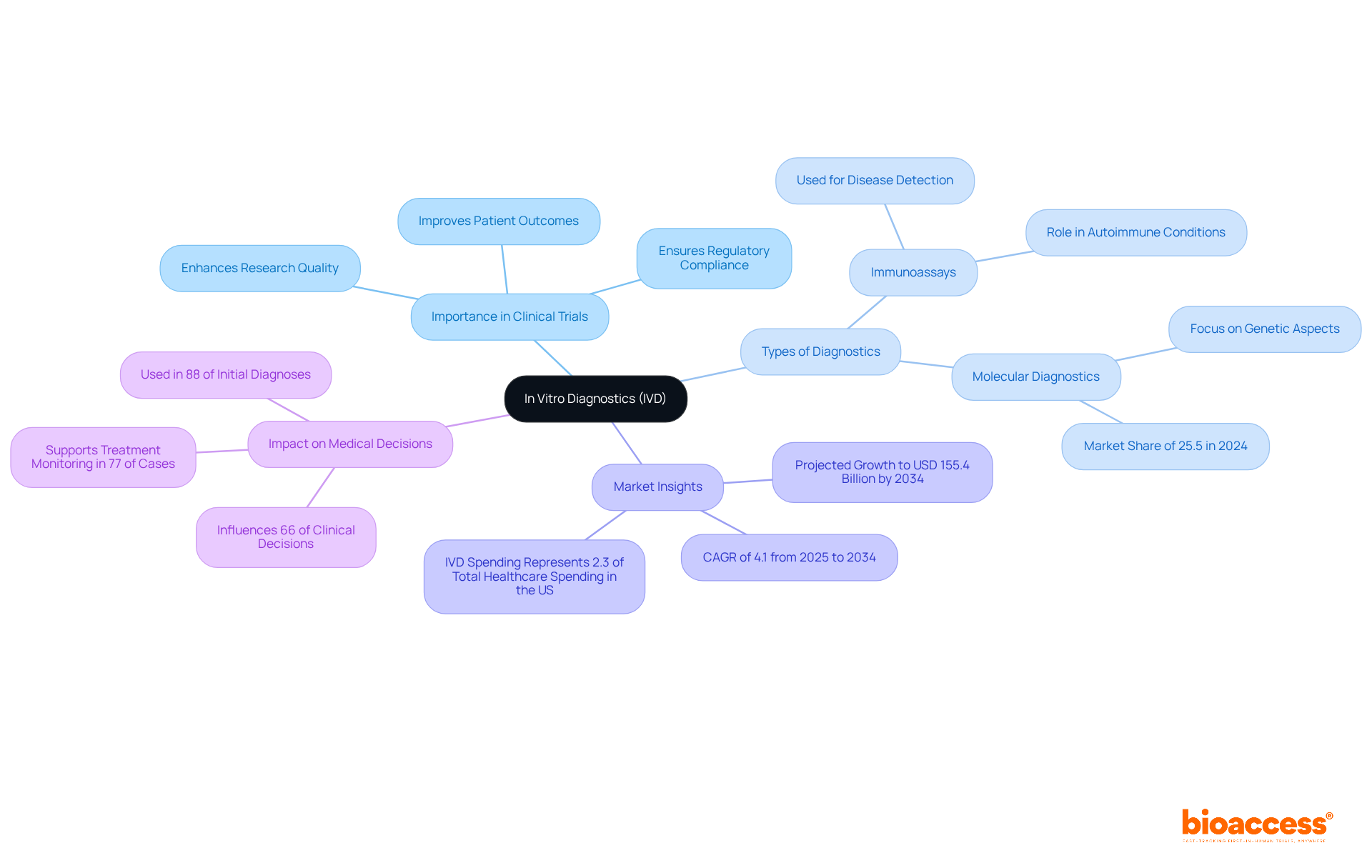
In vitro diagnostics (IVD) play a crucial role in clinical research, encompassing tests performed on samples like blood, urine, or tissue obtained from the human body. These diagnostics are essential, providing vital information that informs treatment decisions and enhances patient care. Recent advancements in IVD technology, particularly in molecular diagnostics and point-of-care testing, have significantly boosted diagnostic accuracy and accessibility. For example, the molecular diagnostics segment accounted for 25.5% of the market share in 2024 and is projected to reach USD 40.8 billion by 2034. This trend reflects a growing reliance on these technologies for precise disease detection and monitoring.
Understanding the various types of diagnostics available—from immunoassays to advanced molecular tests—is critical for selecting and collaborating with an in vitro diagnostics company. Each diagnostic type serves distinct purposes, ranging from initial disease detection to ongoing monitoring of treatment efficacy. The integration of IVDs into medical trials not only enhances research quality but also ensures compliance with regulatory standards, ultimately leading to improved patient outcomes. Statistics indicate that IVD testing influences approximately 66% of medical decisions, underscoring their essential role in patient management. As the IVD landscape continues to evolve, leveraging these technologies will be vital for advancing medical research and optimizing treatment pathways.
When selecting an in vitro diagnostics (IVD) company, it’s crucial to consider several key criteria that can significantly influence the success of your clinical trials:
Regulatory Compliance: Verify that the organization adheres to both local and international regulations, such as FDA or CE marking. This compliance is essential for ensuring the validity and reliability of the diagnostics. Companies like bioaccess provide comprehensive support in navigating these regulatory landscapes, including feasibility and selection of research sites and principal investigators (PIs), as well as review and feedback on study documents to comply with country requirements.
Technological Expertise: Assess the organization’s technological capabilities, including the variety of tests provided and their commitment to innovation in creating new diagnostic solutions. Companies that invest in state-of-the-art technology are better positioned to meet evolving market demands.
Quality Assurance: Investigate the organization’s quality control processes and relevant certifications. A robust quality assurance framework is vital for maintaining high testing standards and ensuring accurate results. Firms such as bioaccess highlight quality through stringent project management and oversight, guaranteeing adherence to industry standards during the testing process.
Experience and Reputation: Look for organizations with a demonstrated history in carrying out medical studies. Their experience can greatly enhance the likelihood of a successful study, as established firms often have refined processes and insights into best practices. Bioaccess, for instance, provides significant expertise in study project management, which can be essential in navigating the intricacies of research studies.
Support Services: Assess the level of support provided, including training, technical assistance, and data management. Comprehensive support can facilitate smoother collaboration and improve the efficiency of the research process. Cultural alignment between your organization and the manufacturer can enhance working relationships through shared values and collaboration. Bioaccess provides tailored support services that align closely with client goals, enhancing the overall research experience.
Cost-Effectiveness: Review the pricing structure to ensure it aligns with your budget while still meeting quality expectations. A transparent pricing model can help avoid hidden costs and optimize your return on investment. Bioaccess emphasizes clear, consistent communication regarding costs, which can enhance the overall value of the partnership.
By carefully assessing these standards, you can select an in vitro diagnostics company that aligns with your research goals, ultimately enhancing the overall investigation process.

To initiate collaboration with your chosen IVD company, follow these essential steps:
Establish Clear Objectives: Clearly define the goals of your collaboration, including specific outcomes expected from the IVD tests. Research indicates that successful partnerships often hinge on well-articulated objectives, significantly enhancing project alignment and outcomes.
Develop a Collaboration Agreement: Draft a formal agreement that outlines roles, responsibilities, timelines, and deliverables. This ensures both parties are aligned and sets a foundation for accountability.
Set Up Communication Channels: Establish regular communication protocols to facilitate updates, feedback, and issue resolution throughout the collaboration. Effective communication is crucial; as noted by industry leaders, it can significantly impact the success of partnerships.
Conduct Kick-off Meetings: Organize initial meetings to introduce teams, discuss project timelines, and clarify expectations. This fosters a collaborative atmosphere and helps build rapport between teams.
Integrate Systems and Processes: Work together with the IVD organization to combine their testing methods with your research protocols. This ensures seamless data flow and compliance, which is vital for maintaining the integrity of the study.
Monitor Progress: Regularly review the collaboration’s progress against established objectives. Make adjustments as necessary to stay on track, as continuous evaluation is key to achieving desired outcomes.
By adhering to these steps, you can establish a strong basis for a fruitful collaboration with your in vitro diagnostics company, ultimately enhancing the quality and efficiency of your studies.

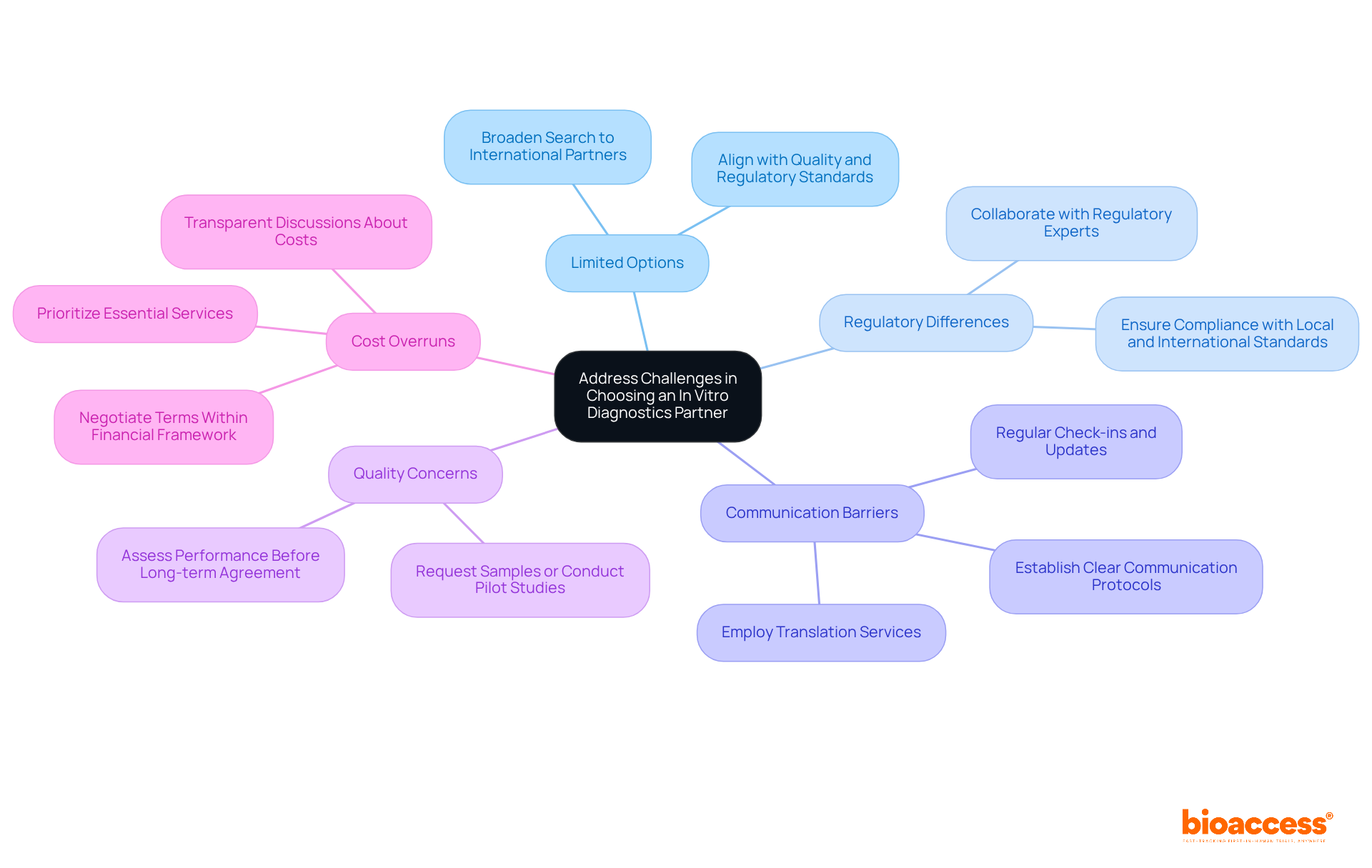
Selecting the right in vitro diagnostics company as a partner is crucial for the success of clinical research, yet it can be fraught with challenges. Here are common issues and effective strategies to navigate them:
Limited options arise in certain regions where the availability of in vitro diagnostics companies may be restricted. To mitigate this, broaden your search to include international partners that align with your quality and regulatory standards. This approach can provide access to innovative solutions and diverse expertise.
Regulatory Differences: Regulatory requirements vary significantly across countries, complicating partnerships. Collaborate with regulatory experts, such as Katherine Ruiz, who can guide you through these complexities, ensuring compliance with local and international standards. This is essential for preserving the integrity of your research studies and accelerating market entry.
Communication Barriers: Language and cultural differences can impede effective collaboration. Establish clear communication protocols and consider employing translation services to facilitate understanding. Regular check-ins and updates can also help bridge any gaps in communication.
Quality Concerns: If there are uncertainties regarding the quality of diagnostics, proactively request samples or conduct pilot studies to assess performance. This due diligence can help ensure that the partner's products meet your medical needs and standards before entering a long-term agreement.
Cost Overruns: Budget constraints can complicate the selection process. Focus on prioritizing essential services and negotiate terms that fit within your financial framework. Transparent discussions about costs and expectations can help prevent overruns and ensure a mutually beneficial partnership.
By anticipating these challenges and implementing proactive strategies, including leveraging comprehensive clinical trial management services such as those offered by bioaccess—feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—you can significantly enhance your chances of selecting a suitable in vitro diagnostics company as your partner. This ultimately contributes to the success of your clinical trial outcomes.
Selecting and collaborating with an in vitro diagnostics (IVD) company is crucial for enhancing the quality and efficiency of clinical trials. Understanding the vital role IVDs play in patient care and research empowers stakeholders to make informed decisions that lead to improved health outcomes. The significance of partnering with a reputable IVD provider cannot be overstated; it directly influences the reliability of diagnostic results and the overall success of clinical studies.
This article highlights several key considerations for choosing an IVD partner, including:
Each factor is essential in ensuring that the selected company meets the specific needs of a clinical trial while adhering to industry standards. Moreover, effective collaboration strategies—such as establishing clear objectives and communication channels—are vital for fostering a productive partnership.
In summary, navigating the complexities of selecting an IVD company demands careful evaluation and proactive planning. By addressing potential challenges and leveraging best practices, researchers can strengthen their partnerships and contribute to the advancement of medical science. Engaging with the right IVD provider not only streamlines the research process but also lays the groundwork for innovative diagnostic solutions that can transform patient care. Taking these steps ensures that the collaboration evolves from a mere contractual agreement into a strategic alliance aimed at achieving meaningful outcomes in healthcare.
What are in vitro diagnostics (IVD)?
In vitro diagnostics (IVD) are tests performed on samples such as blood, urine, or tissue obtained from the human body, providing vital information that informs treatment decisions and enhances patient care.
Why are IVDs important in clinical trials?
IVDs are essential in clinical trials as they enhance research quality, ensure compliance with regulatory standards, and ultimately lead to improved patient outcomes.
What advancements have been made in IVD technology?
Recent advancements in IVD technology, particularly in molecular diagnostics and point-of-care testing, have significantly increased diagnostic accuracy and accessibility.
What is the market trend for molecular diagnostics?
The molecular diagnostics segment accounted for 25.5% of the IVD market share in 2024 and is projected to reach USD 40.8 billion by 2034, reflecting a growing reliance on these technologies for precise disease detection and monitoring.
What types of diagnostics are available?
There are various types of diagnostics available, including immunoassays and advanced molecular tests, each serving distinct purposes from initial disease detection to ongoing monitoring of treatment efficacy.
How do IVDs influence medical decisions?
Statistics indicate that IVD testing influences approximately 66% of medical decisions, underscoring their essential role in patient management.
What is the significance of leveraging IVD technologies in medical research?
Leveraging IVD technologies is vital for advancing medical research and optimizing treatment pathways as the IVD landscape continues to evolve.