

Adaptive design trials are transforming the clinical research landscape, especially in Romania, where regulations are adapting to support this innovative approach. These trials present a unique opportunity for researchers to make real-time adjustments based on interim data, significantly boosting the efficiency and effectiveness of drug development.
However, as the demand for flexibility in research increases, what specific requirements and challenges must researchers navigate to successfully implement adaptive design trials in Romania?
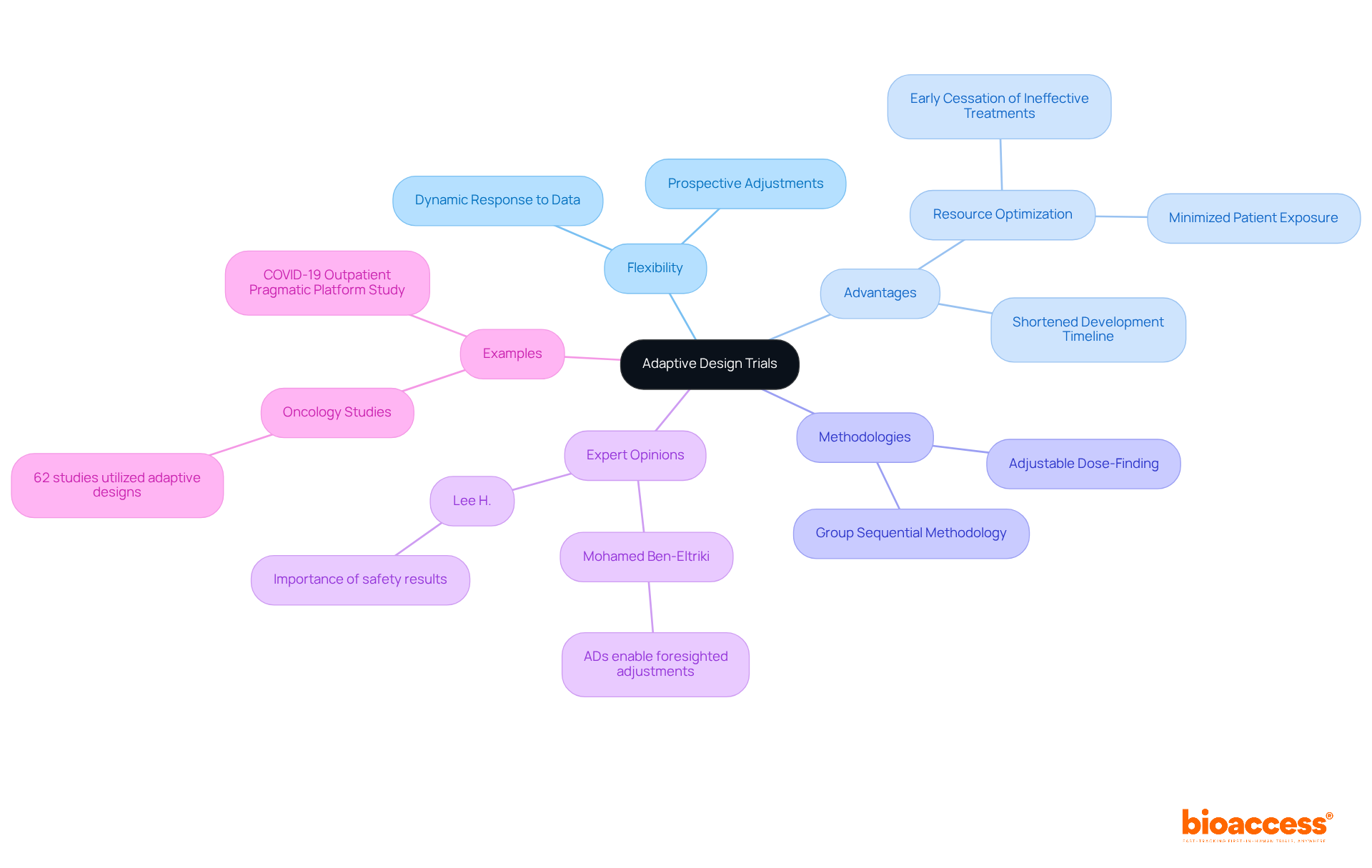
Adaptive design studies represent a pivotal category of clinical research, which must adhere to the requirements for adaptive design trials in Romania, allowing for prospectively planned adjustments to various study elements based on interim data analysis. This flexibility empowers researchers to make informed decisions throughout the study, such as modifying sample sizes, treatment regimens, or endpoints, ultimately enhancing the study's efficiency and effectiveness. Unlike conventional fixed formats, these flexible studies can dynamically respond to gathered information, potentially leading to quicker and more precise insights into a treatment's effectiveness and safety.
The advantages of flexible testing approaches are particularly evident in their ability to optimize resource allocation and shorten the timeline for medication development. For instance, adaptive studies can facilitate the early cessation of ineffective treatments, thereby conserving resources and minimizing patient exposure to non-therapeutic interventions. A notable example is the application of group sequential methodology, which allows for interim analyses that can lead to halting a study for futility or success based on predefined criteria. This strategy has been successfully implemented in numerous oncology studies, demonstrating its potential to streamline the clinical development process.
Experts in the field have underscored the benefits of flexible approaches, asserting that they significantly enhance study efficiency. Mohamed Ben-Eltriki noted, "ADs enable foresighted adjustments to one or more elements of the research based on gathered information from the experiment," emphasizing the core principle of flexible models. Furthermore, the integration of these approaches has been shown to increase the likelihood of identifying effective therapies while reducing the overall costs associated with research studies. As the landscape of medical research continues to evolve, the requirements for adaptive design trials in Romania are increasingly recognized as a crucial strategy for advancing drug development. Notably, flexible approaches were employed in 62 oncology studies, with the adjustable dose-finding method accounting for 63.5% of all phase 1 evaluations. The COVID-19 Outpatient Pragmatic Platform Study exemplifies the practical benefits of flexible approaches in urgent clinical scenarios.
In Romania, the execution of flexible testing must comply with the requirements for adaptive design trials in Romania, which are contingent upon strict adherence to the regulations established by the National Agency for Medicines and Medical Devices (ANMDMR). This process begins with obtaining ethical consent from the relevant ethics boards and presenting a comprehensive study protocol that clearly outlines the flexible characteristics of the research. Compliance with Good Clinical Practice (GCP) guidelines is not just recommended; it is mandatory.
Sponsors must provide detailed justifications for any proposed modifications to the study design, ensuring that these changes maintain the scientific integrity of the research. Notably, the recent introduction of a 30-day silent approval procedure for studies significantly streamlines the regulatory pathway, meeting the requirements for adaptive design trials in Romania and facilitating a quicker start for adaptive studies. This enhancement is particularly vital, given that the average approval duration for ethical submissions hovers around 60 days. The potential for expedited timelines positions Romania as a competitive player in the clinical research arena, making it an attractive option for sponsors and researchers alike.

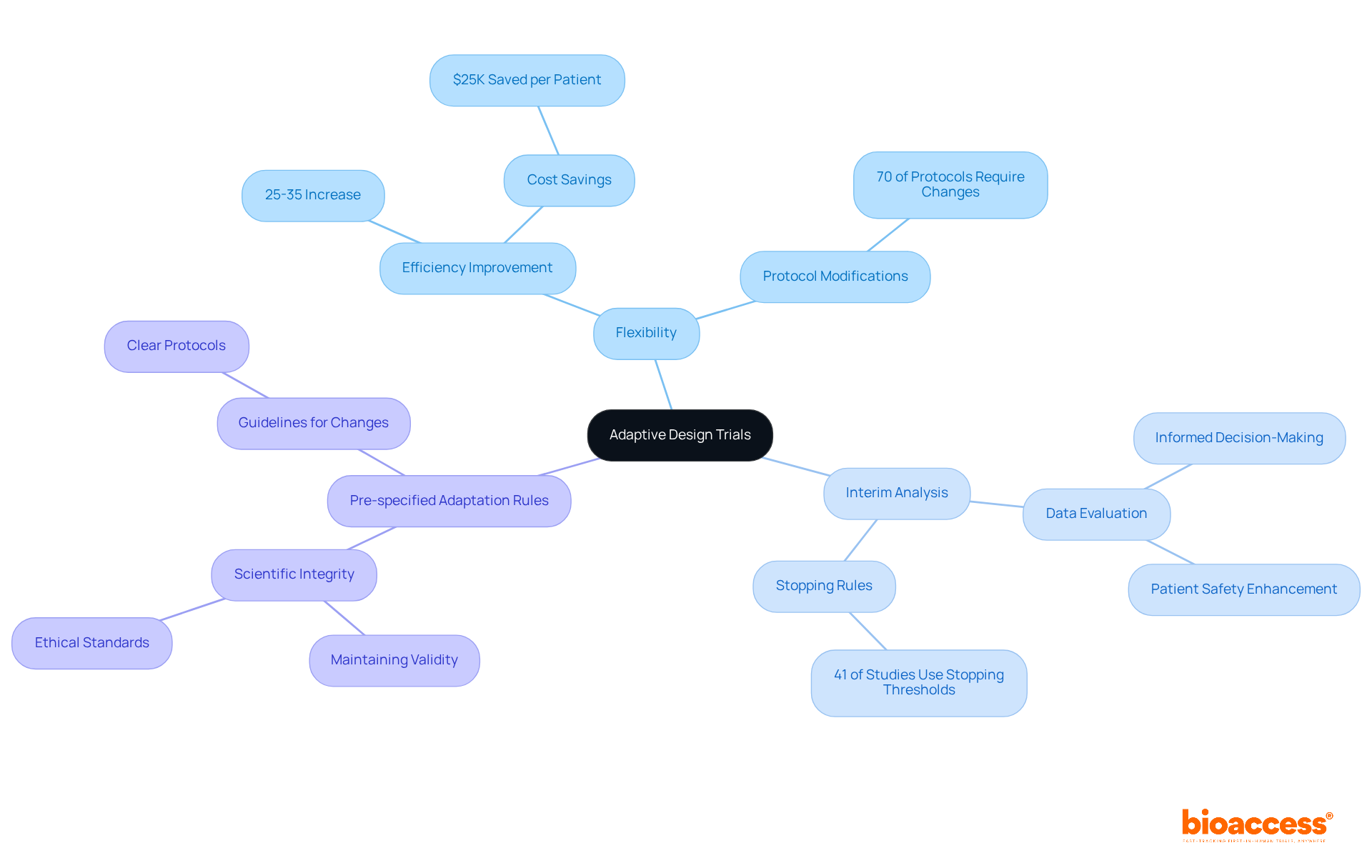
Adaptive design studies stand out in clinical research due to their essential characteristics: flexibility, interim analysis, and pre-specified adaptation rules.
Flexibility allows researchers to adjust study parameters based on real-time data, leading to a remarkable 25-35% increase in experimental efficiency and significant cost savings. Did you know that 70% of clinical study protocols require modifications during the research period? This statistic highlights the critical need for adaptability in today's fast-paced research environment.
Interim analysis is another vital component, enabling the evaluation of data at predetermined intervals. This process informs decisions about whether to continue, modify, or halt the study, enhancing patient safety and optimizing resource utilization. By incorporating interim analysis, researchers can make informed choices that directly impact the success of their studies.
Pre-specified adaptation rules are crucial as they outline the conditions under which changes can be implemented. These rules ensure that the study maintains its scientific integrity and adheres to ethical standards. Together, these characteristics empower adaptive studies to respond effectively to emerging data, potentially accelerating the development of innovative treatments. In a landscape where collaboration is key, understanding and implementing these principles can significantly enhance the outcomes of clinical research.
Adaptive studies are pivotal in clinical research, significantly enhancing the efficiency and effectiveness of drug development. These experiments allow for real-time adjustments based on interim outcomes, which can lead to a reduction in the time and resources required to bring new therapies to market. For example, Mahlich et al. (2021) simulated scenarios showing that flexible approaches can boost Phase III success rates from approximately 62% to between 70% and 80%, potentially shortening overall development timelines and cutting costs by around 14%.
Moreover, adaptive studies minimize patient exposure to ineffective therapies, thereby elevating ethical standards in clinical research. This is particularly crucial in fields like oncology and rare diseases, where traditional testing methods often struggle to address the complexities of treatment efficacy. The BATTLE trial, a landmark study in lung cancer, illustrates this approach by enrolling 341 patients from 2006 to 2009 and utilizing biomarker profiles to customize treatments, achieving a disease control rate of 46% and setting a new standard for personalized therapy.
Expert opinions highlight the ethical implications of flexible strategies, with many advocating for their ability to foster more tailored treatment methods. José Pinheiro emphasizes that "flexible approaches should be regarded only if they contribute positively to the overall drug development process." By enabling researchers to adjust protocols based on patient responses, flexible studies not only enhance patient safety but also raise the overall standard of medical research. As healthcare increasingly shifts towards more patient-centered models, the importance of flexible testing in facilitating timely access to effective therapies cannot be overstated. Furthermore, it is estimated that at least 20% of clinical studies utilized flexible designs, as reported by The Tufts Center for the Study of Drug Development in 2013, reflecting the growing acceptance of these methodologies in the industry. However, it is crucial to recognize the operational challenges associated with the requirements for adaptive design trials in Romania, which necessitate meticulous planning and execution to ensure their success.

Adaptive design trials represent a pivotal advancement in clinical research, particularly within Romania's regulatory framework. By enabling modifications based on interim data, these trials significantly enhance the efficiency and relevance of drug development processes. This adaptability not only fosters informed decision-making but also accelerates the journey toward discovering effective treatments.
Key insights from the article underscore the essential characteristics of adaptive design trials, including:
These elements work synergistically to optimize resource allocation and minimize patient exposure to ineffective therapies, ultimately cultivating a more ethical and efficient research environment. The successful application of adaptive designs in various studies, particularly in oncology, highlights their potential to revolutionize clinical trials.
In light of these findings, embracing adaptive design trials is vital for advancing medical research in Romania and beyond. As the landscape of drug development continues to evolve, stakeholders must prioritize understanding and integrating these methodologies to enhance patient outcomes and streamline the research process. A commitment to adaptive designs not only reflects a progressive approach to clinical trials but also aligns with the broader goal of delivering timely and effective therapies to those in need.
What are adaptive design trials?
Adaptive design trials are a type of clinical research that allows for prospectively planned adjustments to various study elements based on interim data analysis. This flexibility enables researchers to modify aspects such as sample sizes, treatment regimens, or endpoints to enhance the study's efficiency and effectiveness.
How do adaptive design trials differ from conventional studies?
Unlike conventional fixed-format studies, adaptive design trials can dynamically respond to gathered information, allowing for quicker and more precise insights into a treatment's effectiveness and safety.
What are the advantages of using adaptive design trials?
The advantages include optimizing resource allocation, shortening the timeline for medication development, and facilitating the early cessation of ineffective treatments. This approach conserves resources and minimizes patient exposure to non-therapeutic interventions.
Can you provide an example of adaptive design methodology?
An example is group sequential methodology, which allows for interim analyses that can lead to stopping a study for futility or success based on predefined criteria. This strategy has been successfully implemented in numerous oncology studies.
What benefits have experts identified regarding adaptive design trials?
Experts have noted that adaptive design trials significantly enhance study efficiency, increase the likelihood of identifying effective therapies, and reduce overall research costs.
How prevalent are adaptive design approaches in oncology studies?
Flexible approaches have been employed in 62 oncology studies, with the adjustable dose-finding method accounting for 63.5% of all phase 1 evaluations.
What is the significance of the COVID-19 Outpatient Pragmatic Platform Study in relation to adaptive design trials?
The COVID-19 Outpatient Pragmatic Platform Study exemplifies the practical benefits of flexible approaches in urgent clinical scenarios, showcasing how adaptive design can be effectively utilized in real-world situations.