


The article outlines ten significant benefits of decentralized trials for clinical research directors, emphasizing notable improvements in:
These advantages are substantiated by compelling evidence indicating that decentralized trials can:
Consequently, these factors collectively optimize the clinical research process, making a strong case for the adoption of decentralized methodologies in clinical trials.
Decentralized trials are fundamentally reshaping the landscape of clinical research, presenting a compelling solution to enduring challenges in participant recruitment, retention, and diversity. As research directors strive to enhance the efficiency and inclusivity of their studies, these innovative methodologies offer a wealth of opportunities to engage broader patient populations while alleviating logistical burdens.
The key benefits of adopting decentralized trials are significant, yet the question remains: how can these trials be effectively implemented to maximize their transformative potential? This inquiry not only underscores the relevance of decentralized trials but also invites a deeper exploration of their impact on the clinical research landscape.
bioaccess® strategically leverages the regulatory pace of Latin America, the diverse patient demographics of the Balkans, and the efficient routes in Australia to significantly enhance the effectiveness of decentralized trials. This powerful combination facilitates ethical approvals in an impressive 4-6 weeks, with participant enrollment occurring 50% faster than in traditional markets. Such expedited timelines are crucial for research directors seeking to optimize study efficiency and flexibility.
Notably, while the average duration for ethical approvals in distributed studies is recorded at 48 days, bioaccess® consistently surpasses this benchmark, positioning itself as a vital partner in the clinical research landscape. Successful examples of decentralized trials powered by bioaccess® underscore the tangible benefits of this approach, reflecting its commitment to advancing medical innovations swiftly and effectively.

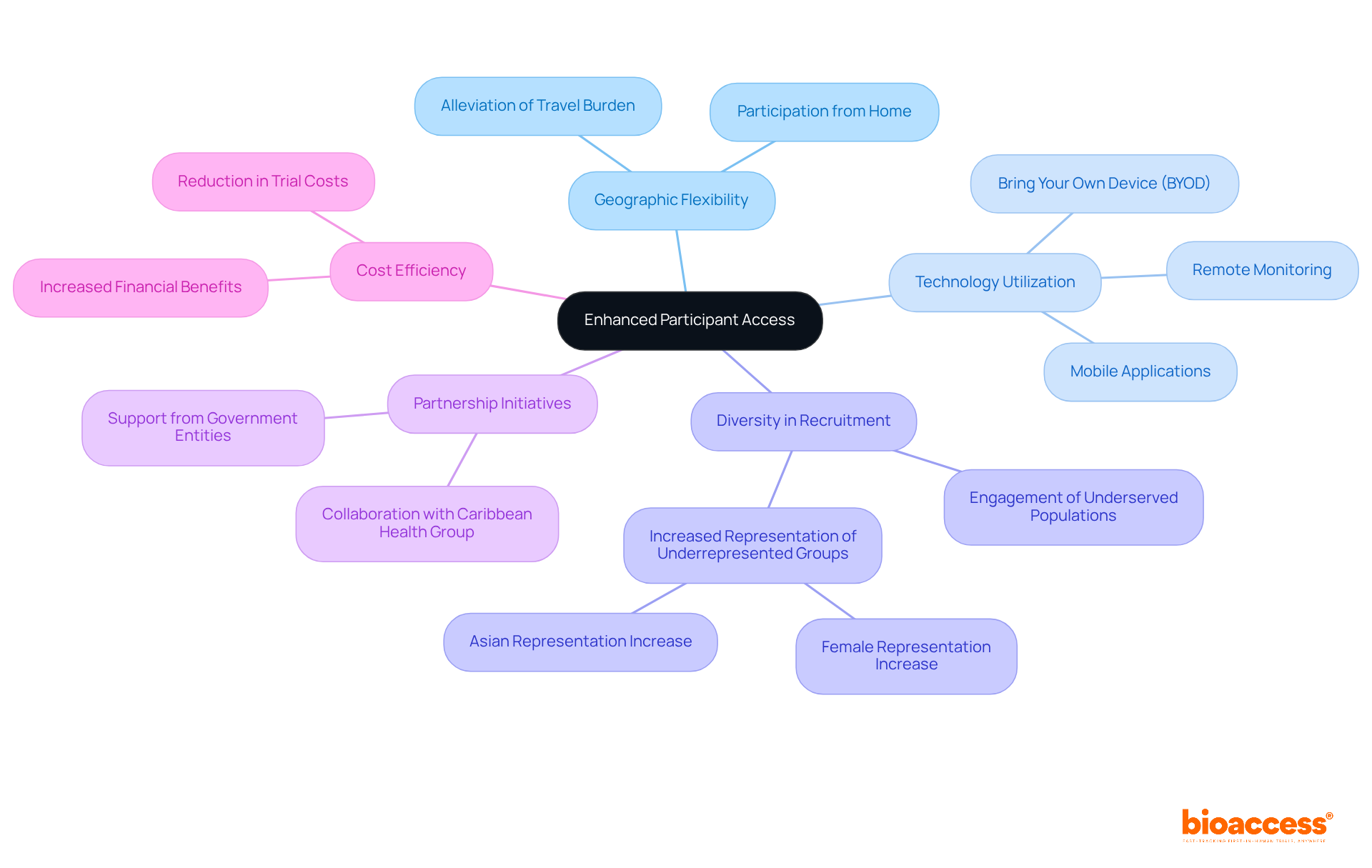
Decentralized trials empower participants to engage from their own locations, significantly alleviating travel burdens. This flexibility not only aids in wider recruitment but also focuses on underrepresented groups, thereby improving the diversity and inclusivity of research.
By leveraging telehealth and remote monitoring technologies, researchers can effectively connect with patients who previously faced barriers to participation. A survey revealed that 94% of patients would probably utilize a mobile application for clinical studies, with 45% considering the 'bring your own device' (BYOD) method more convenient.
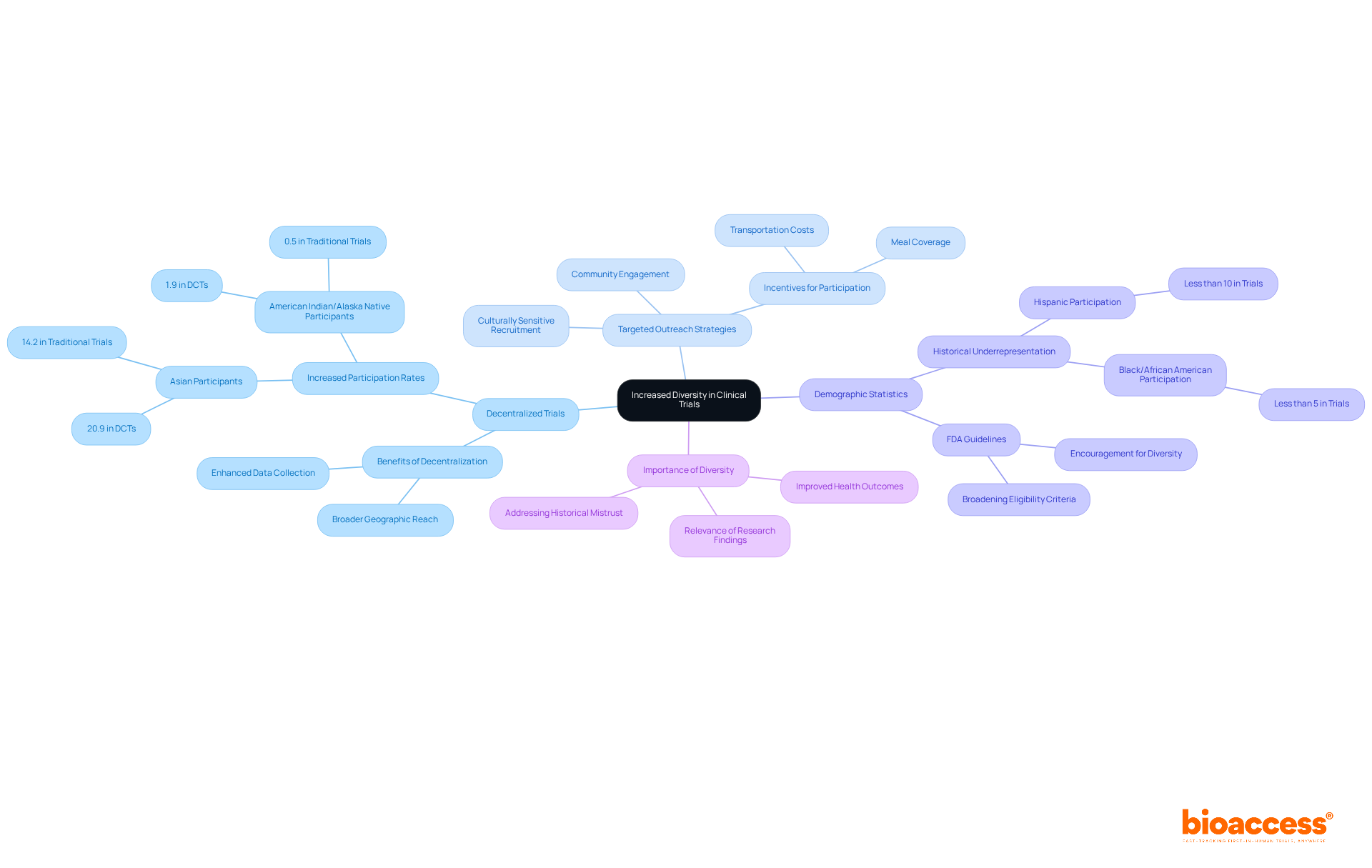
This transition towards distributed methodologies has been demonstrated to enhance recruitment diversity, as indicated by a significant rise in Asian representation in DCT-enabled studies, increasing from 14.2% to 20.9%. Moreover, decentralized trials have been associated with a 10-25% decrease in expenses, enhancing participation and making it more attractive to a broader audience.
Bioaccess™'s partnership with the Caribbean Health Group seeks to improve ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. This initiative has garnered significant backing from Colombia's Minister of Health, emphasizing the government's dedication to making Barranquilla a premier location for medical studies in Latin America.
As Yevgeniya Nusinovich observed, 'Decentralized trials allow individuals to take part in research studies from the comfort of their homes by utilizing telemedicine, mobile health applications, and remote monitoring devices.
Decentralized trials are essential in engaging diverse patient populations, facilitating participation from a broad spectrum of geographic and demographic backgrounds. This inclusive approach not only enriches the data collected but also yields valuable insights into how various populations respond to treatments. For instance, research indicates that decentralized trials have led to a notable increase in the involvement of Asian individuals, rising from 14.2% in traditional trials to 20.9% in trials enabled by decentralized methods. Moreover, the enrollment of American Indian or Alaska Native participants in decentralized trials has nearly quadrupled, highlighting the effectiveness of these strategies.
To enhance diversity, research directors should implement targeted outreach initiatives and culturally sensitive recruitment strategies. Engaging with community organizations and utilizing independent disease registries can significantly broaden the reach to underrepresented populations. Additionally, offering incentives such as transportation costs and meals can help alleviate barriers to participation, thereby fostering a more inclusive environment.
The importance of varied patient groups in medical research cannot be overstated. Experts emphasize that ensuring trials accurately represent the intended population is crucial for producing relevant and applicable research outcomes. This commitment to diversity not only enhances the accuracy of health outcomes but also addresses the historical underrepresentation of minority groups, a significant barrier to effective treatment advancement. By prioritizing inclusivity, clinical research can better serve the needs of all patients, ultimately leading to improved health outcomes across diverse communities.
Decentralized trials significantly enhance patient retention by offering flexible participation options, including remote monitoring and telehealth consultations. These conveniences alleviate the burden on individuals, fostering ongoing engagement throughout the study. Regular communication and personalized assistance, coupled with advanced digital tools, further enrich the experience for participants.
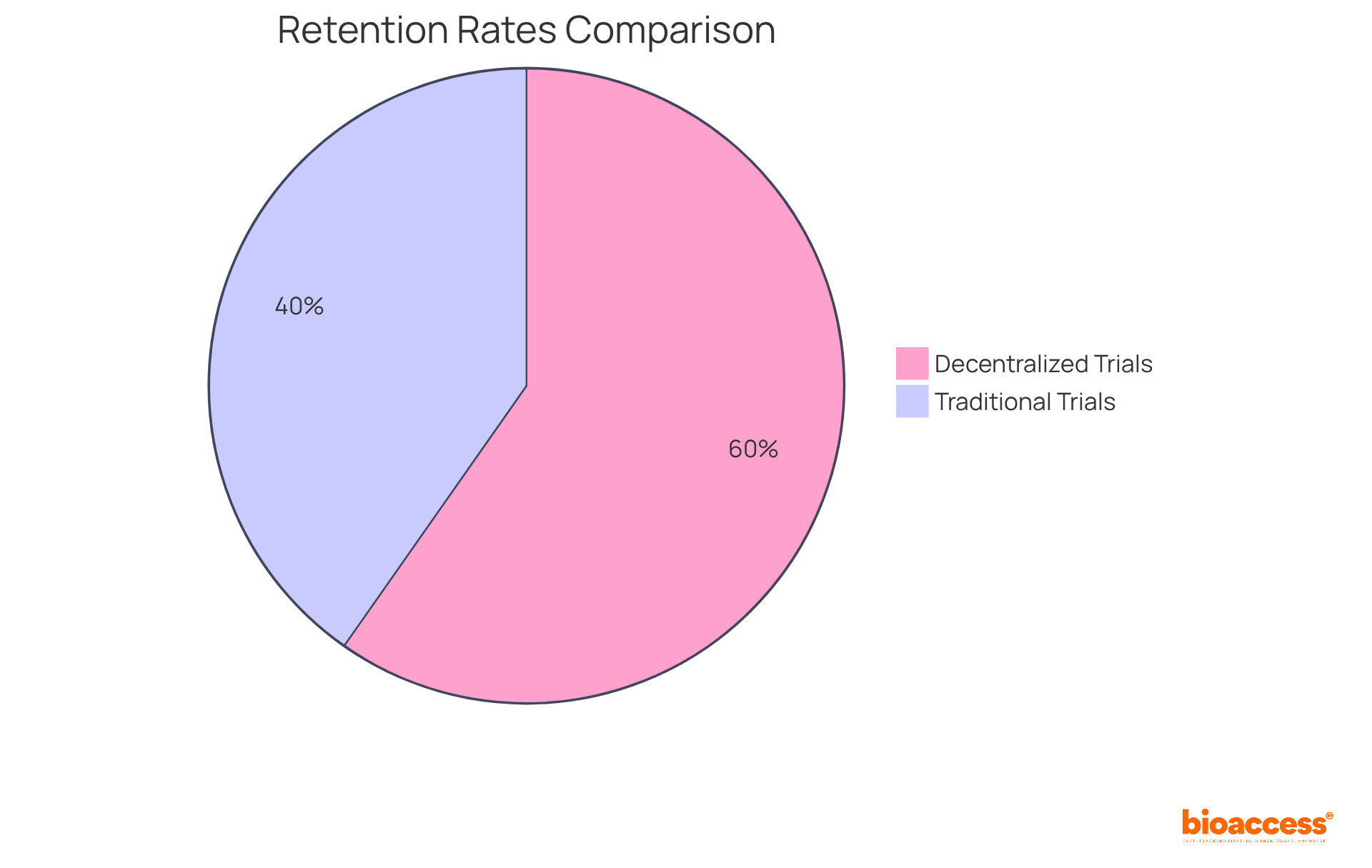
Research indicates that decentralized trials achieve retention rates of 89%, compared to just 60% for traditional models. This remarkable improvement is primarily due to the reduced logistical challenges and increased accessibility that remote monitoring facilitates. As Balaji Maddiboyina, a senior medical reviewer, notes, 'Decentralized trials improve accessibility by minimizing the burden on individuals through remote or local study activities.'
By integrating these strategies, clinical studies can forge stronger connections with participants, ultimately leading to enhanced retention rates and more successful outcomes. At bioaccess, our expertise in managing various types of research, including Early-Feasibility and First-In-Human studies, empowers us to implement these patient retention strategies effectively, ensuring that individuals remain engaged and informed throughout the study process.
Decentralized trials greatly improve the recruitment of individuals by utilizing digital platforms and remote engagement strategies. This innovative approach facilitates faster identification and enrollment of qualified candidates, often achieving recruitment timelines that are 50% shorter than conventional methods. For instance, virtual studies can enroll an average of 51 participants per month, compared to just 13 in traditional studies, underscoring the challenges that conventional methods face in meeting recruitment goals. By utilizing social media, online registries, and targeted outreach, clinical research directors can effectively amplify their recruitment efforts.
The integration of AI and data analytics further streamlines the recruitment process, enabling precise targeting and improved alignment of candidates to selection criteria. Notably, a recent NIH migraine study completed its recruitment four times quicker than anticipated, exemplifying the broader trend of decentralized trials enhancing recruitment timelines. Additionally, the use of digital tools has been shown to significantly reduce recruitment costs, with some studies reporting acquisition costs that are 12 times lower compared to traditional media.
Moreover, comprehensive medical study management services, such as those offered by bioaccess, play a critical role in supporting decentralized trials. Services including feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting ensure that trials are not only efficient but also compliant with local regulations. As the landscape of clinical studies evolves, embracing these digital innovations and robust management services is essential for achieving quicker, more effective, and inclusive participant enrollment.

Decentralized trials yield substantial cost reductions by minimizing the need for physical locations and their associated overhead expenses. By leveraging remote monitoring and telehealth visits, operational costs can be markedly decreased.
For example, a precision medicine company reported savings of approximately $20 million by reducing the number of physical sites from over 100 to just 25 for a study on age-related macular degeneration. Furthermore, decentralized trials promote faster recruitment and retention rates, which further enhances budget efficiency.
The typical implementation cost for a phase II DCT study is around $473,000, while for phase III, it escalates to $1,042,000. However, the return on investment (ROI) for incorporating DCT elements in phase II studies is estimated to be 4.62 times the additional cost, demonstrating that the initial investment can lead to significant financial returns.
By adopting decentralized trials, research directors can optimize their budgets and allocate resources more effectively, ultimately resulting in more successful study outcomes.

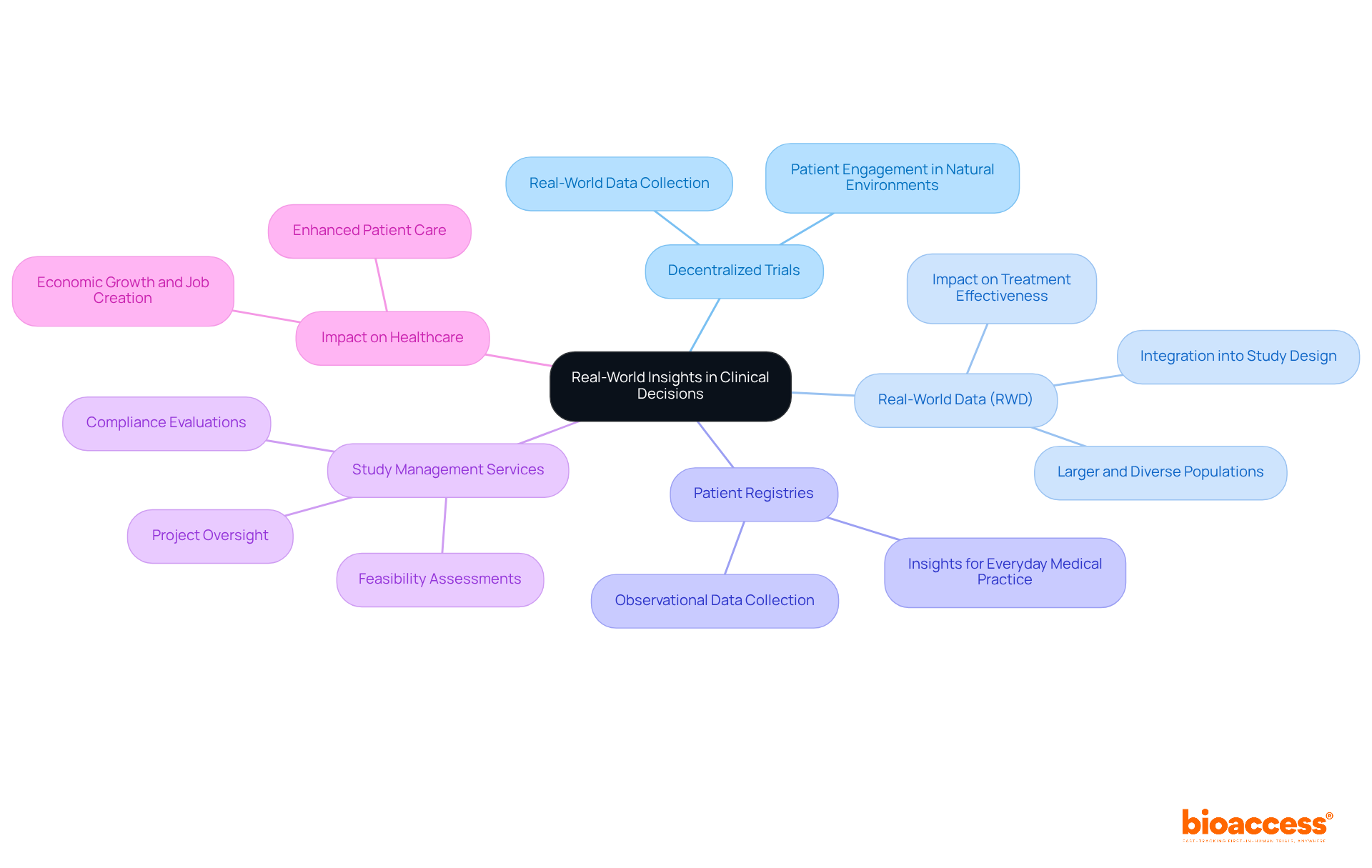
Decentralized trials present a unique opportunity to gather real-world data (RWD) that profoundly influences clinical decisions. By allowing participants to engage in their natural environments, researchers can collect data that accurately reflects genuine patient experiences and outcomes. This methodology not only enhances the significance of study results but also plays a crucial role in developing more effective therapies tailored to real-world scenarios.
Research indicates that patient registries can encompass larger and more diverse populations compared to traditional controlled studies, yielding insights that are more pertinent to everyday medical practice. Furthermore, the integration of real-world evidence into study design fosters a deeper understanding of how participant environments impact outcomes, ultimately driving improvements in treatment effectiveness and patient care.
Comprehensive research study management services, such as those provided by bioaccess—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—are vital in optimizing decentralized trials. These services ensure that studies are structured and executed efficiently, leveraging RWD to inform medical decisions and enhance patient care.
Case studies illustrating the role of real-world evidence in payer-provider collaboration demonstrate how RWD can contribute to job creation, economic growth, and healthcare enhancement in local communities.
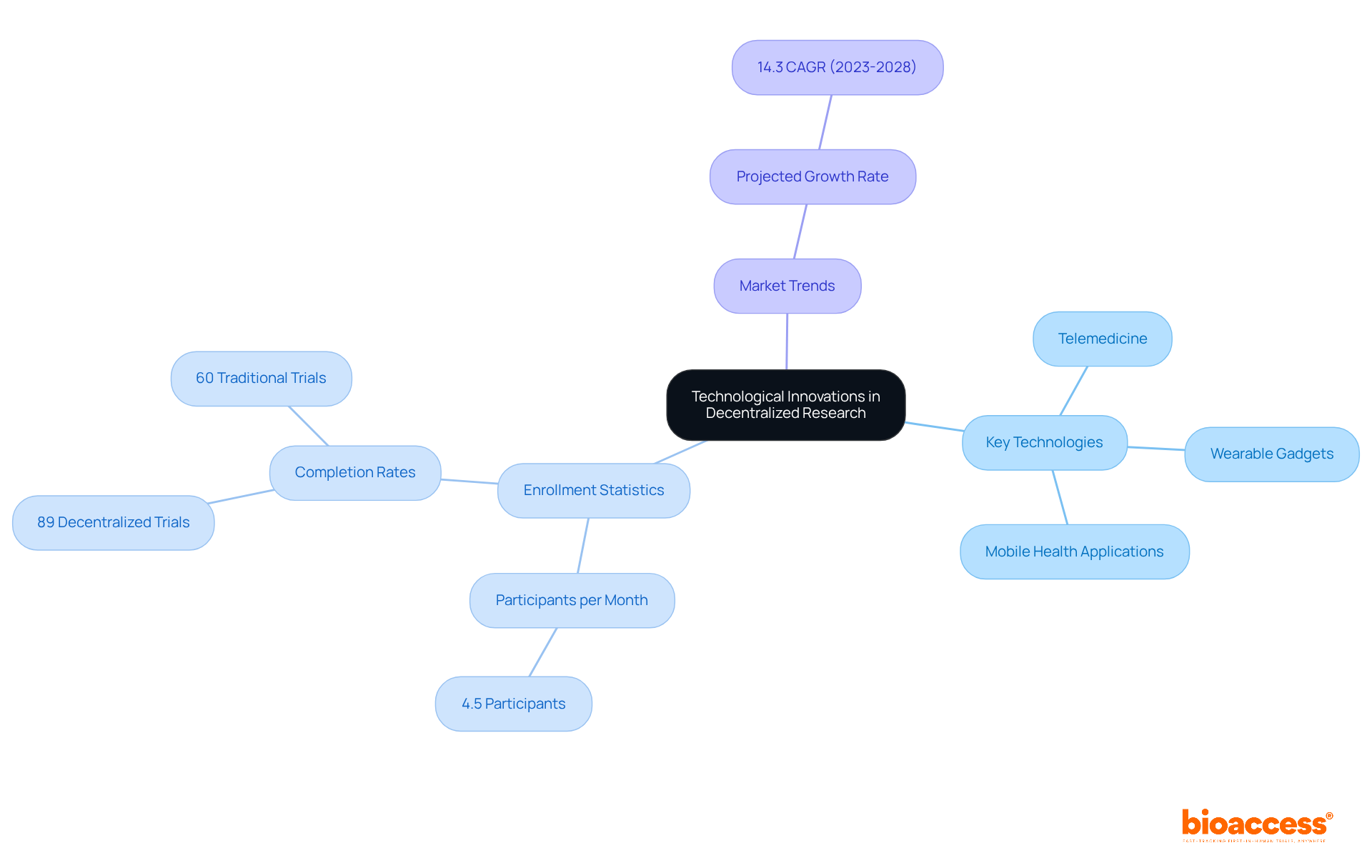
Technological advancements, including telemedicine, wearable gadgets, and mobile health applications, are pivotal for enabling decentralized trials in Latin America. These tools facilitate remote observation, effective data gathering, and improved engagement of individuals, allowing studies to be conducted outside conventional clinical settings.
Research indicates that distributed studies can enlist participants at a rate of 4.5 individuals each month, significantly higher than the 1.25 individuals per month typical of entirely in-person studies. Furthermore, 89% of patients who selected decentralized trials completed their studies, compared to only 60% in traditional settings, underscoring the effectiveness of these approaches.
Conversations with industry leaders emphasize that understanding local market dynamics and cultural nuances is essential for successful patient recruitment and retention in these studies. Moreover, the wearable device market is projected to expand at a compound annual growth rate of 14.3% from 2023 to 2028, highlighting the increasing importance and investment in wearable technology within research environments.
As the landscape of clinical research evolves, the integration of telemedicine and wearable technology will be crucial for enhancing study efficiency in decentralized trials and ensuring a positive experience for participants, particularly in the diverse and rapidly growing Latin American market.
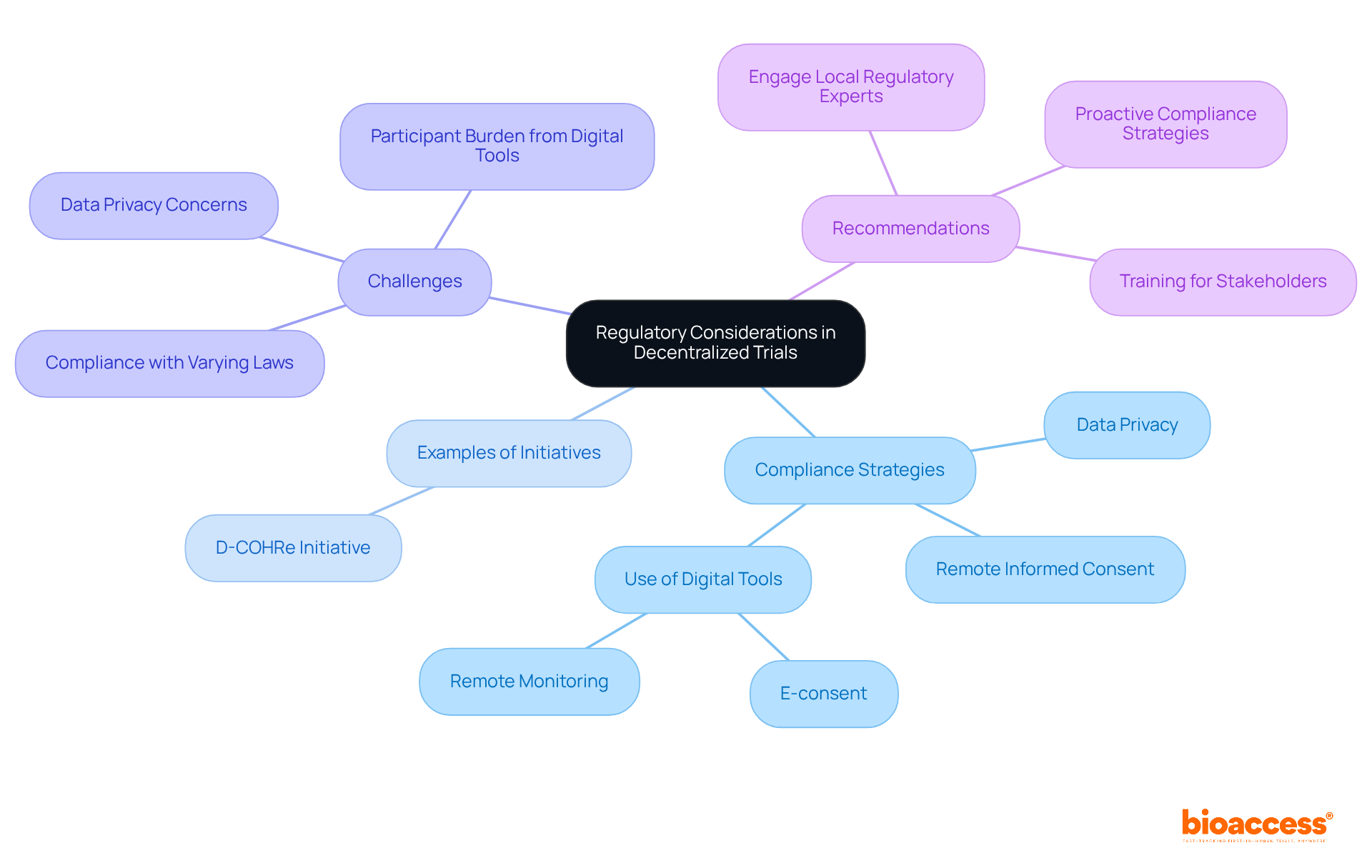
Navigating regulatory considerations is essential for the successful execution of distributed studies. Clinical research leaders must ensure that all study activities comply with both local and international regulations, particularly regarding data privacy and subject consent. The distributed medical research market, valued at USD 9.39 billion in 2025 and expected to attain USD 18.62 billion by 2030, underscores the increasing importance of adherence in this evolving landscape.
To effectively manage compliance, researchers should stay informed about the evolving regulatory frameworks. For instance, the FDA's recent guidance updates emphasize the significance of risk-based oversight and remote informed consent, critical for ensuring the safety of subjects and data integrity in distributed studies. Additionally, the integration of digital tools, such as e-consent and remote monitoring, can streamline compliance processes while enhancing data accuracy.
Examples of navigating rules in distributed health research include the D-COHRe initiative, which aims to enhance access to distributed studies through collaboration with regulatory bodies. Moreover, the challenges of data privacy remain a top concern, as companies must ensure compliance with varying privacy laws across regions. Engaging with local regulatory experts can provide valuable insights into market nuances, facilitating a smoother compliance journey.
Ultimately, a proactive strategy for regulatory adherence is crucial as the sector adopts decentralized trials. This enables researchers to leverage the advantages of these innovative methodologies while safeguarding participant rights and data integrity.
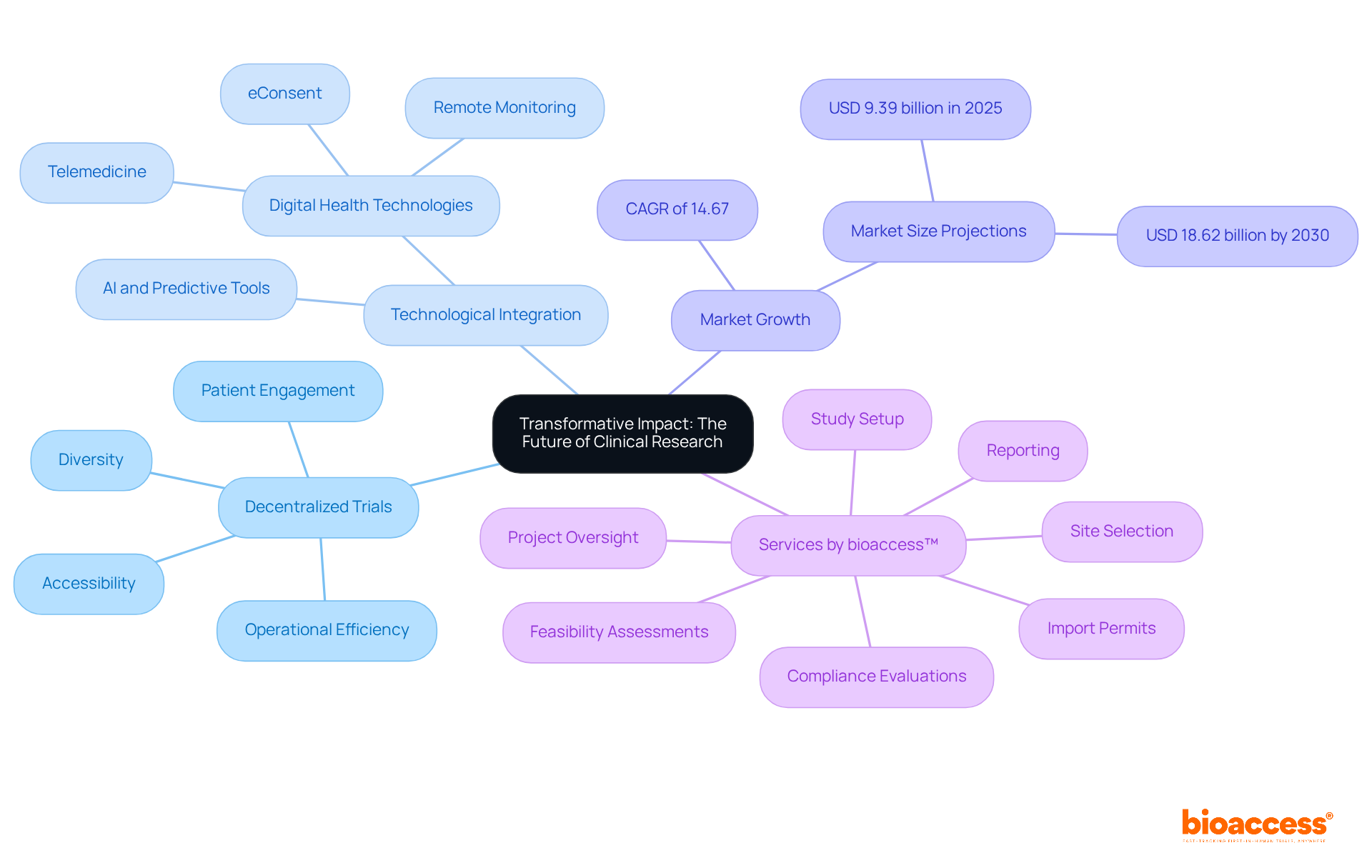
Decentralized trials are poised to revolutionize medical research by significantly enhancing accessibility, diversity, and operational efficiency. As the industry adapts to these changes, implementing distributed methodologies will be crucial for meeting the evolving needs of patients and stakeholders.
By leveraging advanced technologies and innovative strategies, research directors can boost patient engagement and streamline processes, positioning their organizations for success in this dynamic research landscape. Notably, the distributed research market is projected to grow from USD 9.39 billion in 2025 to USD 18.62 billion by 2030, reflecting a compound annual growth rate (CAGR) of 14.67%. This growth underscores the increasing recognition of decentralized trials as a means to improve the recruitment and retention of diverse patient groups, alleviating the strain on traditional research techniques.
Additionally, the integration of digital health technologies, such as telemedicine and remote monitoring, is expected to enhance patient accessibility, making participation in research studies more feasible for individuals facing challenges in conventional settings. However, significant hurdles remain, such as the absence of harmonized international data governance that must be addressed.
Organizations like bioaccess™ are at the forefront of this transformation, offering comprehensive management services for research studies, including:
These services not only simplify the research process but also support local economies through job creation and healthcare enhancement. Furthermore, the collaboration between bioaccess™ and Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia exemplifies the potential of decentralized trials to foster international partnerships and improve market access.
As organizations embrace these transformative trends, they will enhance the efficiency of clinical trials and contribute to a more inclusive, patient-centric research environment.
Decentralized trials signify a transformative shift in clinical research, delivering numerous benefits that enhance efficiency, accessibility, and participant engagement. By leveraging advanced technologies and innovative methodologies, these trials streamline recruitment and retention processes, ensuring a broader and more diverse patient population contributes to critical research. This modern approach addresses historical disparities in clinical studies, thereby improving the overall quality and applicability of research outcomes.
Key insights from the article reveal that decentralized trials can significantly reduce recruitment times, lower costs, and enhance patient retention through flexible participation options. The integration of telehealth and remote monitoring technologies is vital in overcoming geographic barriers, enabling researchers to connect with participants who may have previously faced challenges in accessing traditional clinical trials. Furthermore, the emphasis on real-world data collection enriches the research landscape, providing insights that more accurately reflect patient experiences and treatment responses.
As the clinical research landscape evolves, embracing decentralized trials is crucial for research directors aiming to optimize their studies and meet the needs of diverse patient populations. The future of clinical research transcends mere efficiency; it embodies inclusivity and patient-centered approaches. By adopting these innovative methodologies, organizations can enhance their research capabilities, improve health outcomes, and ultimately drive advancements in medical science that benefit all communities.
What is bioaccess® and how does it enhance decentralized trials?
Bioaccess® leverages the regulatory pace of Latin America, diverse patient demographics of the Balkans, and efficient routes in Australia to enhance decentralized trials. It facilitates ethical approvals in 4-6 weeks and participant enrollment occurs 50% faster than in traditional markets.
How does bioaccess® compare to traditional methods regarding ethical approvals?
The average duration for ethical approvals in distributed studies is 48 days, while bioaccess® consistently surpasses this benchmark, achieving approvals in a much shorter timeframe.
What are the benefits of decentralized trials for participant access?
Decentralized trials allow participants to engage from their own locations, reducing travel burdens and improving recruitment, particularly for underrepresented groups. This approach enhances diversity and inclusivity in research.
How do telehealth and remote monitoring technologies contribute to decentralized trials?
These technologies enable researchers to connect with patients who face barriers to participation, facilitating broader engagement in clinical studies.
What percentage of patients are likely to use a mobile application for clinical studies?
A survey indicated that 94% of patients would probably utilize a mobile application for clinical studies.
What impact do decentralized trials have on recruitment diversity?
Decentralized trials have increased Asian representation in studies from 14.2% to 20.9% and have nearly quadrupled the enrollment of American Indian or Alaska Native participants.
How do decentralized trials affect study expenses?
Decentralized trials have been associated with a 10-25% decrease in expenses, making participation more attractive to a broader audience.
What initiatives has bioaccess™ undertaken to improve recruitment in Colombia?
Bioaccess™ partnered with the Caribbean Health Group to enhance ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
Why is diversity important in clinical trials?
Engaging diverse patient populations enriches data collection and provides valuable insights into how various groups respond to treatments, ultimately leading to improved health outcomes for all communities.
What strategies can research directors implement to enhance diversity in trials?
Research directors should employ targeted outreach initiatives, culturally sensitive recruitment strategies, and offer incentives like transportation costs and meals to alleviate barriers to participation.