


Remote patient monitoring (RPM) in clinical trials presents substantial advantages, such as enhanced patient engagement, cost reduction, and improved data quality. These benefits collectively streamline the research process and elevate outcomes. This article underscores how RPM enables real-time data collection, diminishes operational expenses, and nurtures continuous communication between researchers and participants. Ultimately, these elements contribute to more efficient and patient-centric clinical trials.
Remote patient monitoring (RPM) transcends mere technological advancement; it signifies a paradigm shift in the conduct of clinical trials. As the healthcare landscape evolves, the integration of RPM into clinical research stands to enhance efficiency, elevate patient engagement, and diminish operational costs. Yet, amid these advantages, a crucial question emerges: how can researchers adeptly leverage these tools to surmount the traditional obstacles of clinical trials? This article delves into the multifaceted benefits of remote patient monitoring, illuminating its transformative influence on the future of clinical research and the opportunities it creates for both patients and researchers.
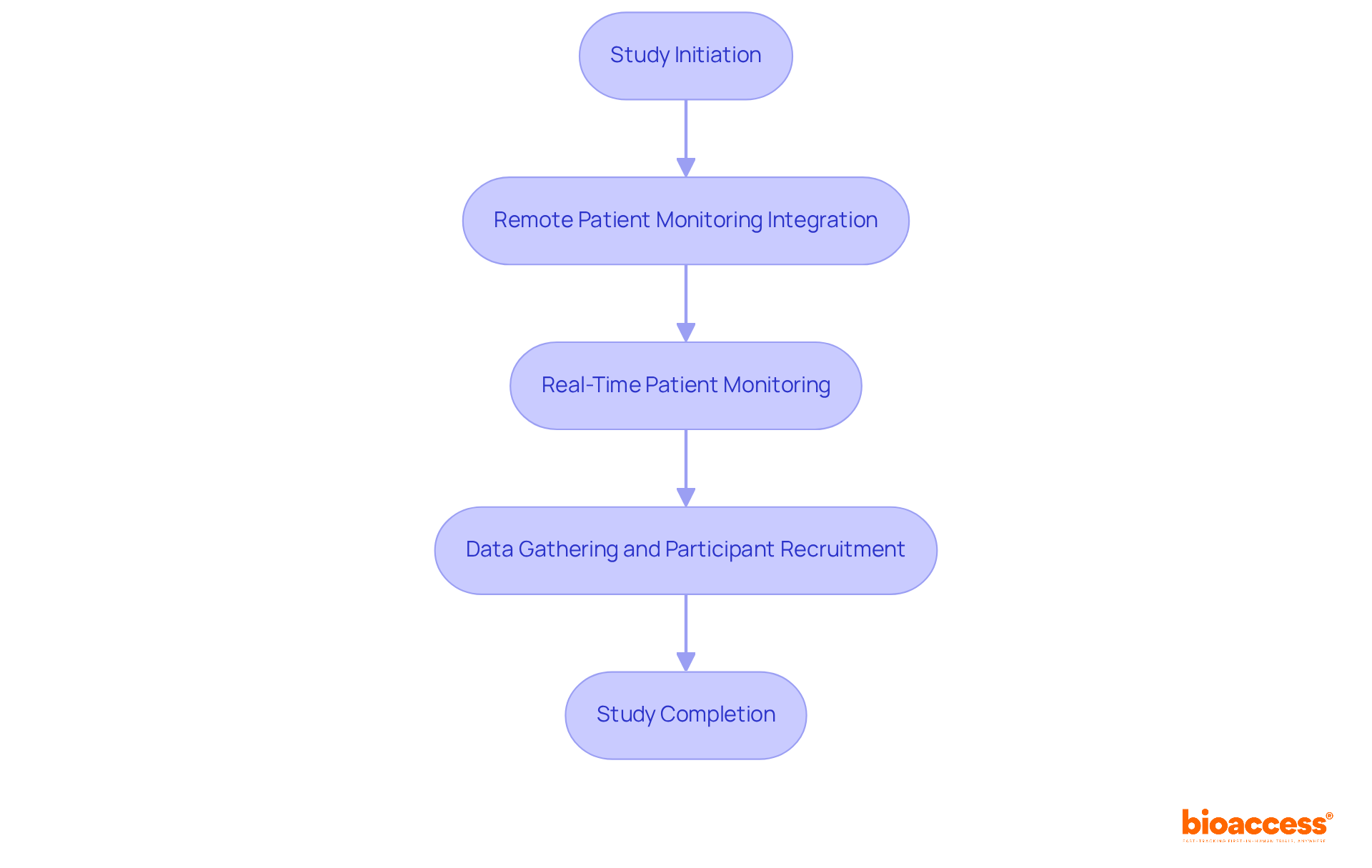
bioaccess® effectively integrates remote patient monitoring clinical trials into its research processes, which leads to a significant reduction in participant recruitment and data gathering timelines. By leveraging remote patient monitoring clinical trials, bioaccess® enhances study efficiency, facilitating real-time patient monitoring that accelerates the overall timeline from study initiation to completion. This innovative approach not only expedites research studies but also reinforces the organization's commitment to ethical and prompt authorizations.
Furthermore, bioaccess® offers comprehensive services, including rapid site activation with pre-qualified networks, ensuring that studies are executed efficiently and effectively. As the remote health monitoring market is projected to grow substantially, with an anticipated 115.5 million users by 2027, bioaccess® is strategically positioned to capitalize on this trend, ensuring that remote patient monitoring clinical trials are conducted more swiftly and efficiently in delivering valuable health solutions across LATAM, Eastern Europe, and Australia.
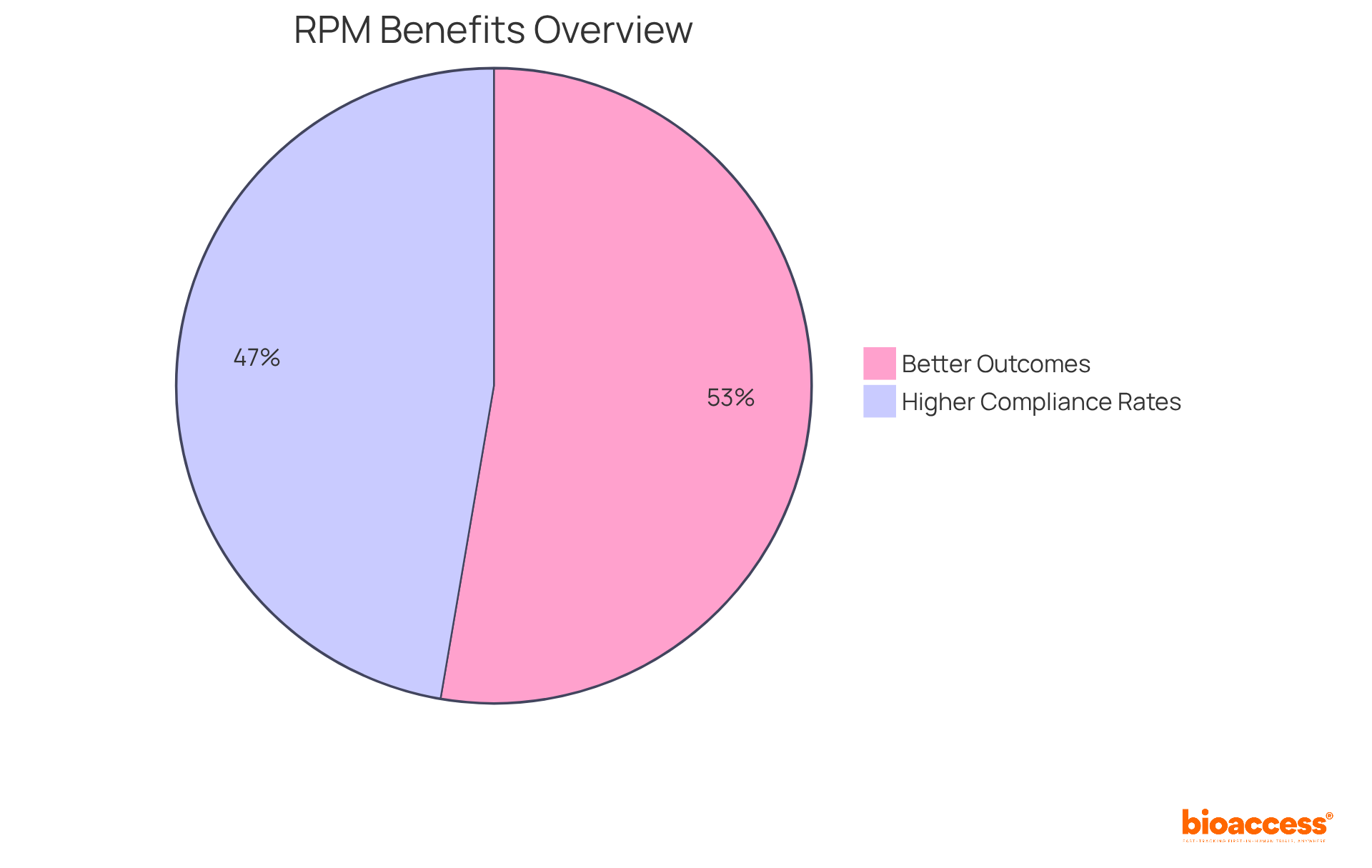
Remote patient monitoring clinical trials significantly enhance engagement throughout the trial process by promoting continuous communication between individuals and researchers. This ongoing interaction not only fosters a sense of connection and support but also leads to improved adherence to study protocols.
Research indicates that:
Furthermore, the increased engagement facilitated by RPM contributes to higher retention rates, effectively minimizing the risk of delays associated with participant dropout. In fact, studies have demonstrated that remote patient monitoring clinical trials can reduce chronic care admissions by up to 41%, according to a survey of 25 healthcare providers, underscoring their effectiveness in maintaining participant involvement.
As Lucy Lamboley, Vice President of Operations, asserts, "Remote monitoring of individuals is a valuable method for providing continuous care for individuals, helping practitioners forge effective paths for supporting individuals and managing chronic disease from a distance."
Moreover, considering that a single hospital readmission costs around USD 15,200, implementing RPM can enhance outcomes for individuals while lowering healthcare expenses.
To optimize the advantages of remote patient monitoring clinical trials in research studies, investigators should consider incorporating these tools early in the study framework to enhance participant involvement and adherence.
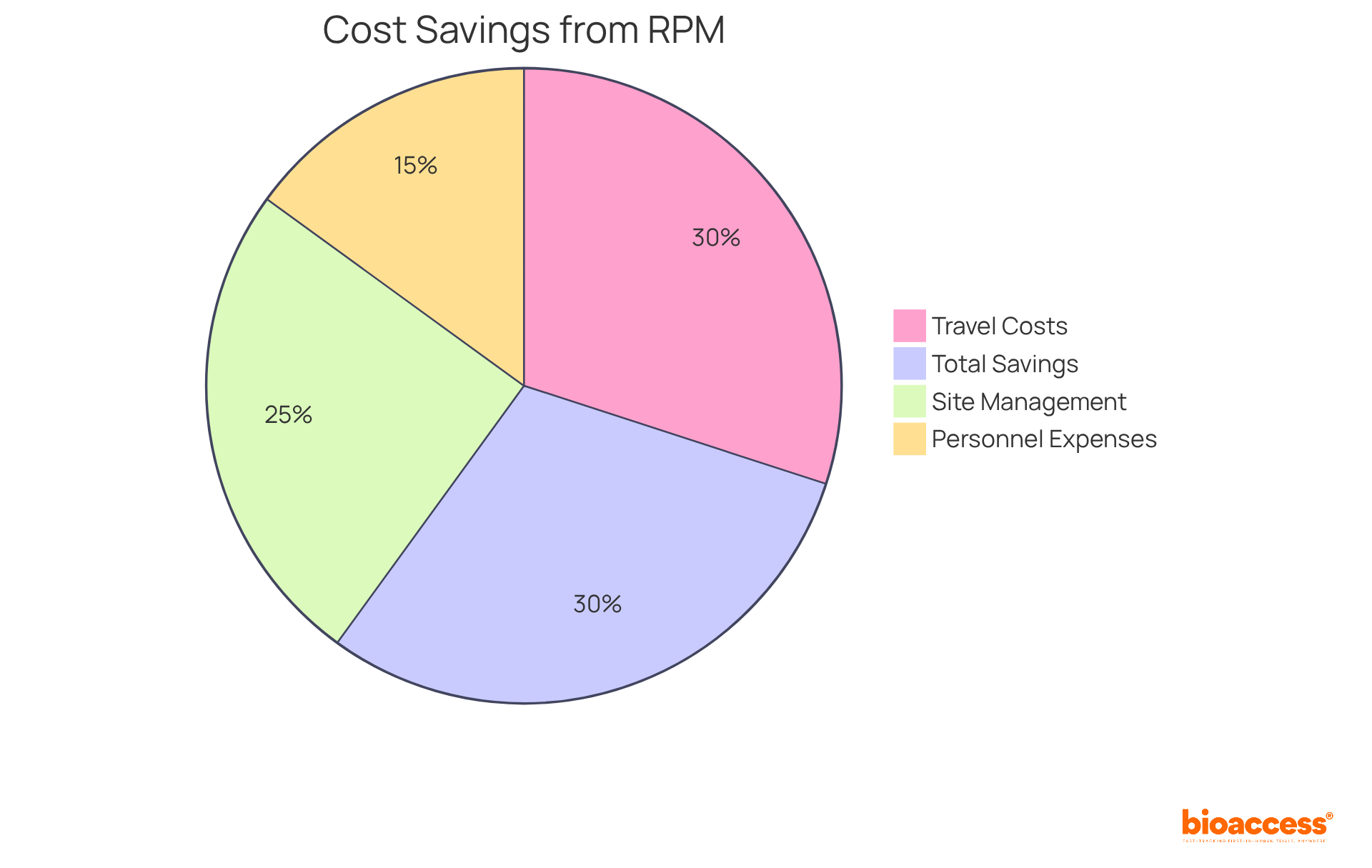
Remote patient monitoring clinical trials are revolutionizing medical studies by significantly reducing operational expenses. By decreasing the need for in-person visits and optimizing data collection processes, remote patient monitoring clinical trials generate substantial savings in travel costs, site management, and personnel expenses. Research indicates that remote patient monitoring clinical trials can lead to a decrease in total study costs by approximately 30% during specific phases of the examination. This financial efficiency empowers sponsors to reallocate resources more effectively, potentially facilitating investment in additional studies or enhancing existing remote patient monitoring clinical trials.
In 2025, the financial impact of remote patient monitoring clinical trials on research budgets is expected to be considerable, with average operational cost savings reaching up to $11,472 per patient compared to standard care. A notable instance is Deaconess Health, which implemented an RPM program that resulted in a 50% reduction in 30-day readmission rates, leading to significant cost savings. Furthermore, the integration of RPM technologies has demonstrated operational savings, as claims for RPM codes surged by 1,298% from 2019 to 2022, reflecting an increasing acknowledgment of its value.
Clinical trial sponsors are progressively recognizing these operational savings with remote patient monitoring clinical trials, with many reporting enhanced budget flexibility and improved study feasibility. As the adoption of remote patient monitoring clinical trials continues to escalate, its role in reducing operational expenses will be pivotal for the future of clinical research.
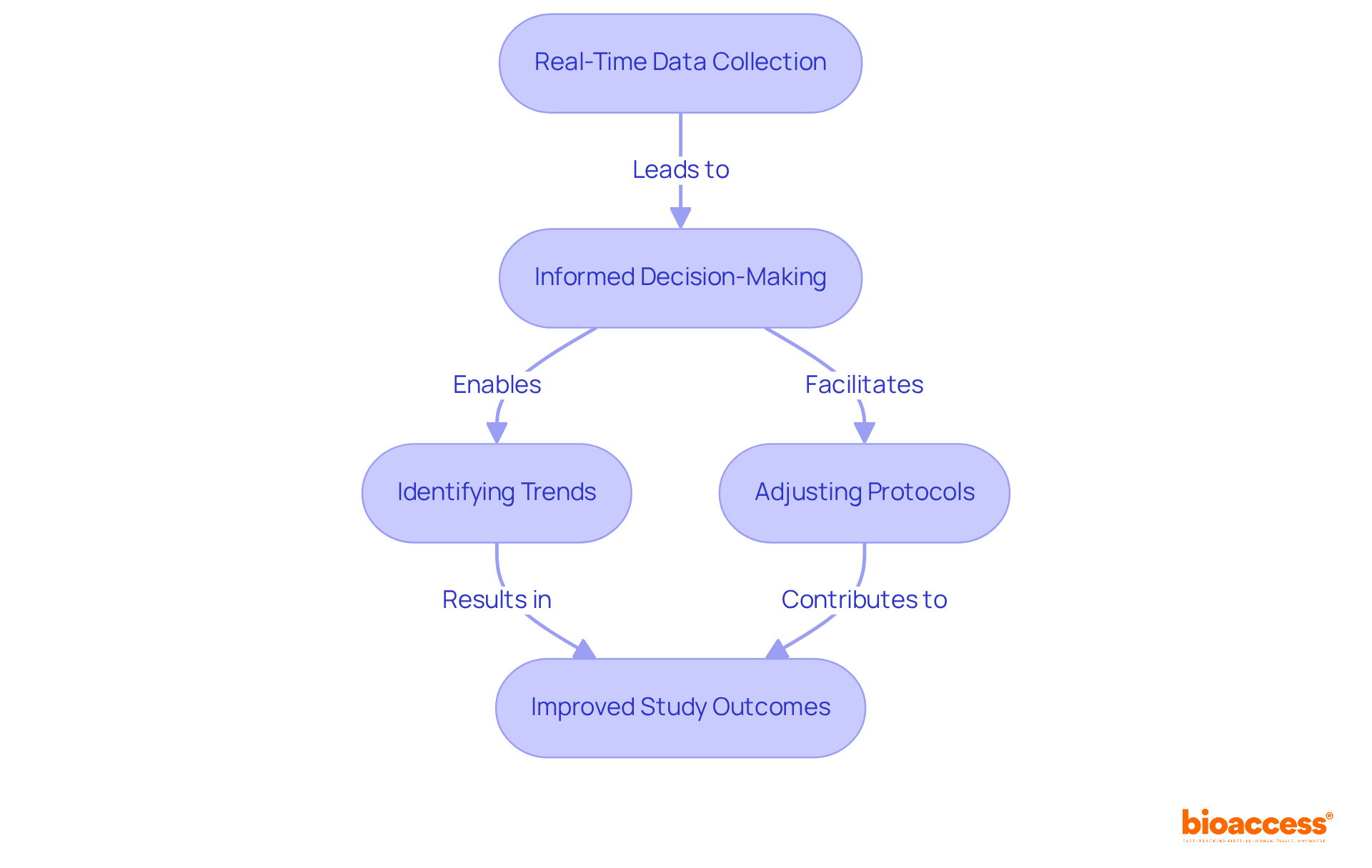
Remote patient monitoring clinical trials significantly enhance the collection of real-time data, empowering researchers to make swift, informed decisions. This urgency is crucial for identifying trends, assessing responses, and adjusting protocols as necessary, ultimately leading to improved study outcomes.
Industry specialists assert that the integration of real-time information into remote patient monitoring clinical trials is no longer a luxury but a necessity for effective decision-making. For example, predictive modeling can foresee potential challenges, enabling proactive risk mitigation and adherence to regulatory standards.
Notably, bioaccess® facilitates the enrollment of treatment-naive cardiology or neurology groups 50% faster than Western locations, resulting in $25K savings per individual with FDA-ready data—no rework, no delays.
The application of advanced analytics optimizes data management in remote patient monitoring clinical trials, minimizing the time needed for data cleaning and analysis, which accelerates development timelines.
Real-world data (RWD) has emerged as an essential element in this context, offering insights that inform treatment adherence and compliance, vital for understanding medication impacts.
By leveraging these technologies and comprehensive study management services—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting—bioaccess® enhances efficiency and ensures participant safety, ultimately leading to more successful outcomes.
Remote individual monitoring (RPM) is a crucial advancement that empowers researchers to continuously track health metrics, facilitating the early detection of adverse events or complications. This proactive strategy significantly enhances safety, as timely interventions can be implemented based on real-time data, thereby reducing the risk of serious health concerns during clinical trials.
Research indicates that RPM can lower hospital readmissions by as much as 76%, while admissions for chronic care complications decreased by 19% to 41% among 25 surveyed organizations, demonstrating its effectiveness in managing care. Furthermore, healthcare professionals have observed that RPM not only improves outcomes for individuals but also fosters a sense of connection and engagement, which is essential for adherence to treatment protocols.
For instance, a survey conducted by the University of Pittsburgh Medical Center reported satisfaction rates exceeding 90% among individuals involved in RPM programs, reflecting the positive impact of continuous monitoring on their overall healthcare experience. Additionally, each Medicare beneficiary can generate over $1,000 in reimbursement annually by participating in RPM, underscoring the financial benefits associated with this approach.
By incorporating remote patient monitoring clinical trials into research studies, scientists can ensure a more adaptive and patient-focused method, ultimately resulting in improved health outcomes and increased safety for participants.
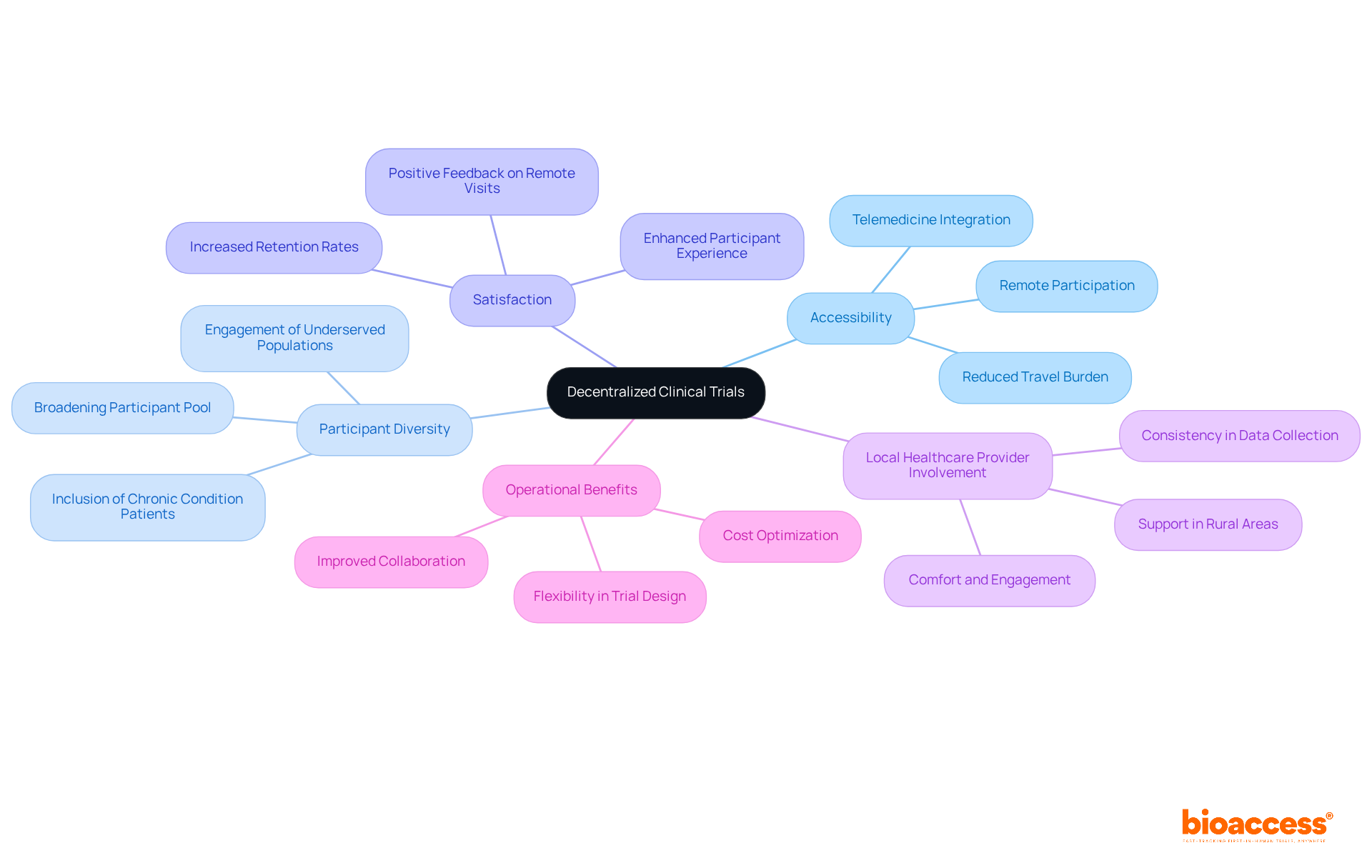
Remote patient monitoring clinical trials serve as a cornerstone of decentralized clinical trials (DCTs), empowering patients to engage in research from the comfort of their homes. This innovative strategy significantly broadens accessibility, enabling a diverse array of participants—including those with chronic conditions or mobility challenges—to contribute to clinical investigations. By alleviating the logistical burdens associated with traditional site visits, RPM fosters inclusivity and diversity within participant populations, ultimately yielding more representative and generalizable outcomes.
Research indicates that permitting even a single remote visit can markedly enhance participant satisfaction and retention. Furthermore, incorporating local healthcare providers into DCTs amplifies individual comfort and engagement, particularly in rural or underserved regions. This model not only optimizes operational costs but also aligns with the FDA's recent guidance, which underscores the necessity of preserving rigor and compliance in decentralized environments.
Clinical study directors have noted that the flexibility afforded by RPM facilitates a more patient-centric approach, crucial for addressing the evolving needs of various populations. As clinical research increasingly gravitates towards decentralized methodologies, remote patient monitoring clinical trials will be vital for achieving successful trial outcomes and enriching participant experiences.
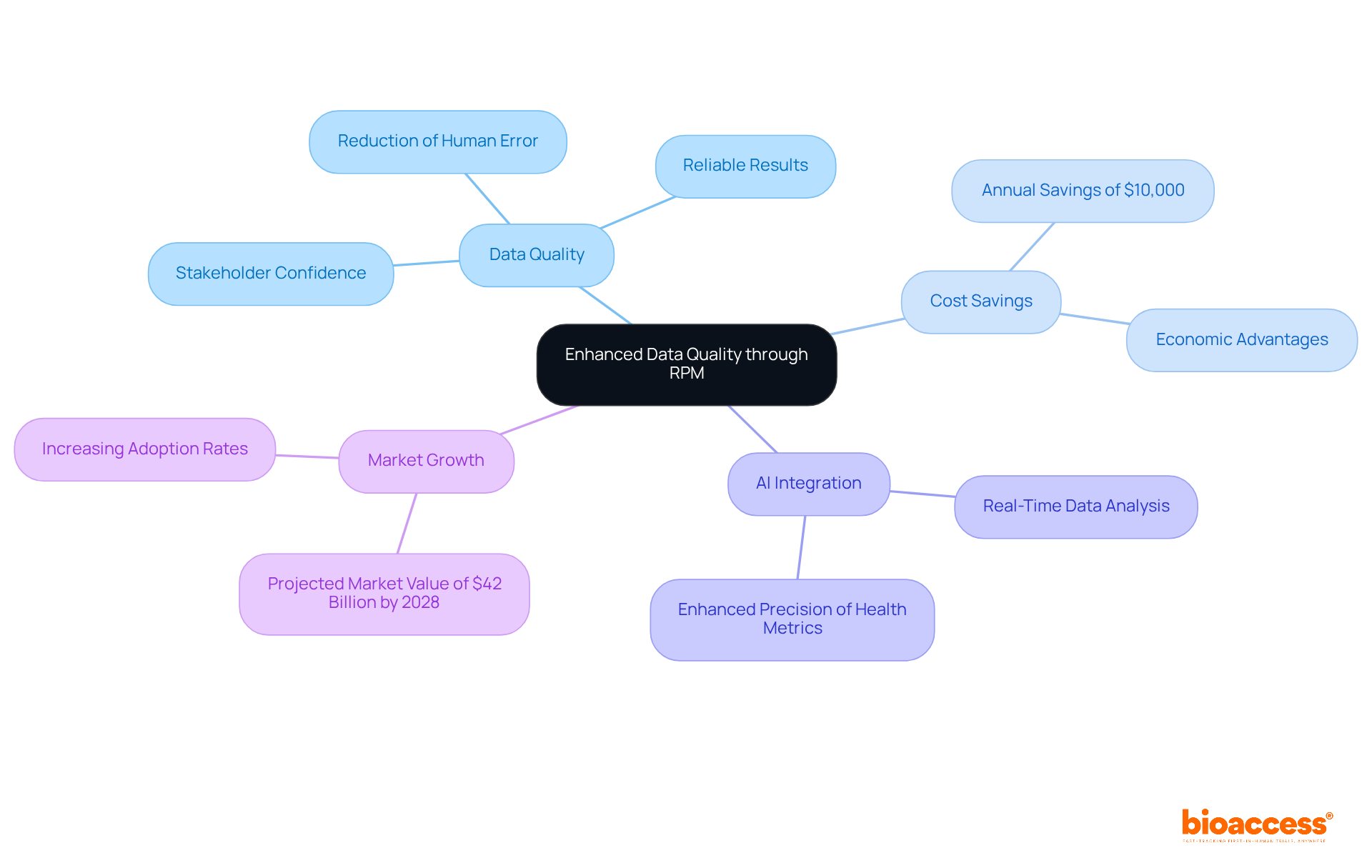
Remote individual monitoring (RPM) significantly enhances data quality by reducing human error linked to manual data entry, thereby ensuring that information is gathered consistently and accurately. This improvement is crucial for achieving reliable results, which are essential for regulatory submissions and scientific publications.
For example, studies indicate that RPM implementation can result in an 85% reduction in hospital readmissions, highlighting its effectiveness in maintaining patient engagement and adherence to study protocols. Furthermore, the integration of artificial intelligence within RPM systems allows for real-time data analysis, enhancing the precision of health metrics collected during evaluations.
Researchers have observed that minimizing human error in data collection not only bolsters the integrity of findings but also instills greater confidence among stakeholders in the results presented. As reliance on remote patient monitoring clinical trials increases, the impact on data accuracy in medical research becomes increasingly apparent, paving the way for more robust and credible scientific outcomes.
At bioaccess, our comprehensive research study management services—including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting—ensure that the integration of RPM is executed effectively, further improving the quality and reliability of research.
As Dr. Michael Fragala notes, 'The integration of artificial intelligence (AI) is enhancing RPM by sifting through data to detect early signs of trouble.' Moreover, RPM has been shown to reduce medical costs by $10,000 annually for individuals with heart failure, underscoring its economic advantages. The market for remote patient monitoring clinical trials is projected to reach $42 billion by 2028, emphasizing its growing significance and acceptance in medical studies.
Remote health monitoring systems (RPM) are exceptionally scalable, which allows remote patient monitoring clinical trials to adapt to diverse sizes and complexities. Whether a study encompasses a small cohort or thousands of participants, remote patient monitoring clinical trials can be customized to meet specific needs, ensuring that every individual receives the required monitoring and support.
With bioaccess®'s innovative approach, medical studies can achieve participant enrollment 50% faster, particularly within cardiology and neurology groups, while also realizing significant cost savings of $25K per participant through FDA-ready data. This adaptability not only enhances the efficacy of trials but also addresses common recruitment challenges faced by Medtech and biopharma startups.
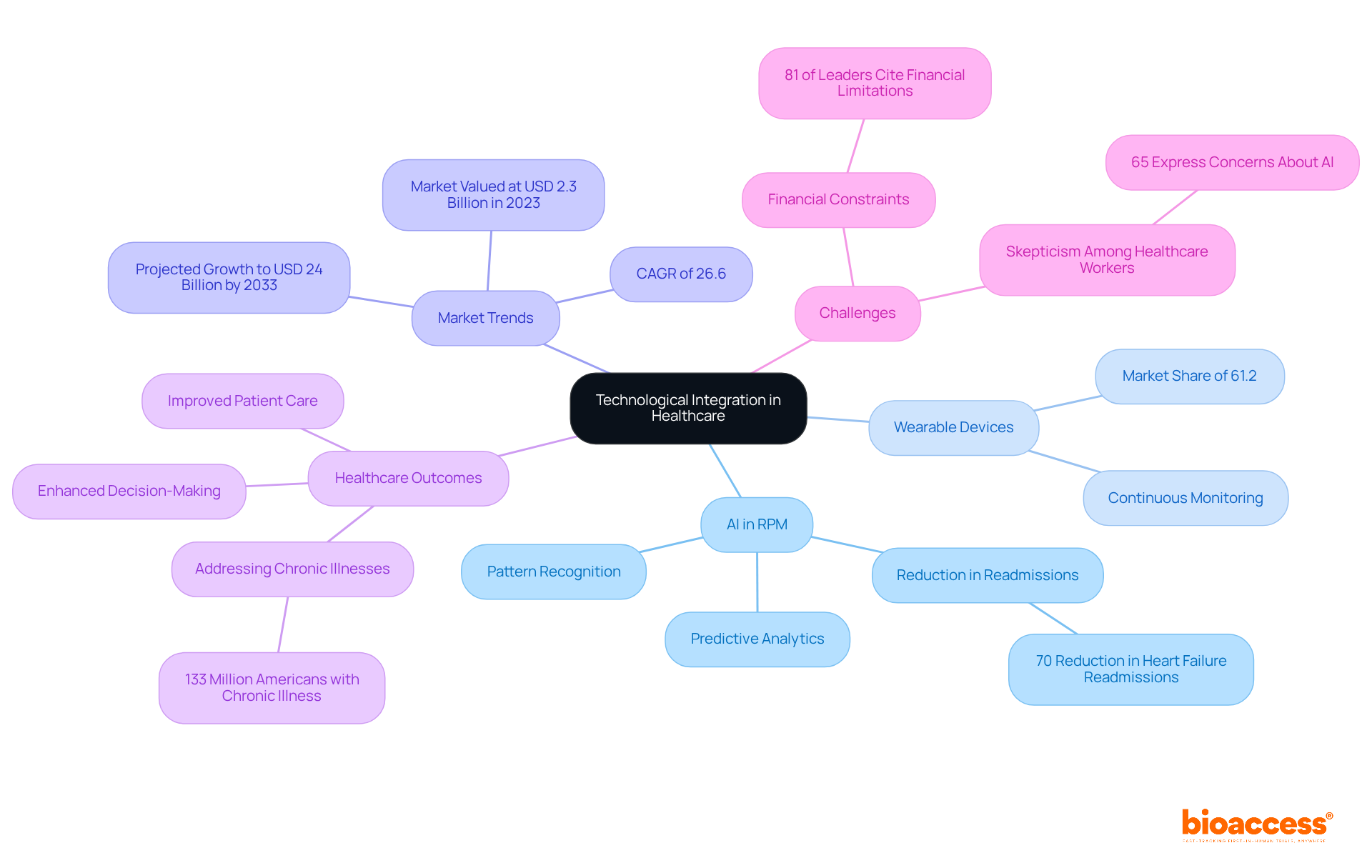
The incorporation of artificial intelligence (AI) and wearable devices into remote patient monitoring clinical trials significantly enhances data collection and analysis, leading to improved management of individuals and clinical trial results. AI algorithms excel at identifying patterns and predicting health outcomes, enabling proactive interventions that can mitigate complications. For instance, wearable devices continuously collect health data, facilitating real-time monitoring of individuals' conditions. This capability is especially vital in managing chronic diseases, where timely adjustments to treatment plans can significantly enhance outcomes for individuals.
In 2023, the wearable device segment captured a substantial market revenue share of 61.2%, underscoring its pivotal role in RPM. Significantly, AI-powered technologies have demonstrated their efficiency in decreasing hospital readmissions; for instance, Biofourmis technology achieved an impressive 70% reduction in 30-day readmissions among individuals with heart failure. Such advancements not only improve patient care but also simplify research processes, allowing quicker and more precise data collection.
Healthcare leaders acknowledge the significance of incorporating wearables into remote patient monitoring clinical trials, with 85% planning to invest in generative AI technologies over the next three years. This investment indicates a growing recognition of AI's potential to revolutionize medical research. As one technology leader observed, the integration of diagnostics and monitoring signifies a considerable advancement in remote care platforms, enabling a more thorough approach to managing individuals.
The effect of wearable devices on medical study results is significant. By supplying ongoing health information, these devices allow researchers to observe individual responses in real time, which enhances the effectiveness of remote patient monitoring clinical trials and leads to more informed decision-making. As the market for AI in remote patient monitoring clinical trials is anticipated to expand at a compound annual growth rate (CAGR) of 26.6% from 2024 to 2033, the future of medical studies will progressively depend on these technological advancements to provide improved outcomes for individuals. Furthermore, with the AI in RPM market valued at USD 2.3 billion in 2023 and expected to reach around USD 24 billion by 2033, the significance of these technologies in healthcare is undeniable. Additionally, 81% of healthcare leaders indicate financial limitations as a significant obstacle influencing their capacity to integrate new technologies, including AI, emphasizing the hurdles to applying these innovations in research. Approximately 133 million Americans suffer from at least one chronic illness, highlighting the critical need for effective RPM solutions.
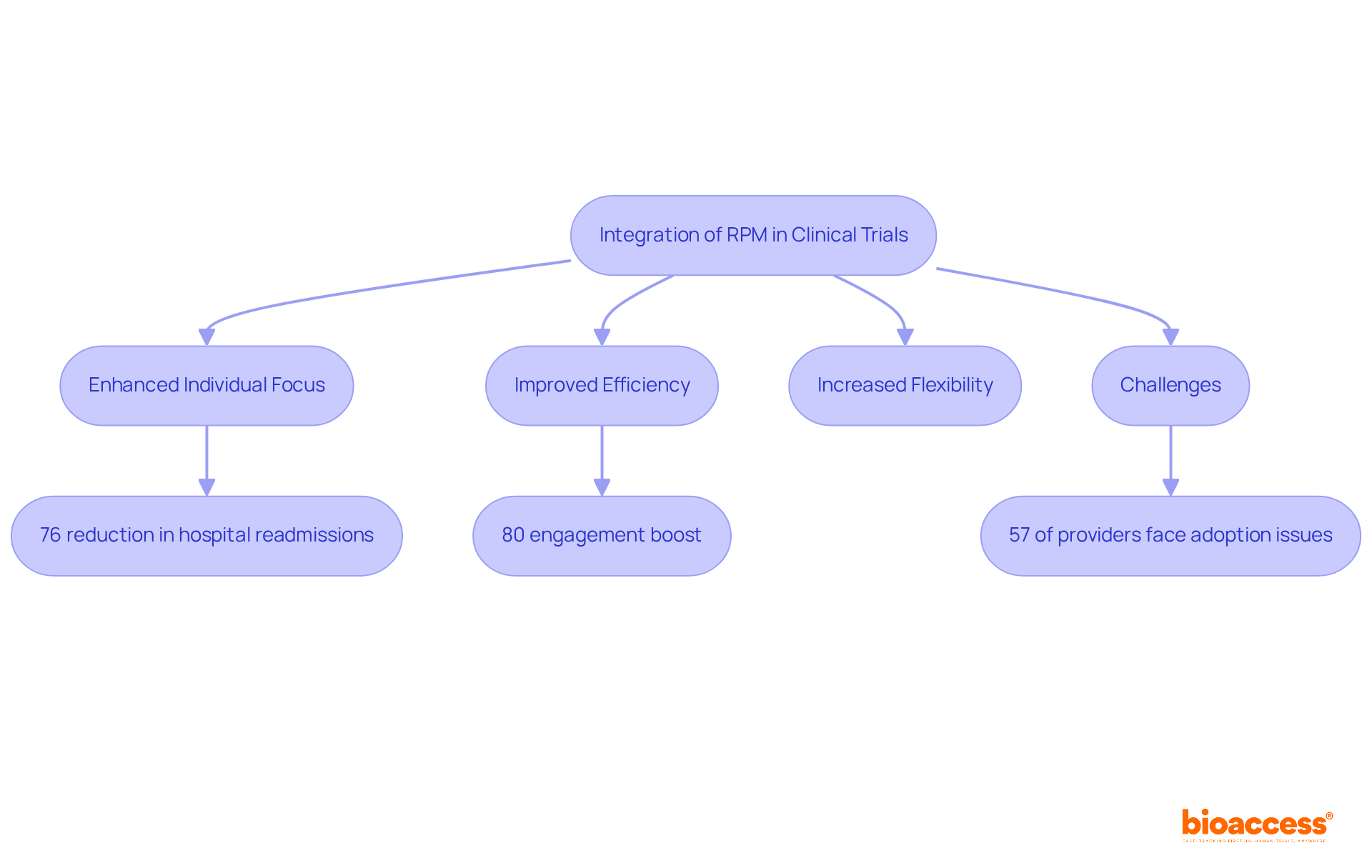
Remote patient monitoring clinical trials are revolutionizing the clinical trial process by enhancing focus on individuals, improving efficiency, and increasing flexibility. The integration of RPM technologies significantly enriches individual experiences and outcomes, while also accelerating the timelines for introducing innovative therapies to the market. For instance, research indicates that RPM can reduce hospital readmissions by as much as 76% and hospitalizations by up to 25% for individuals with chronic illnesses, demonstrating its effectiveness in managing chronic conditions and boosting engagement levels by nearly 80%.
The future of medical studies will increasingly rely on remote patient monitoring clinical trials to facilitate real-time data collection and individual monitoring, enabling timely interventions and tailored care plans. This transition not only enhances the quality of data collected but also empowers individuals to take an active role in managing their health. As a result, the framework of medical studies is evolving to be more patient-centric, focusing on personal needs and preferences.
Examples of successful participant-focused studies utilizing RPM include the Boston Scientific LATITUDE platform, which allows for the remote observation of implanted cardioverter-defibrillators, providing healthcare providers with real-time information that enhances participant management. Moreover, projections suggest that the number of individuals using RPM solutions will grow from 75 million in 2023 to 115.5 million globally by 2027, underscoring the increasing necessity of these technologies in clinical trials.
Industry leaders are recognizing the transformative potential of RPM, with many highlighting its ability to improve health outcomes and reduce costs. For example, MSI International reports that 80% of Americans support the use of remote health monitoring. Additionally, satisfaction rates with RPM exceed 90%, reflecting strong approval among users. As the landscape of medical research evolves, remote patient monitoring clinical trials will play a crucial role in shaping the future of trials, ensuring they are more adaptable to individual needs and capable of delivering innovative treatments more effectively.
However, it is essential to address the challenges healthcare providers encounter when implementing RPM systems, as 57% of providers find it challenging to adopt these technologies, particularly for older patients. This balanced perspective underscores both the promise and the obstacles of RPM in clinical research.
Remote patient monitoring (RPM) is revolutionizing clinical trials, delivering numerous benefits that enhance efficiency, participant engagement, and overall study outcomes. By incorporating RPM into clinical research, organizations can streamline processes, reduce operational costs, and adopt a more patient-centric approach. The integration of these technologies not only accelerates data collection and analysis but also cultivates a stronger connection between researchers and participants, ultimately resulting in improved health outcomes.
Insights from the article reveal how RPM enhances patient compliance and retention, drives significant cost reductions, and enables the collection of real-time data. The flexibility provided by remote monitoring facilitates decentralized trials, increasing accessibility for diverse populations. Moreover, the integration of advanced technologies such as AI and wearables improves data quality and supports informed decision-making throughout the research process.
As the medical research landscape evolves, embracing remote patient monitoring will be essential for fostering innovative solutions and enhancing patient care. Stakeholders in clinical trials are urged to leverage these advancements, not only to boost operational efficiencies but also to prioritize patient safety and satisfaction. The future of clinical trials hinges on the ability to adapt to individual needs, with RPM leading the way in this transformative journey.
What is bioaccess® and how does it utilize remote patient monitoring in clinical trials?
bioaccess® integrates remote patient monitoring into its clinical trial processes, significantly reducing participant recruitment and data gathering timelines while enhancing study efficiency through real-time patient monitoring.
What are the benefits of remote patient monitoring clinical trials?
The benefits include accelerated timelines from study initiation to completion, improved participant engagement, higher compliance rates, and reduced operational expenses.
How does remote patient monitoring enhance patient engagement during clinical trials?
It promotes continuous communication between participants and researchers, fostering a sense of connection and support, which leads to better adherence to study protocols and higher retention rates.
What statistics support the effectiveness of remote patient monitoring?
Research indicates that 49% of healthcare professionals report better outcomes due to remote patient monitoring, and 44% observe higher compliance rates. Additionally, it can reduce chronic care admissions by up to 41%.
How does remote patient monitoring contribute to cost reduction in clinical trials?
By minimizing the need for in-person visits and optimizing data collection, remote patient monitoring can decrease total study costs by approximately 30% during specific phases, resulting in significant savings in travel, site management, and personnel expenses.
What financial impact is expected from remote patient monitoring in clinical trials by 2025?
It is expected that average operational cost savings will reach up to $11,472 per patient compared to standard care, leading to enhanced budget flexibility for sponsors.
What is the projected growth of the remote health monitoring market?
The remote health monitoring market is projected to grow substantially, with an anticipated 115.5 million users by 2027.
How can investigators optimize the advantages of remote patient monitoring in their studies?
Investigators should consider incorporating remote patient monitoring tools early in the study framework to enhance participant involvement and adherence.