


The article delineates ten significant benefits of virtual clinical trials for research directors, underscoring their capacity to enhance patient recruitment, lower research costs, and increase study flexibility. These advantages arise from the integration of advanced technology and the removal of geographical barriers, which together facilitate faster enrollment and more efficient data collection processes. Ultimately, this transformation is reshaping the clinical research landscape, making it imperative for stakeholders to recognize and adapt to these advancements.
Virtual clinical trials are revolutionizing the landscape of medical research. They provide unprecedented access to diverse patient populations and significantly enhance the efficiency of study processes. Research directors stand to gain a multitude of advantages from this innovative approach, including:
However, as the shift towards virtual trials accelerates, research leaders must navigate the challenges and ethical considerations that accompany this transformation. How can they effectively address these issues while maximizing the potential benefits of this emerging paradigm?
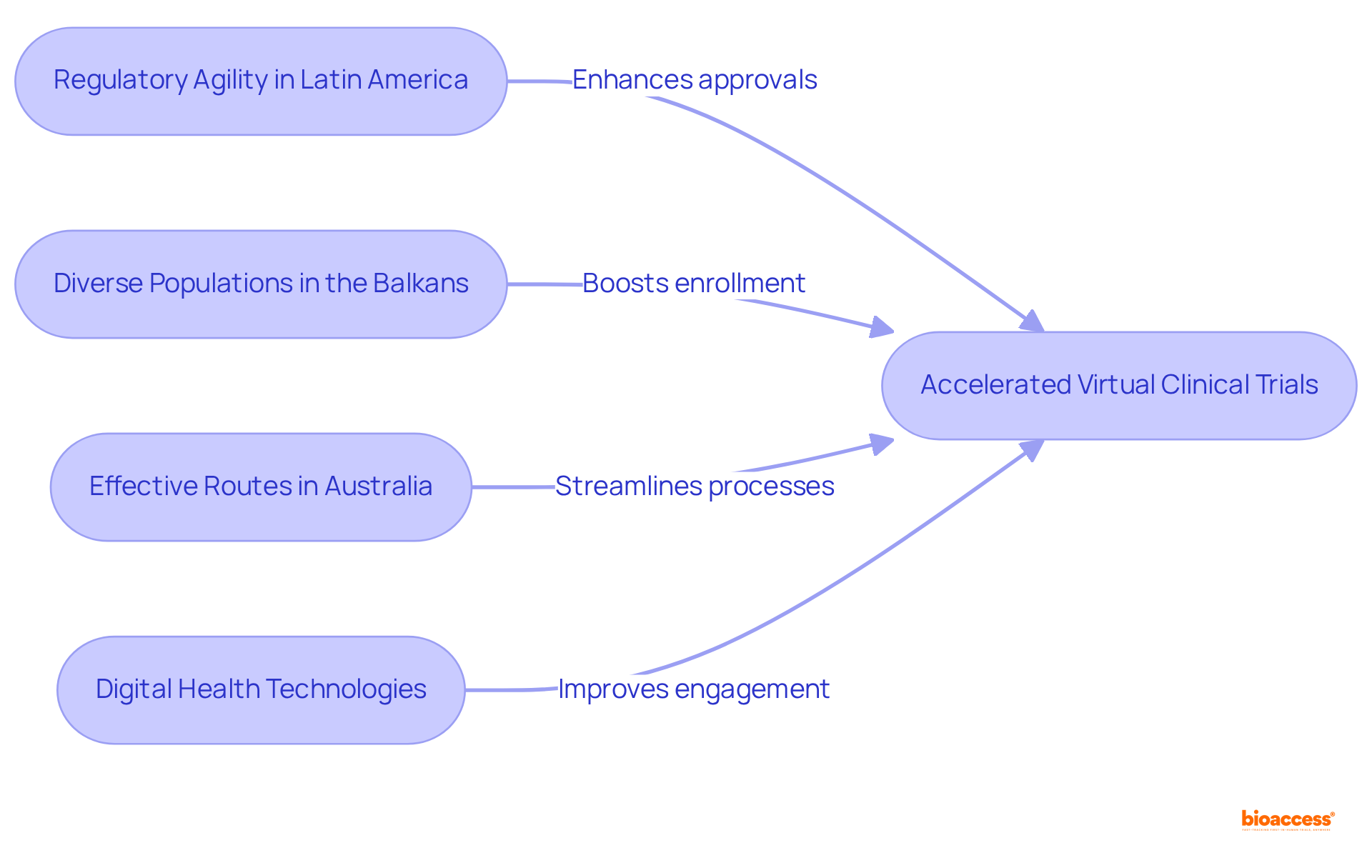
bioaccess® leverages the regulatory agility of Latin America, the diverse populations of the Balkans, and the effective routes in Australia to enhance virtual clinical trials. This strategic amalgamation facilitates ethical approvals within an impressive timeframe of 4-6 weeks and boosts enrollment rates by 50%. Such agility positions bioaccess® as an indispensable partner for research directors seeking to expedite their studies, ensuring timely access to critical data and insights. Recent advancements in virtual clinical trials, including the integration of digital health technologies and telehealth solutions, further underscore the significance of this approach, as the industry increasingly embraces innovative methods to streamline processes and elevate user engagement.
Virtual clinical trials significantly enhance patient enrollment by eliminating geographical barriers, allowing individuals to participate from the comfort of their homes. This flexibility not only boosts enrollment rates but also aligns seamlessly with the schedules and lifestyles of potential participants.
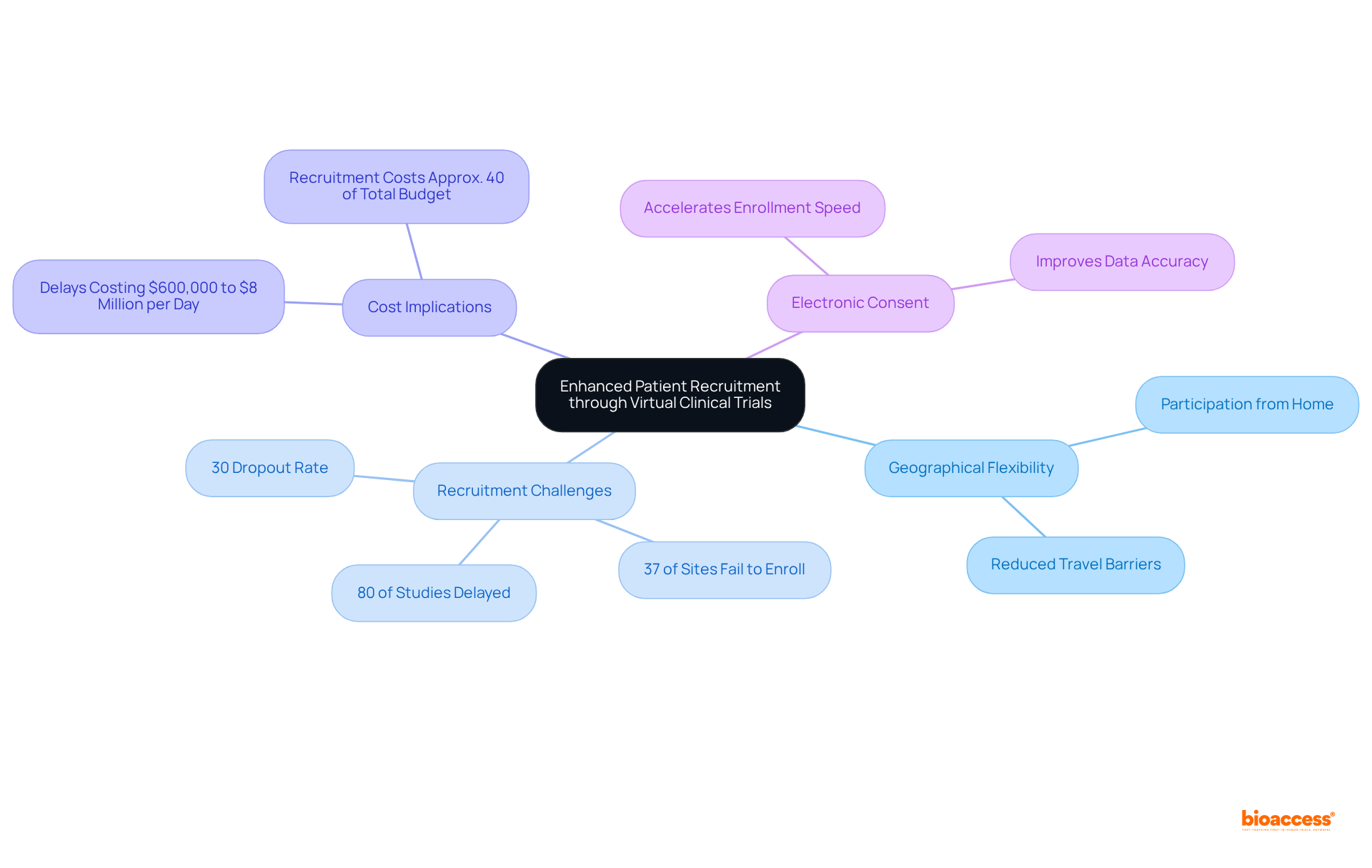
Research indicates that approximately 80% of studies are delayed or terminated due to recruitment challenges, underscoring the urgent need for innovative solutions. Digital platforms are crucial in reaching diverse populations, ensuring a more representative sample. Notably, 37% of research sites fail to enroll sufficient participants, which highlights the importance of these platforms in overcoming recruitment hurdles.
Furthermore, delays can cost sponsors between $600,000 and $8 million for each day a study postpones a product’s development and launch, reinforcing the necessity for improved recruitment strategies. The implementation of electronic consent (e-consent) has been shown to accelerate enrollment speed, streamlining the recruitment process.
Consequently, virtual clinical trials not only enhance accessibility but also foster greater inclusivity in medical research, ultimately leading to more successful outcomes.
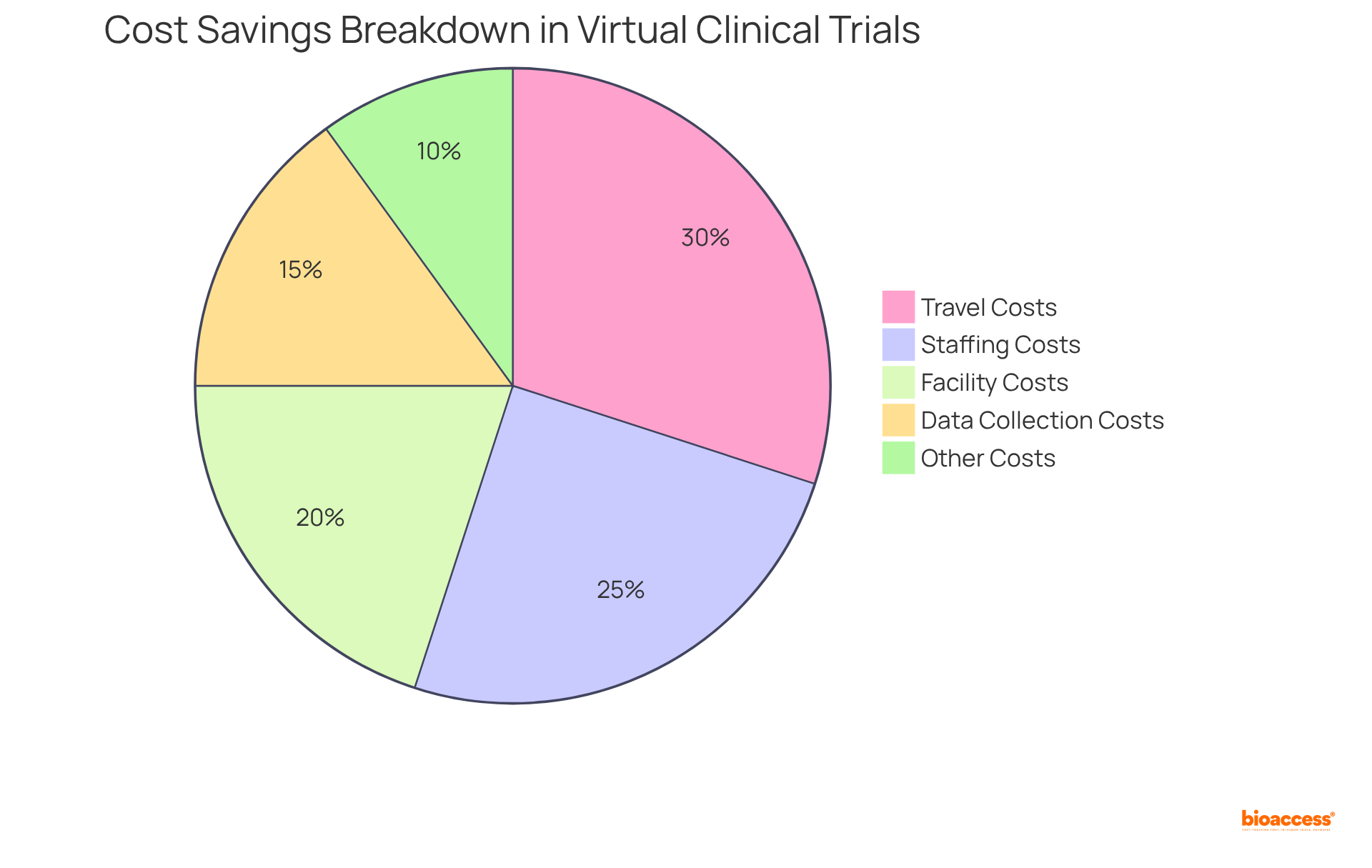
Virtual clinical trials notably reduce research expenses by minimizing the need for physical locations, travel costs, and associated overheads. By leveraging technology for virtual clinical trials and data collection, organizations can significantly lower staffing and facility costs, leading to overall reduced project budgets.
For instance, virtual clinical trials can decrease study costs by approximately 25-30%, particularly regarding expenses tied to participant travel and the personnel required for on-site observation. The typical budget reductions for virtual clinical trials can range from $4,300 to $600,000, depending on the study's characteristics.
The cost-effectiveness of virtual clinical trials is particularly appealing for startups and smaller research organizations. Furthermore, with 50% of individuals more likely to participate in studies that offer home care alternatives, retention rates soar to 95%, thereby enhancing the financial viability of these innovative approaches.
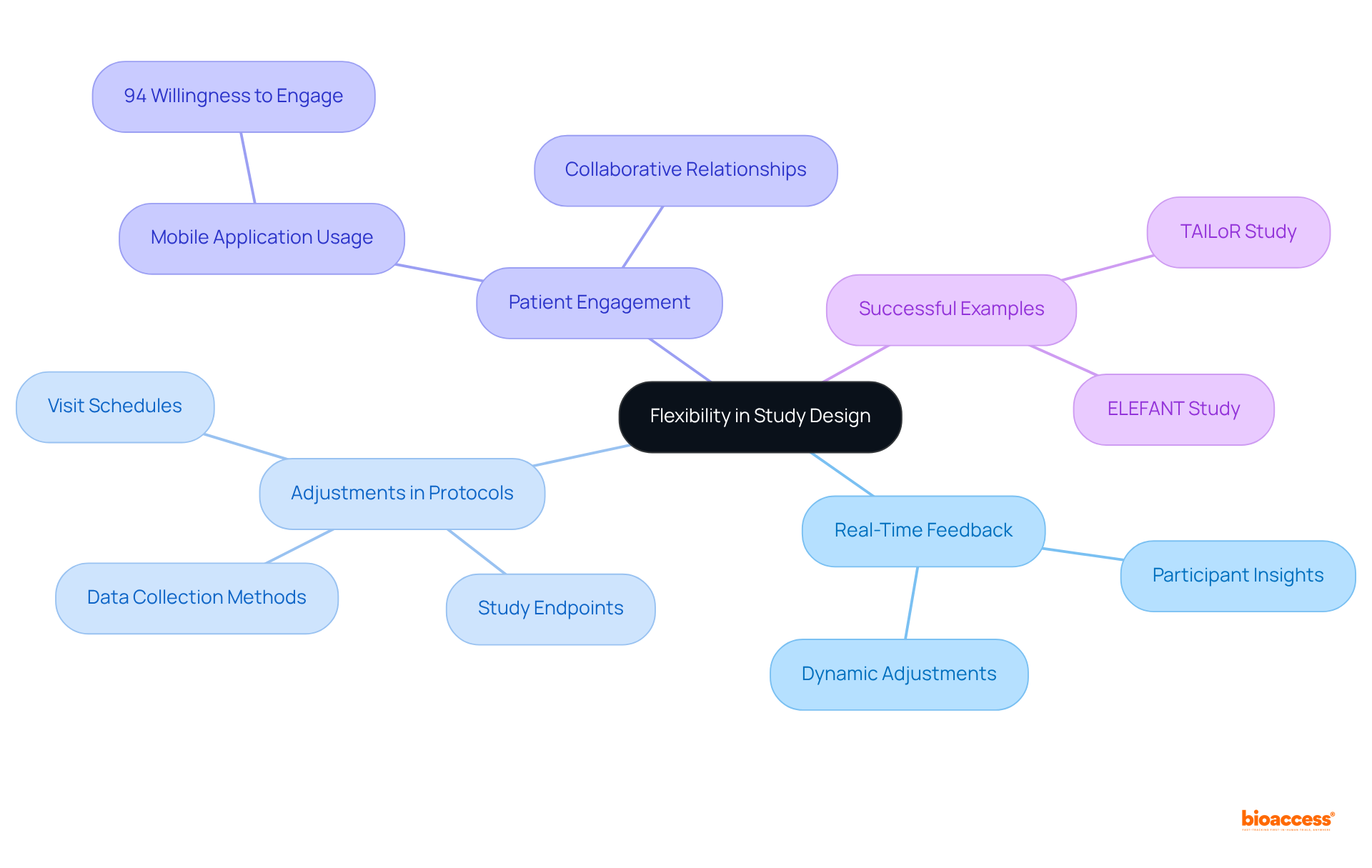
Virtual studies present exceptional adaptability in study design, enabling researchers to modify protocols based on real-time feedback from participants. This flexibility encompasses adjustments to visit schedules, data collection methods, and even study endpoints, all tailored to meet patient preferences and needs. Such responsiveness not only boosts participant satisfaction but also significantly enhances retention rates.
Notably, a Medidata survey revealed that 94% of respondents expressed a willingness to engage with mobile applications for study participation. As we approach 2025, the ability to dynamically modify research protocols is becoming increasingly vital, particularly in virtual environments where patient-focused strategies are essential.
Successful examples of adaptive protocols in virtual studies, such as the ELEFANT and TAILoR studies, illustrate how integrating patient insights can yield more effective and efficient research outcomes, ultimately fostering a more collaborative relationship between researchers and participants.
The integration of technology in virtual clinical trials significantly enhances data gathering through electronic health records, mobile applications, and wearable devices. These innovative tools facilitate real-time data capture and monitoring, thereby reducing the risk of data loss and ensuring greater accuracy. Such advancements in technology not only enable more effective analysis and reporting but also play a crucial role in improving overall outcomes.
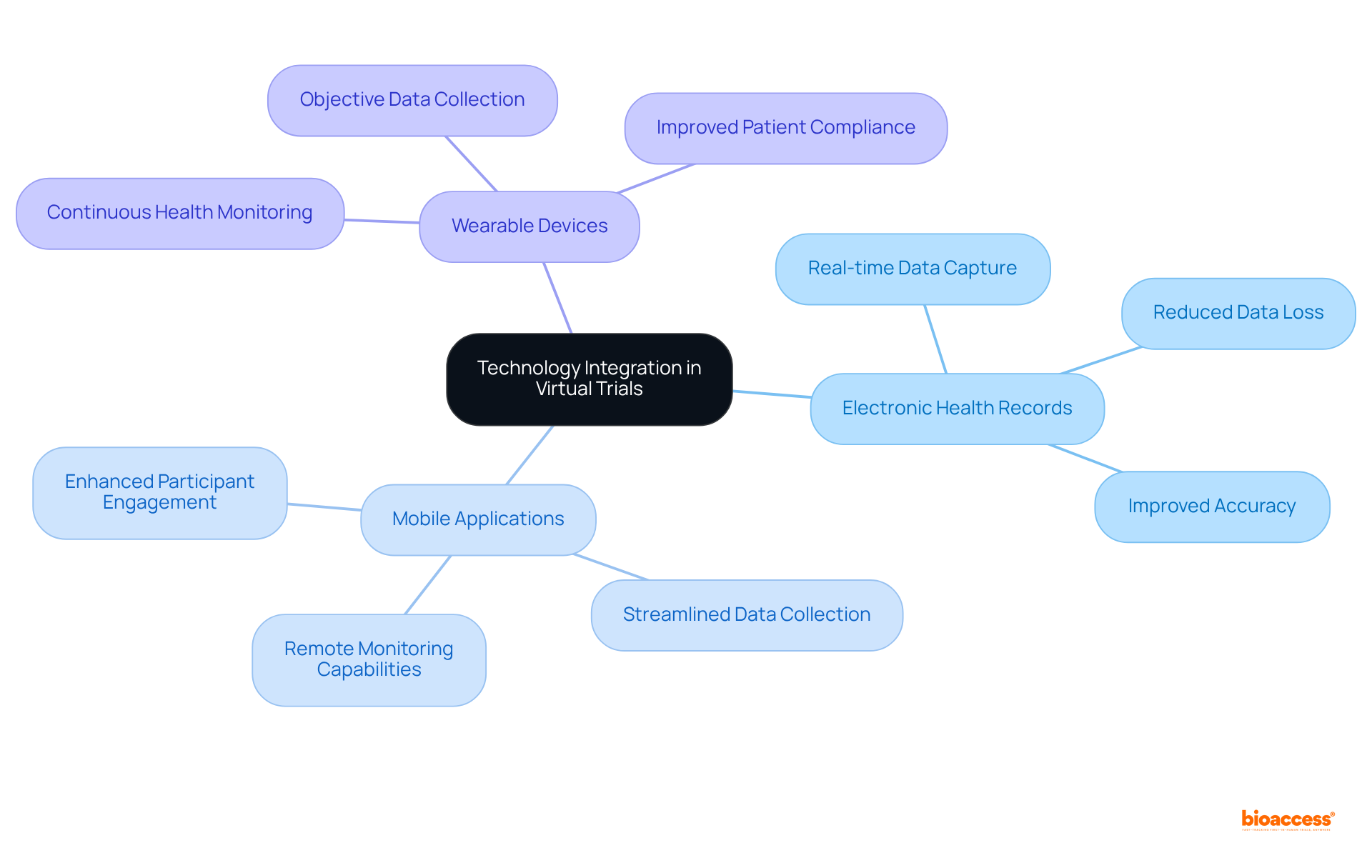
Virtual clinical trials significantly enhance research timelines by streamlining patient recruitment, data collection, and monitoring processes. By employing remote evaluations and advanced digital tools for data management, these studies can progress at an accelerated pace, facilitating quicker analysis and reporting of results.
For instance, online recruitment strategies yield 4.17 times more individuals per day compared to traditional methods, with the median cost per enrollee notably lower at $72 versus $199 for offline recruitment. This efficiency proves vital for expediting the introduction of innovative therapies to the market.
Furthermore, in 2022/23, there were 952,789 individuals involved in research studies in England, an increase of over 220,000 compared to pre-pandemic figures, underscoring the growing importance of virtual clinical trials in enhancing participation. The integration of AI and machine learning can boost enrollment by 10 to 20 percent, compressing development timelines by an average of six months per asset.
Additionally, setbacks in medical studies can cost firms over $35,000 daily for each study, highlighting the financial implications of inefficiencies in traditional approaches. As virtual clinical trials continue to evolve, they are poised to transform the landscape of medical research, ensuring that innovative therapies reach patients more swiftly.
However, it is essential to acknowledge that participation in research studies carries potential risks, which must be thoroughly assessed. As Samruddhi Yardi noted, 'Clinical studies are essential for progressing medical understanding and enhancing healthcare by evaluating the safety and effectiveness of new treatments before they are widely available.
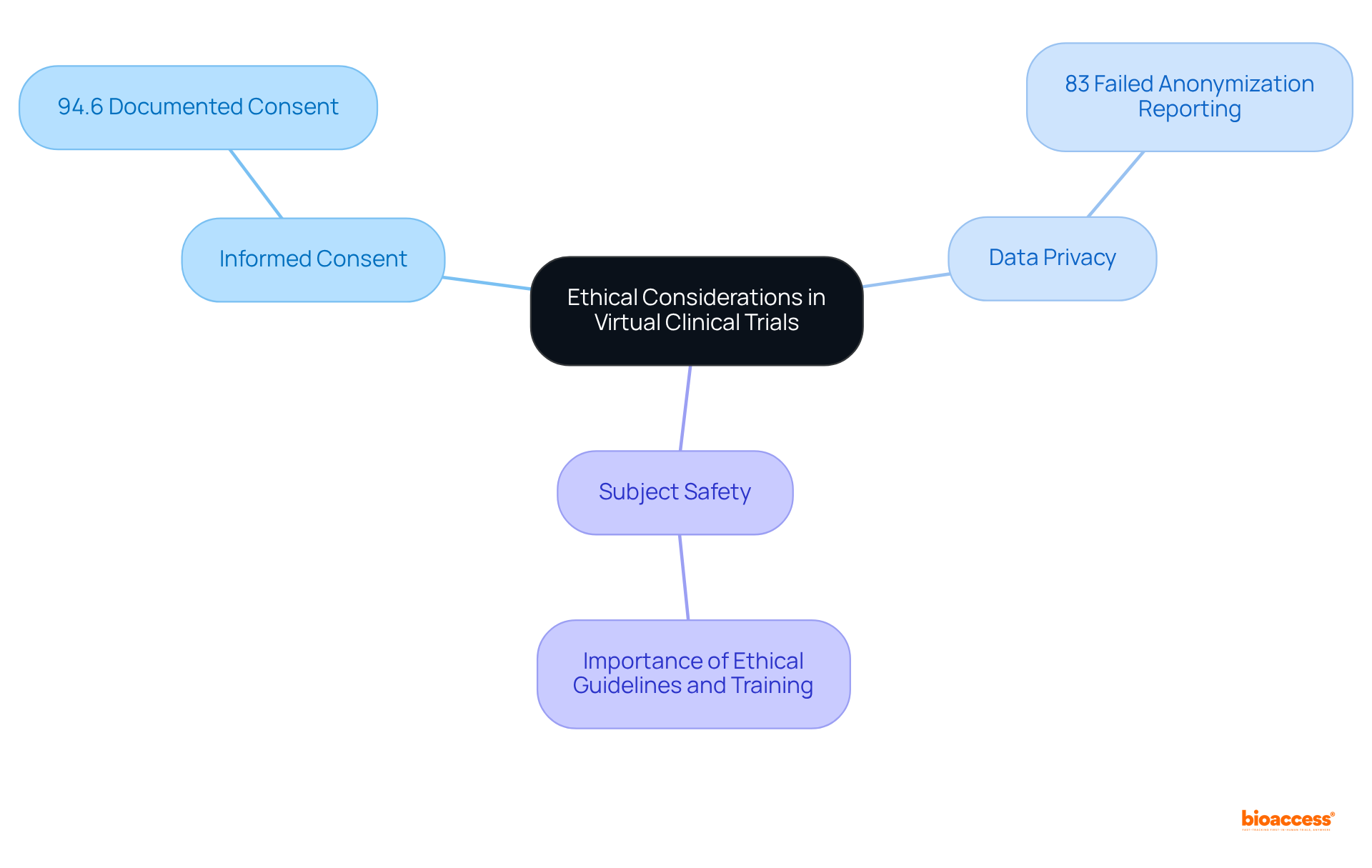
Carrying out virtual clinical trials necessitates a stringent approach to ethical considerations, particularly regarding informed consent, data privacy, and subject safety. Research directors must ensure that all interactions during virtual clinical trials adhere to regulatory standards, thereby guaranteeing that individuals are fully informed about the study's nature and their rights.
For instance, as of 2023, informed consent was documented in 94.6% of research studies, highlighting the critical importance of transparency in the consent process. Furthermore, compliance in remote clinical research is vital; studies indicate that 83% of studies failed to adequately report on the anonymization of data, potentially undermining trust among subjects.
To ensure compliance, directors should implement robust training for staff on ethical guidelines and utilize electronic consent (e-consent) tools, which have gained popularity for their ability to streamline the consent process while ensuring user comprehension.
By prioritizing these ethical standards, research leaders can foster trust and integrity in the research process, ultimately enhancing participation and study success.
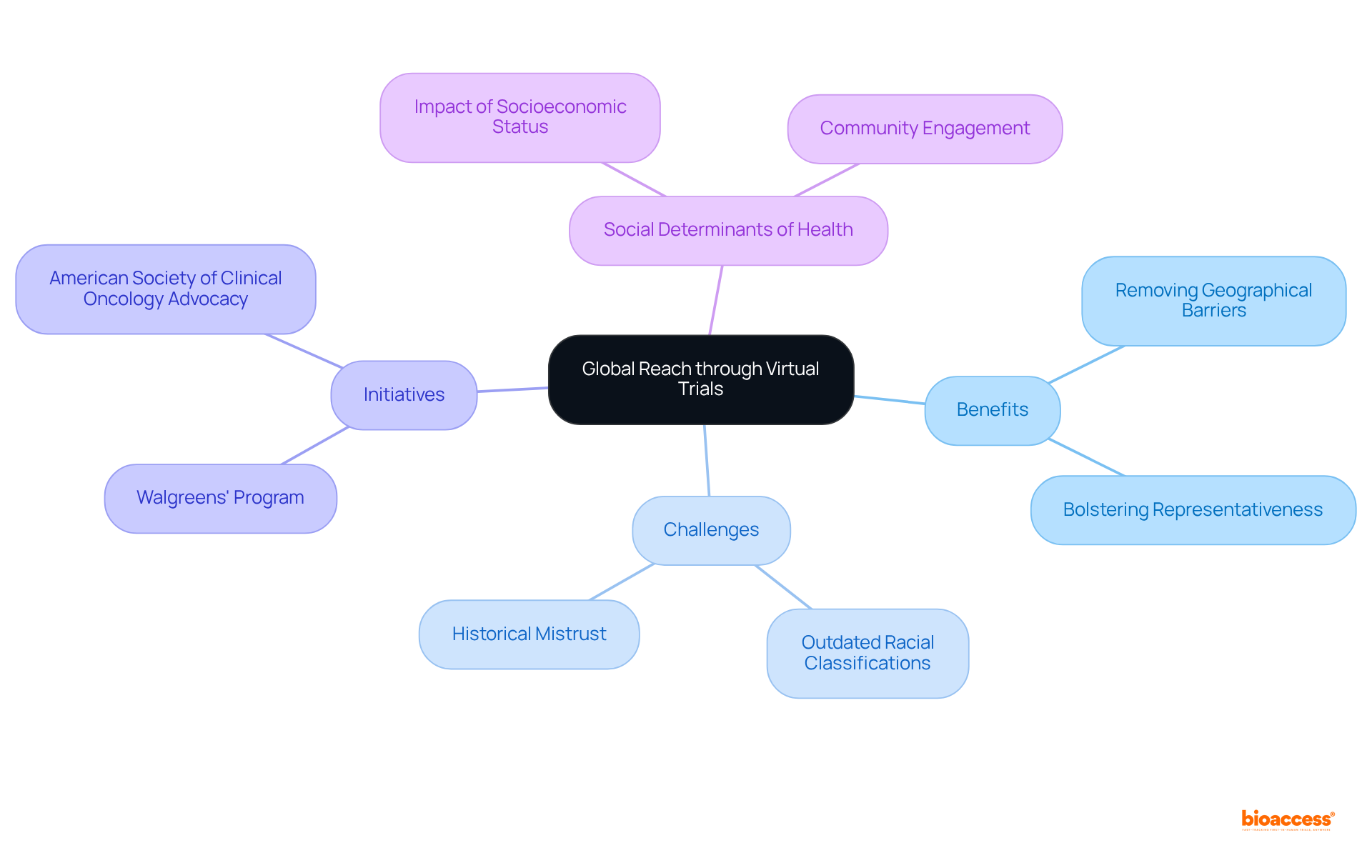
Virtual clinical trials significantly enhance researchers' access to diverse patient groups by removing geographical barriers. This inclusivity not only facilitates the recruitment of participants from varied backgrounds but also bolsters the representativeness of study outcomes.
However, challenges such as outdated racial classifications in medical studies can hinder effective recruitment and representation. By utilizing digital platforms, researchers can engage underrepresented groups effectively, ensuring that findings are relevant and applicable to a broader audience.
For instance, initiatives like Walgreens' research program aim to improve racial and ethnic diversity by incorporating both in-person and virtual participation methods. Additionally, the American Society of Clinical Oncology advocates for enhanced equity in cancer studies, underscoring the necessity for diverse representation.
It is also essential to consider social determinants of health (SDOH), which significantly influence participation in studies, especially among marginalized communities. As we approach 2025, addressing historical inequalities—including the legacy of distrust stemming from events such as the Tuskegee Syphilis Experiment—will be crucial for ensuring that medical research reflects the demographics of the wider community.
Engaging with experts like Arthur L. Caplan, who stresses the importance of inclusivity, can provide valuable insights into overcoming these challenges. Ultimately, the focus on reaching diverse groups through virtual clinical trials will be vital for promoting fairness in medical research.
Virtual clinical trials effectively address various constraints related to conventional studies, such as difficulties in participant recruitment, high dropout rates, and logistical obstacles. For instance, only 31% of 114 studies in the UK achieved enrollment targets, underscoring the challenges in patient recruitment.
By facilitating remote involvement, these virtual clinical trials significantly reduce the challenges associated with travel and time commitments, leading to improved retention and adherence. Research indicates that 90% of follow-up participants expressed a willingness to engage again if the study were extended, with virtual experiments achieving retention rates exceeding 90%.
Furthermore, the integration of technology, including telemedicine and mobile applications, enhances communication and enables real-time tracking of participant involvement in virtual clinical trials, thereby boosting the overall efficiency of the study.
This innovative approach effectively addresses frequent logistical challenges encountered in conventional studies, such as lengthy onboarding processes and high attrition rates, with bioaccess® achieving enrollment 50% faster than traditional methods, ultimately resulting in more robust and reliable data collection.
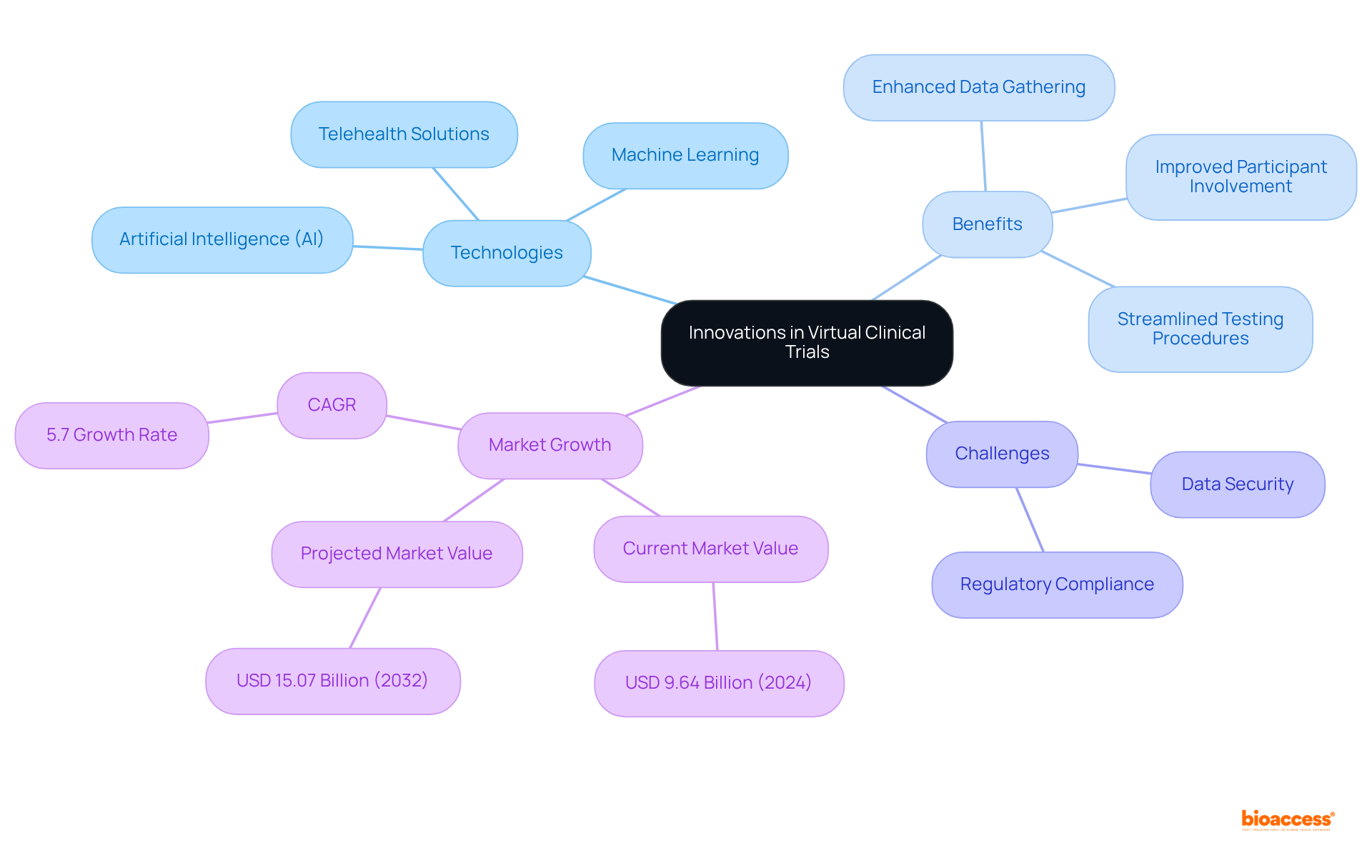
The future of virtual clinical trials is advancing rapidly, driven by innovations in technology such as artificial intelligence (AI), machine learning, and telehealth solutions. These advancements are enhancing data gathering techniques, improving participant involvement, and streamlining testing procedures. For example, AI and machine learning are progressively employed to examine extensive datasets, allowing for more accurate selection of individuals and real-time tracking of results. Furthermore, telehealth solutions enable remote consultations and evaluations, allowing participants to take part from the comfort of their homes, thus enhancing recruitment and retention rates.
Significantly, fewer than 5% of adult cancer patients enroll in research studies, emphasizing the essential function of remote involvement in tackling recruitment issues. Statistics suggest that the virtual medical trials market was valued at USD 9.64 billion in 2024 and is expected to expand at a compound annual growth rate (CAGR) of 5.7%, reaching USD 15.07 billion by 2032. This growth is driven by the increasing demand for decentralized research models and the integration of advanced technologies in virtual clinical trials. However, challenges such as data security and regulatory compliance remain significant concerns that must be addressed.
As research directors navigate this evolving landscape, embracing these innovations while being mindful of potential obstacles will be crucial for maximizing the effectiveness and efficiency of clinical studies. Ultimately, this will lead to faster and more reliable outcomes, making collaboration essential for overcoming the challenges faced in clinical research.
Virtual clinical trials are revolutionizing the medical research landscape, presenting research directors with a multitude of benefits that enhance efficiency, inclusivity, and adaptability. By harnessing technology and innovative methodologies, these trials not only bolster patient recruitment and retention but also markedly reduce costs and streamline processes. This transition towards virtual studies signifies a critical juncture in clinical research, fostering a more responsive and patient-centered approach.
The key advantages emphasized throughout the article include:
As the future of clinical trials progresses, embracing innovations and tackling the challenges associated with virtual studies will be imperative. Research directors are urged to prioritize ethical considerations, ensure compliance, and leverage the potential of emerging technologies to optimize their research impact. By doing so, they can contribute to a more equitable and efficient medical research landscape, paving the way for expedited and more reliable outcomes that benefit both researchers and patients alike.
What is bioaccess and how does it enhance virtual clinical trials?
bioaccess® leverages the regulatory agility of Latin America, diverse populations of the Balkans, and effective routes in Australia to enhance virtual clinical trials. This combination facilitates ethical approvals within 4-6 weeks and boosts enrollment rates by 50%.
What are the advantages of virtual clinical trials in patient recruitment?
Virtual clinical trials enhance patient enrollment by eliminating geographical barriers, allowing individuals to participate from home. This flexibility aligns with participants' schedules and lifestyles, leading to higher enrollment rates and greater inclusivity in medical research.
What challenges do traditional studies face regarding patient recruitment?
Approximately 80% of studies are delayed or terminated due to recruitment challenges, with 37% of research sites failing to enroll sufficient participants. Delays can cost sponsors between $600,000 and $8 million for each day a study is postponed.
How does electronic consent (e-consent) impact the recruitment process?
The implementation of electronic consent (e-consent) accelerates enrollment speed, streamlining the recruitment process and addressing challenges in participant recruitment.
What are the cost benefits of virtual clinical trials?
Virtual clinical trials reduce research expenses by minimizing the need for physical locations, travel costs, and associated overheads. They can decrease study costs by approximately 25-30%, leading to budget reductions ranging from $4,300 to $600,000.
Why are virtual clinical trials appealing to startups and smaller research organizations?
The cost-effectiveness of virtual clinical trials is particularly appealing to startups and smaller organizations as they significantly lower staffing and facility costs, enhancing financial viability. Additionally, 50% of individuals are more likely to participate in studies that offer home care alternatives, leading to retention rates of 95%.