


The article addresses the critical strategies for implementing Corrective Action Preventive Action (CAPA) in clinical research, underscoring its relevance in enhancing compliance and operational efficiency. It highlights the necessity of:
Statistical evidence supports the assertion that robust CAPA practices correlate with improved trial outcomes, establishing a compelling case for their adoption. By integrating these strategies, organizations can navigate the complexities of clinical research more effectively, ultimately leading to superior results.
In the intricate world of clinical research, the stakes are high, and the margin for error is razor-thin. Effective Corrective and Preventive Action (CAPA) strategies are not merely regulatory requirements; they are essential for safeguarding patient safety and ensuring the integrity of clinical trials. This article delves into ten pivotal CAPA strategies that can streamline processes, enhance compliance, and ultimately lead to better outcomes in clinical research. As organizations strive for efficiency, how can they balance the need for rapid action with the rigorous demands of regulatory standards?
bioaccess® leverages its extensive understanding of regulatory frameworks and clinical trial dynamics to expedite the execution of Corrective and Preventive Measures. With over 20 years of expertise in Medtech, bioaccess® ensures that corrective and preventive action processes are not only compliant but also remarkably efficient, significantly reducing the time from issue identification to resolution. This capability is vital in the fast-paced realm of clinical trials, where timely interventions can substantially enhance patient safety and uphold study integrity.
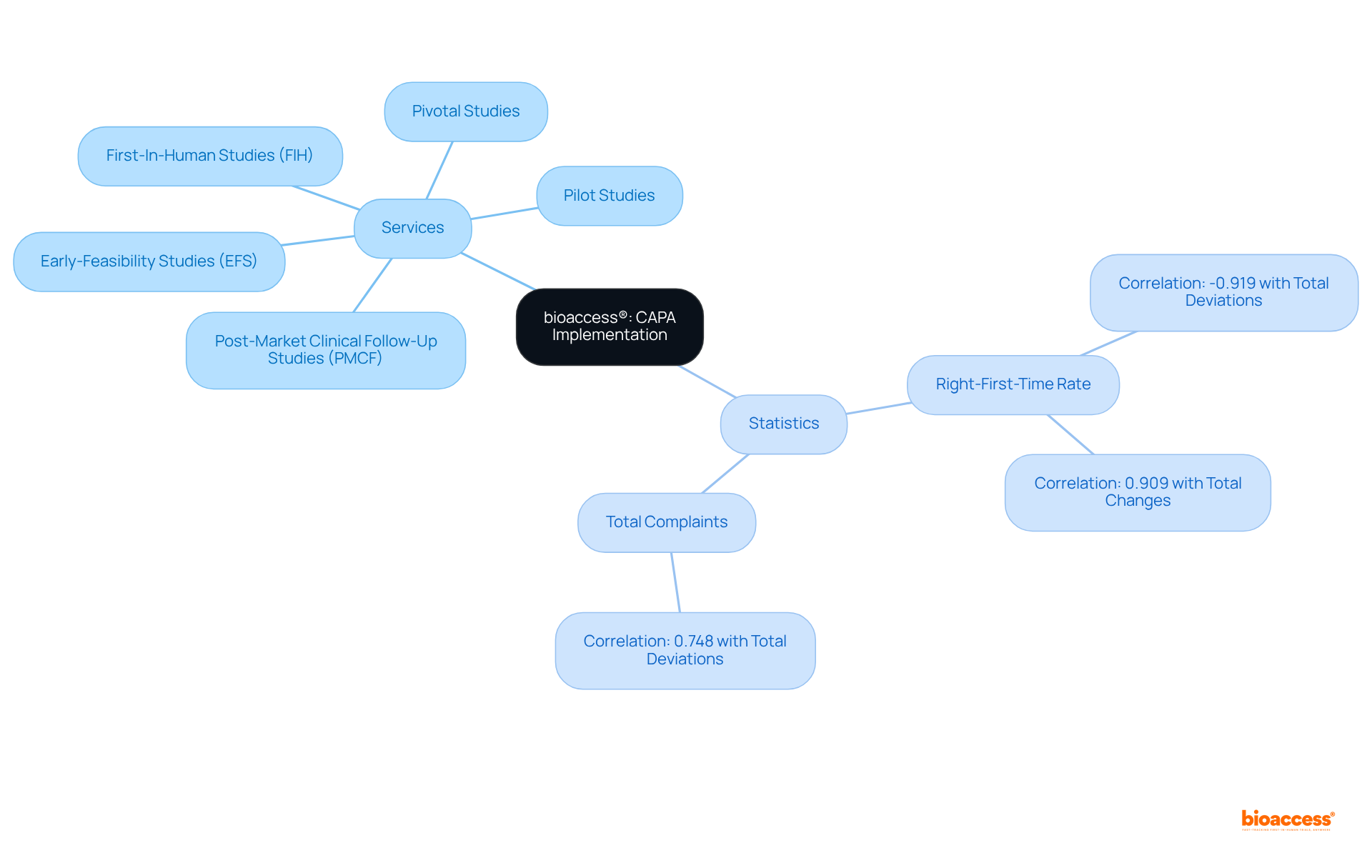
Our comprehensive clinical trial management services encompass:
All meticulously tailored to address the unique needs of each study. Statistics reveal that effective corrective and preventive action strategies can elevate the right-first-time rate, evidenced by a notable negative correlation of -0.919 between the right-first-time rate and total deviations. Furthermore, the correlation of 0.748 between total complaints and total deviations underscores the importance of robust corrective and preventive action strategies in enhancing overall trial efficiency.
Recent advancements in corrective and preventive actions, such as the implementation of automated regulatory mapping systems and enhanced data analytics, further underscore the necessity for a systematic approach. This allows organizations to navigate complexities while maintaining high standards in clinical research. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, plays an instrumental role in ensuring compliance and efficiency in these critical operations.
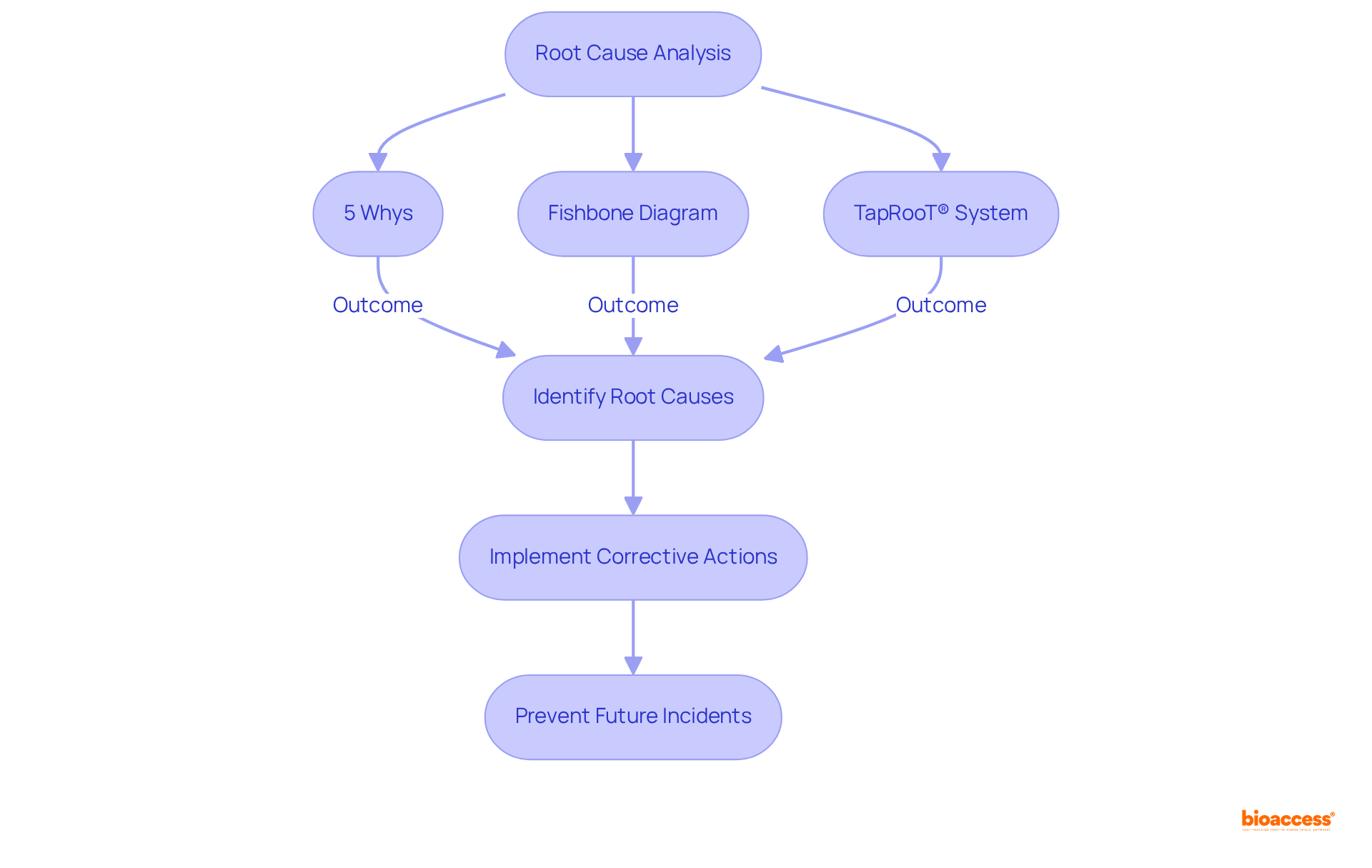
Conducting a thorough root cause analysis (RCA) is essential for effective capa corrective action preventive action implementation in clinical research. This process involves identifying the fundamental reasons behind deviations or non-compliance events, which is critical for ensuring study quality. Techniques such as the '5 Whys' and fishbone diagrams can be employed to dissect issues systematically. By pinpointing the root causes, organizations can implement capa corrective action preventive action measures that not only address the immediate issue but also prevent future incidents. Notably, the TapRooT® system is designed to avoid blame and focus on understanding incidents, a crucial aspect of effective RCA.
The significance of RCA is underscored by the alarming statistic that medical errors rank among the top causes of death and disability worldwide. This highlights the urgent need for effective RCA in clinical research. However, it is important to acknowledge that 82% of recommendations from RCA reports have been classified as weak, indicating a pressing need for more robust capa corrective action preventive action practices. The Joint Commission mandates RCA for reported sentinel events, further emphasizing its regulatory importance in healthcare settings. Incorporating insights from authoritative sources can enhance the credibility of RCA practices, ensuring that organizations not only comply with regulations but also strive for excellence in patient safety.
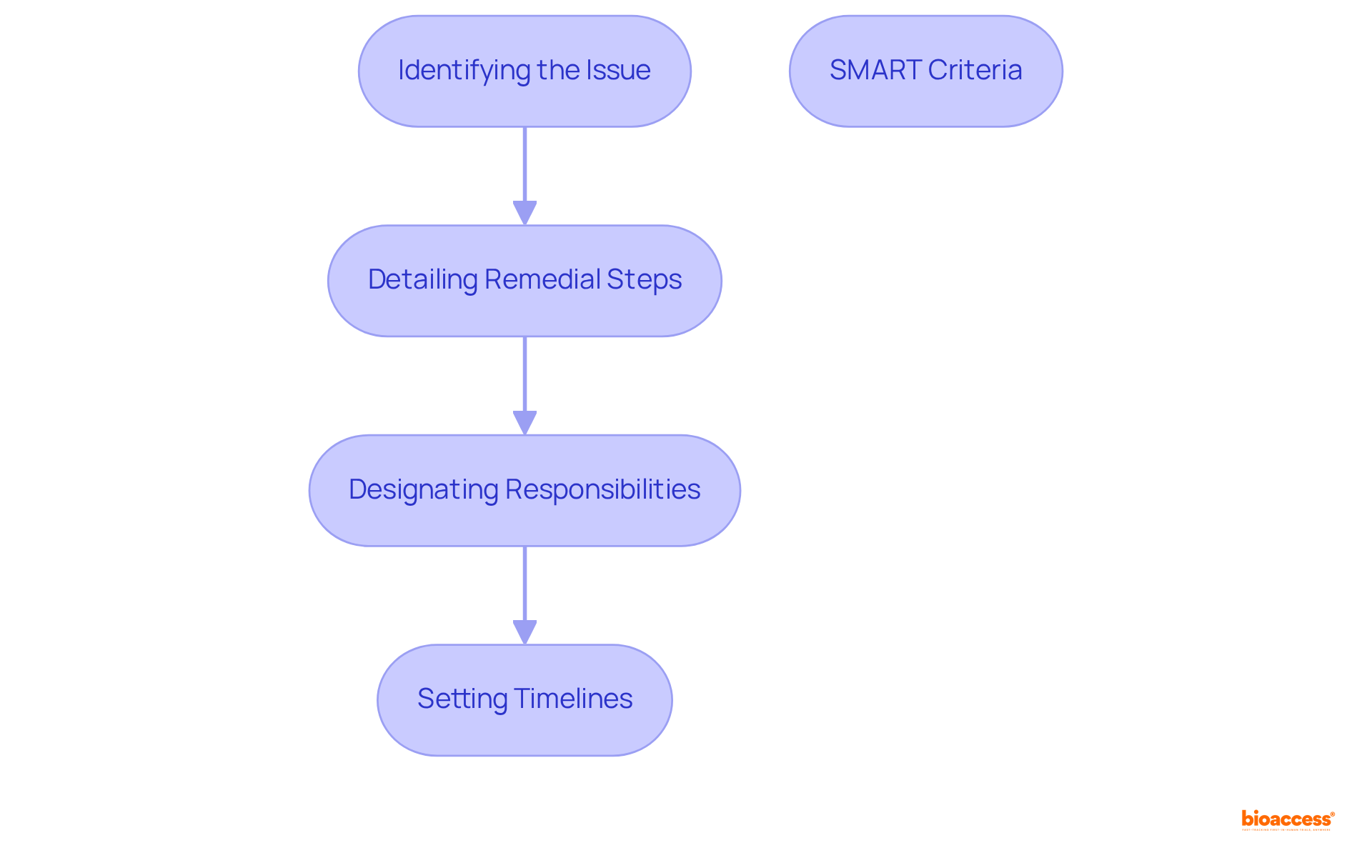
Creating a thorough capa corrective action preventive action plan is paramount in clinical research, encompassing several essential elements:
Each step must adhere to the SMART criteria—specific, measurable, achievable, relevant, and time-bound. As Mark Durivage observes, 'Capa corrective action preventive action issues continue to be among the leading Form 483 observational findings by the FDA,' underscoring the critical need for a well-organized capa corrective action preventive action plan to ensure compliance and regulatory adherence.
Moreover, the plan should incorporate preventive measures aimed at mitigating the risk of recurrence. This may include implementing training programs or revising protocols based on findings from root cause analyses. For instance, the case study titled 'SMART Approach to Effectiveness Checks' illustrates how organizations can enhance their corrective and preventive action systems by applying the SMART methodology, ultimately leading to improved compliance and operational efficiency.
Regular evaluations and revisions of the capa corrective action preventive action plan are essential, ensuring that it remains relevant and effective in addressing ongoing challenges in clinical research. Additionally, documenting the corrective and preventive actions process is critical for accountability and thoroughness, highlighting the importance of maintaining a clear log of measures taken and their outcomes.
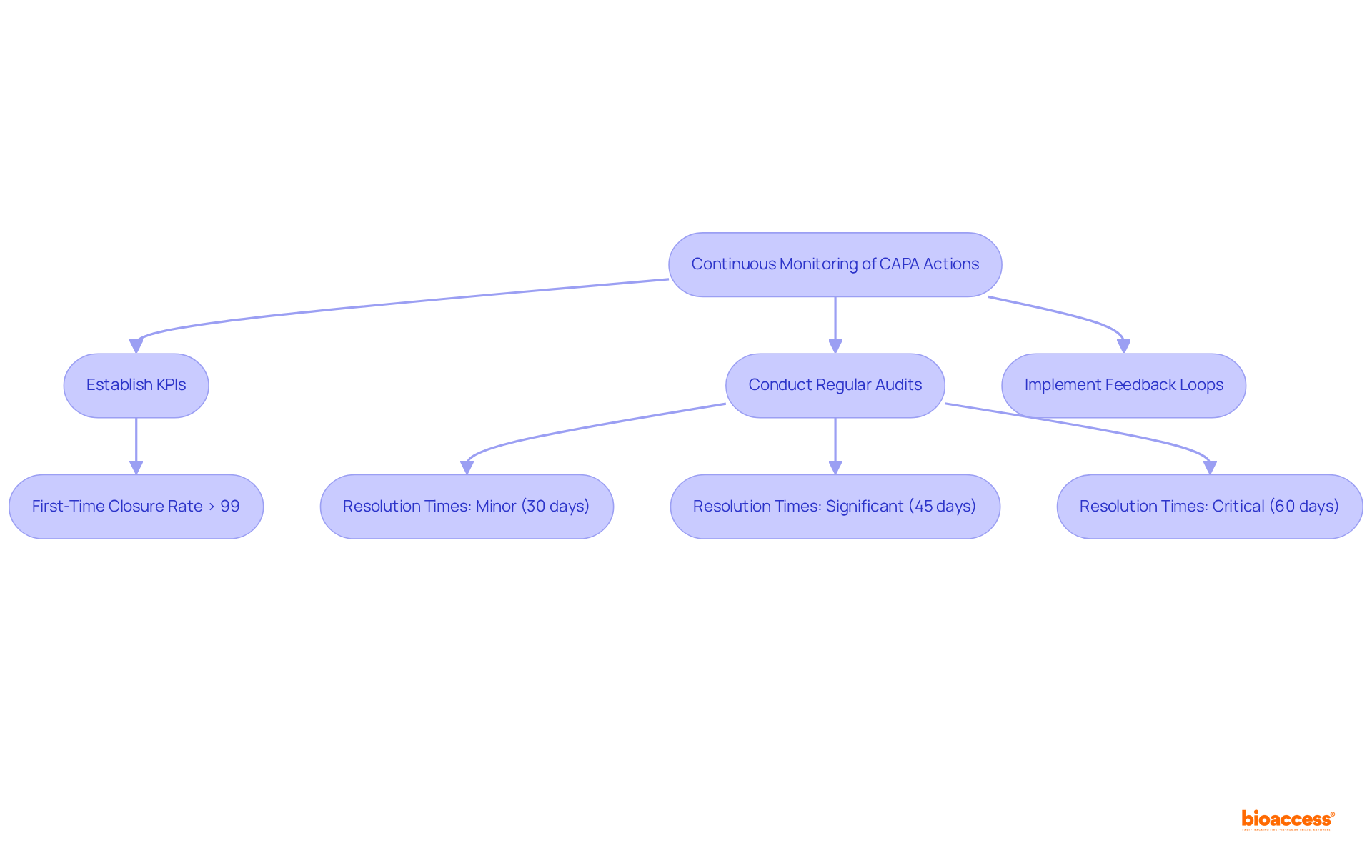
Ongoing oversight systems are crucial for evaluating the efficacy of corrective and preventive measures in clinical research. By establishing key performance indicators (KPIs), organizations can systematically measure the success of these measures. Regular audits and feedback loops are vital for pinpointing areas that require improvement, thereby ensuring that CAPA actions achieve their intended outcomes.
For instance, organizations often aim for a first-time closure rate exceeding 99%, with target resolution times of:
Through a proactive monitoring strategy, organizations can swiftly address emerging challenges, preserving the integrity and quality of their clinical trials. This commitment to continuous evaluation not only enhances compliance but also cultivates a culture of quality within the organization.
Incorporating capa corrective action preventive action processes within Quality Management Systems (QMS) is essential for ensuring compliance with regulatory standards in clinical research. At bioaccess, our extensive clinical trial management services include:
This integration not only streamlines documentation but also enhances the tracking and reporting of capa corrective action preventive action, allowing organizations to respond promptly to quality issues. By embedding capa corrective action preventive action into the quality management system, organizations can create a cohesive framework that fosters continuous improvement and cultivates a culture of quality. This strategy not only bolsters compliance but also enhances overall operational efficiency in clinical research.
For instance, organizations that utilize capa corrective action preventive action management have reported improvements in product compliance performance, with median compliance rates rising from 96% to 99%. Such advancements underscore the importance of a well-integrated capa corrective action preventive action system in maintaining high standards of quality and safety in medical device development.
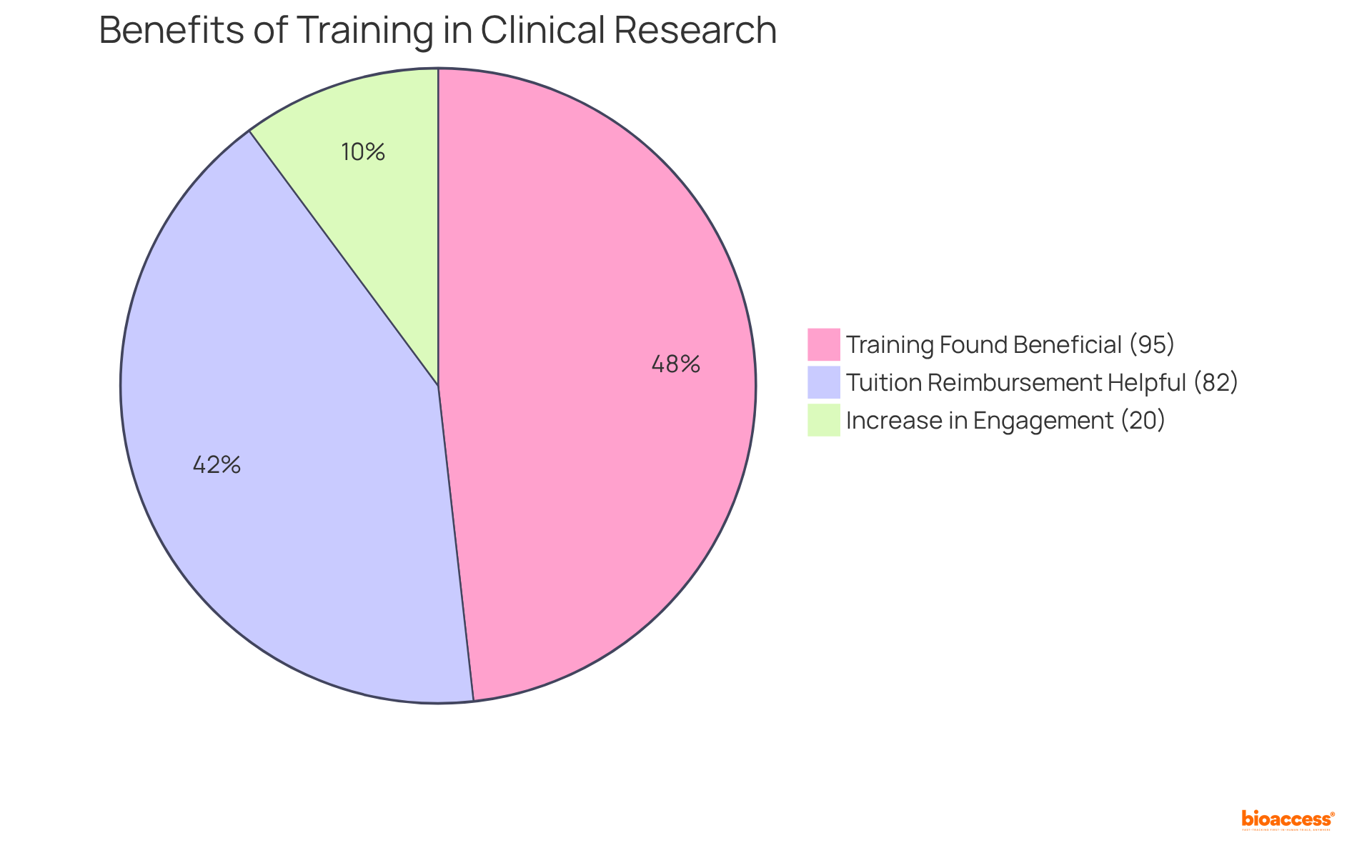
Enabling your team through training and education is paramount for the successful execution of CAPA (Corrective Action Preventive Action) processes in clinical research. Regular training sessions must encompass the principles of CAPA, root cause analysis techniques, and specific organizational procedures. A study has shown that 95% of participants found training beneficial for their subsequent responsibilities, underscoring its critical role in the field. Furthermore, 82% of employees reported that tuition reimbursement programs enhanced their job effectiveness, emphasizing the value of investing in employee education.
By cultivating a culture of continuous learning, organizations can ensure that team members possess the essential knowledge and skills to identify issues, implement CAPA, and elevate the overall quality of clinical research. Companies that commit to employee education experience a remarkable return on investment, with reported savings of $2.29 for every dollar spent. Additionally, personalized learning paths can lead to a reported 20% increase in engagement, while knowledge retention test scores indicate that those applying training information score significantly higher (3.70) compared to those who do not (3.19).
Implementing effective training approaches, such as microlearning, can significantly enhance engagement and retention, ultimately resulting in more successful outcomes. As Winston Churchill aptly noted, 'Success is not final, failure is not fatal: It is the courage to continue that counts.
Engaging stakeholders throughout the capa corrective action preventive action process is paramount for achieving improved outcomes. This engagement encompasses team members, management, and external partners in discussions about identified issues and proposed solutions. By cultivating open communication and collaboration, organizations can harness a wealth of diverse perspectives and expertise, resulting in more effective capa corrective action preventive action strategies. Regular stakeholder meetings are crucial in facilitating this engagement, ensuring alignment on goals and responsibilities among all parties involved.
Implementing prompt capa corrective action preventive action measures is essential for reducing risks related to non-compliance or deviations in clinical research. Organizations like bioaccess establish comprehensive clinical trial management services that encompass:
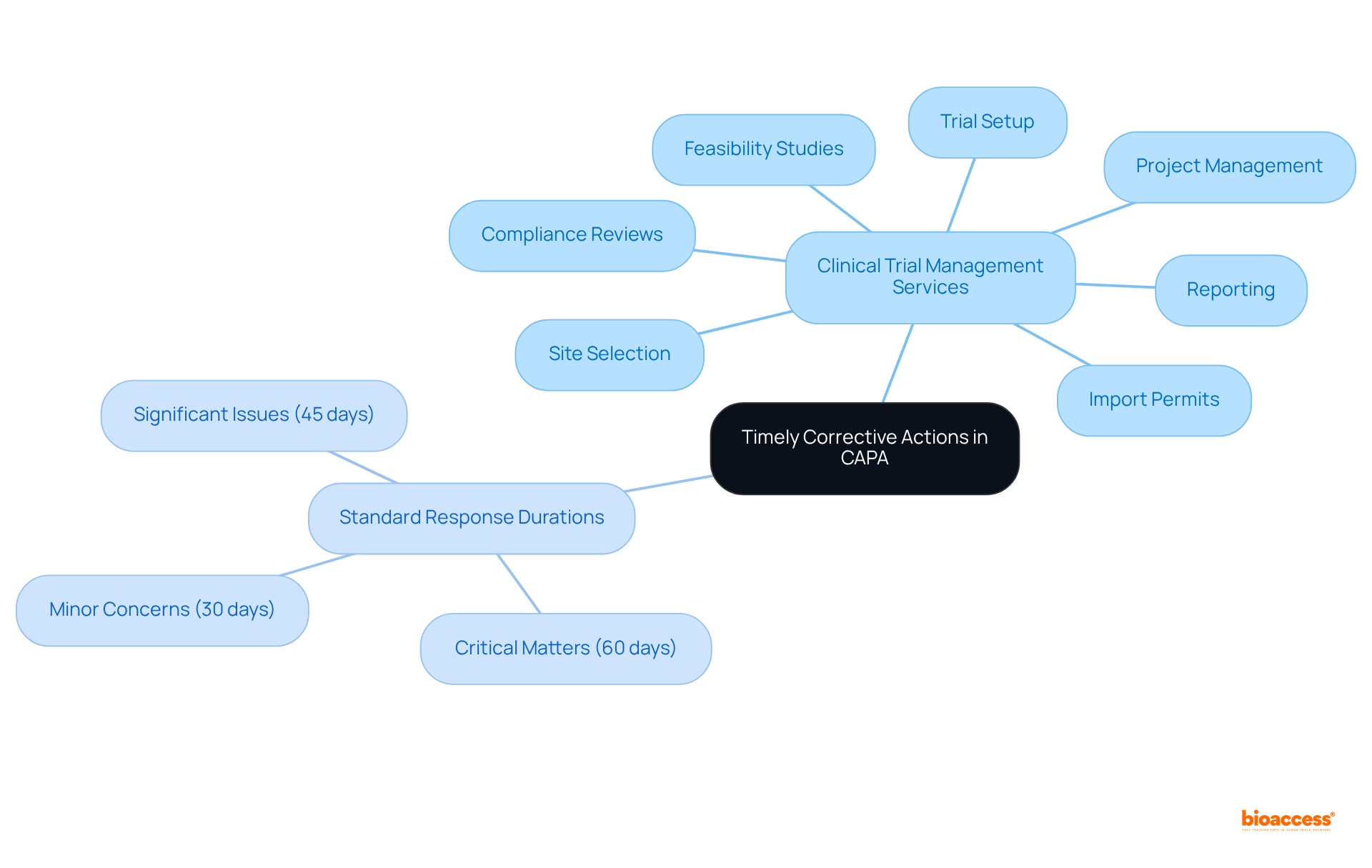
These services ensure that clear protocols are established for escalating concerns and initiating remedial measures without hesitation. A standard response duration for corrective measures in quality management is set at:
With 30 days as the default maximum time frame for a complete response, including root cause identification and validation. This proactive approach not only protects participant safety but also helps maintain the integrity of the study. Frequent evaluations of the capa corrective action preventive action system can assist in recognizing bottlenecks, and organizations are urged to track overdue actions, as any action open longer than 90 days is deemed overdue. By utilizing automated systems such as PERFEQTA, which increases visibility and optimizes response times, organizations can enhance the effectiveness of their capa corrective action preventive action management and promote ongoing enhancement in quality management.
Keeping detailed records throughout the capa corrective action preventive action process is crucial for adherence and responsibility. This includes documenting the identification of issues, root cause analyses, corrective actions taken, and the effectiveness of those actions. Precise records not only uphold regulatory standards but also provide a valuable reference for future capa corrective action preventive action.
Implementing a centralized documentation system can streamline this process and ensure that all relevant information is easily accessible. Notably, 51% of EQMS users have automated document control, indicating a trend towards automation in capa corrective action preventive action documentation.
Organizations that implement capa corrective action preventive action management systems have reported compliance rates rising to 100% - 99%, in contrast to 97% - 85% for those lacking such systems. This contrast underscores the significant impact of automation on compliance rates.
Furthermore, utilizing statistical methods like Failure Mode and Effects Analysis (FMEA) and Statistical Process Control (SPC) can enhance the quality of documentation by providing data-driven insights into quality issues. The 8D methodology also provides a systematic method for addressing issues that can be incorporated into the capa corrective action preventive action process.
By prioritizing effective record-keeping and ensuring all changes are documented in the capa corrective action preventive action log, organizations can not only meet regulatory standards but also foster a culture of continuous improvement and proactive risk management.
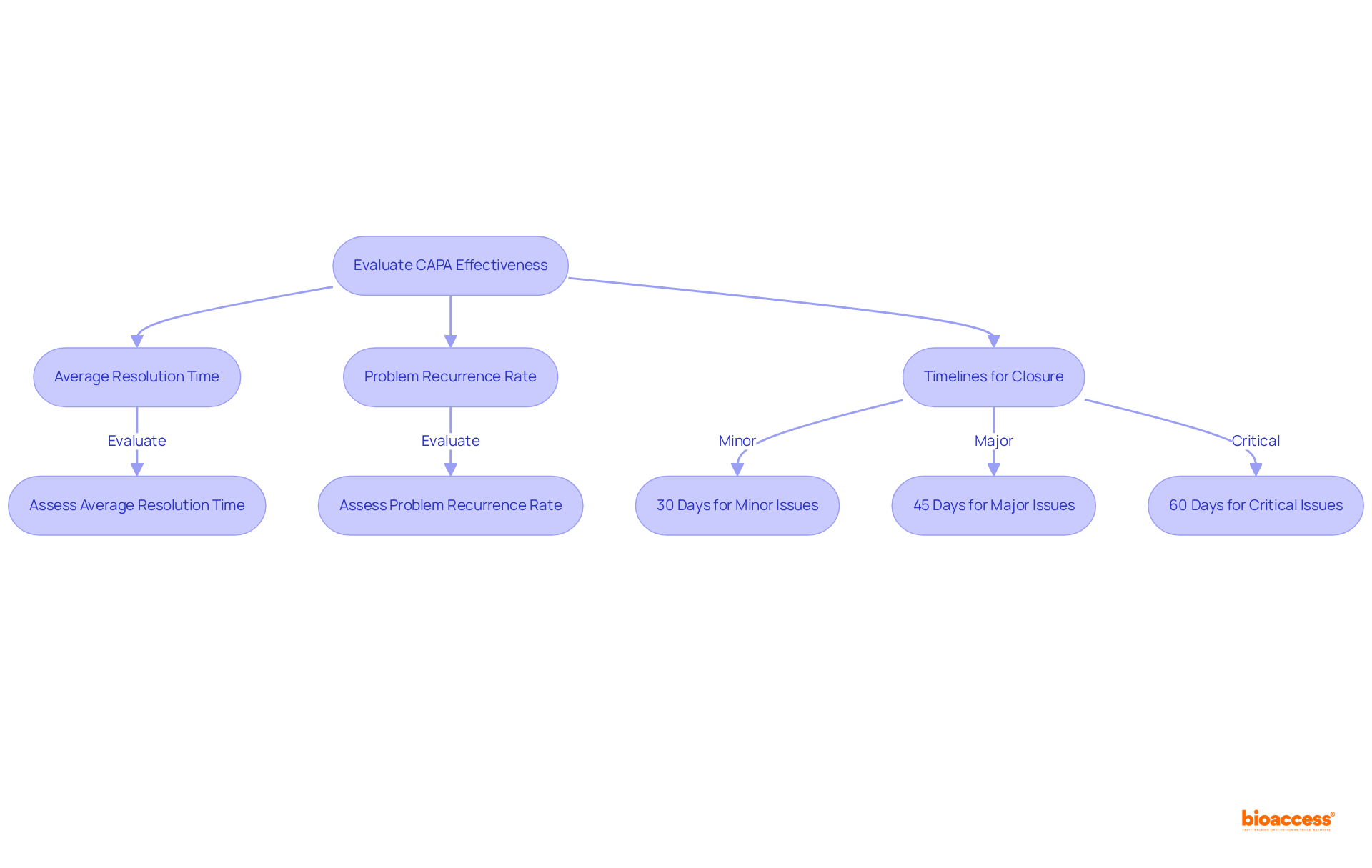
Frequent assessment of capa corrective action preventive action measures is vital for promoting ongoing enhancement in clinical research. Organizations must examine data connected to the results of capa corrective action preventive action to assess their effectiveness in resolving recognized problems. Key metrics for evaluating the effects include:
CAPAs should be completed within a timeframe proportional to the risk they address according to ISO 14971, ensuring that closure targets are established—typically 30 days for minor issues, 45 days for major issues, and 60 days for critical issues—to ensure timely resolution and compliance with industry standards.
Regular evaluations of capa corrective action preventive action metrics can uncover trends and emphasize areas requiring additional improvement. For instance, tracking the percentage of corrective actions finished by their designated deadlines can reflect the effectiveness of the corrective action system. Furthermore, monitoring the allocation of remedial measures across different operational areas assists in root cause analysis, enabling organizations to prioritize their efforts efficiently. It is also crucial to monitor the age of open CAPAs, adhering to best practice guidelines of closing them within 60 days to maintain compliance.
By promoting a culture of responsibility and ongoing education, organizations can greatly enhance their capa corrective action preventive action processes and overall quality management, ultimately resulting in better outcomes in clinical trials. Reporting capa corrective action preventive action metrics in quality management evaluations is essential for thorough oversight, and education centered on remediation and root cause analysis can further enhance performance. A high recurrence rate may indicate ineffective corrective actions or improperly addressed root causes, underscoring the importance of timely and effective CAPA management.
Implementing effective Corrective and Preventive Action (CAPA) strategies is crucial for enhancing the quality and efficiency of clinical research. This article underscores the importance of integrating CAPA within clinical trial management, not only to address immediate issues but also to foster a culture of continuous improvement and compliance. By understanding the intricacies of CAPA processes, organizations can significantly mitigate risks, enhance patient safety, and maintain the integrity of their studies.
Key insights from the discussion emphasize the necessity of:
Each strategy contributes to a robust framework that ensures timely responses to deviations and non-compliance, ultimately leading to improved operational efficiency. Furthermore, the integration of CAPA within Quality Management Systems (QMS) and the emphasis on training and stakeholder involvement are vital for cultivating a collaborative environment that supports effective CAPA implementation.
In summary, organizations engaged in clinical research must prioritize the establishment and refinement of CAPA processes to achieve optimal outcomes. By committing to best practices—such as timely corrective actions, thorough documentation, and regular evaluations of CAPA effectiveness—they can enhance compliance with regulatory standards and improve the overall quality of their trials. Embracing a proactive approach to CAPA not only safeguards participant safety but also drives innovation and excellence in clinical research.
What is bioaccess® and how does it assist in clinical research?
bioaccess® is a company that leverages its extensive understanding of regulatory frameworks and clinical trial dynamics to expedite the execution of Corrective and Preventive Measures (CAPA) in clinical research. With over 20 years of expertise in Medtech, they ensure that CAPA processes are compliant and efficient, significantly reducing the time from issue identification to resolution, which is crucial for patient safety and study integrity.
What clinical trial management services does bioaccess® offer?
bioaccess® offers a range of clinical trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Each service is tailored to meet the unique needs of individual studies.
How do effective CAPA strategies impact clinical trials?
Effective CAPA strategies can significantly elevate the right-first-time rate in clinical trials. There is a notable negative correlation of -0.919 between the right-first-time rate and total deviations, indicating that robust CAPA practices enhance overall trial efficiency.
What is the importance of Root Cause Analysis (RCA) in CAPA implementation?
Conducting a thorough RCA is essential for effective CAPA implementation, as it identifies the fundamental reasons behind deviations or non-compliance events. This process is critical for ensuring study quality and helps organizations implement measures that address immediate issues and prevent future incidents.
What techniques can be used for Root Cause Analysis?
Techniques such as the '5 Whys' and fishbone diagrams can be employed to systematically dissect issues during Root Cause Analysis. The TapRooT® system is also designed to focus on understanding incidents rather than placing blame.
Why is Root Cause Analysis significant in clinical research?
RCA is significant because medical errors rank among the top causes of death and disability worldwide. Effective RCA practices are necessary to enhance patient safety and quality in clinical research. However, it’s noted that 82% of recommendations from RCA reports are classified as weak, indicating a need for more robust CAPA practices.
What are the key elements of a CAPA plan in clinical research?
A comprehensive CAPA plan should include identifying the issue, detailing remedial steps, designating responsibilities, and setting timelines. Each element must adhere to the SMART criteria—specific, measurable, achievable, relevant, and time-bound.
How can organizations ensure their CAPA plans remain effective?
Organizations should regularly evaluate and revise their CAPA plans to ensure they remain relevant and effective in addressing ongoing challenges in clinical research. Documenting the CAPA process is also critical for accountability and thoroughness.
What role do preventive measures play in CAPA plans?
Preventive measures in CAPA plans aim to mitigate the risk of recurrence of issues. This may include implementing training programs or revising protocols based on findings from root cause analyses.