


The article titled "10 Class II Medical Devices Examples You Should Know" serves to illuminate the critical role of Class II medical devices in healthcare. These devices encompass essential tools such as:
Each device is meticulously highlighted for its specific medical function and significance, emphasizing the stringent regulatory requirements and clinical studies necessary to ensure both safety and efficacy. This underscores the indispensable role these devices play in enhancing patient care and improving treatment outcomes.
The landscape of medical technology is rapidly evolving, with Class II medical devices playing a pivotal role in enhancing patient care and treatment outcomes. These devices, which encompass essential tools such as infusion pumps, surgical staplers, and defibrillators, are subject to rigorous regulatory scrutiny to ensure their safety and efficacy.
As innovators strive to bring these critical solutions to market, the challenge lies in navigating complex clinical research processes efficiently. Stakeholders must consider how to leverage advancements in clinical trial management to expedite the development and approval of these life-saving devices.
bioaccess® excels in expediting clinical research for Class II medical devices examples by expertly navigating the regulatory landscapes of Latin America, the Balkans, and Australia. This comprehensive approach includes:
With ethical approvals obtained in an impressive 4-6 weeks and participant enrollment occurring 50% quicker than in conventional markets, bioaccess® provides a streamlined route for innovators eager to introduce their products to market swiftly. This agility is crucial for companies striving to meet the rising demand for Class II medical devices examples, ensuring timely access to essential healthcare solutions.
Industry leaders recognize that enhancing regulatory efficiency is vital for fostering innovation, and bioaccess® stands at the forefront of this transformation, facilitating quicker research studies that ultimately improve outcomes for individuals. Furthermore, bioaccess®'s partnership with Caribbean Health Group aims to establish Barranquilla as a key location for clinical studies in Latin America, supported by Colombia's Minister of Health.

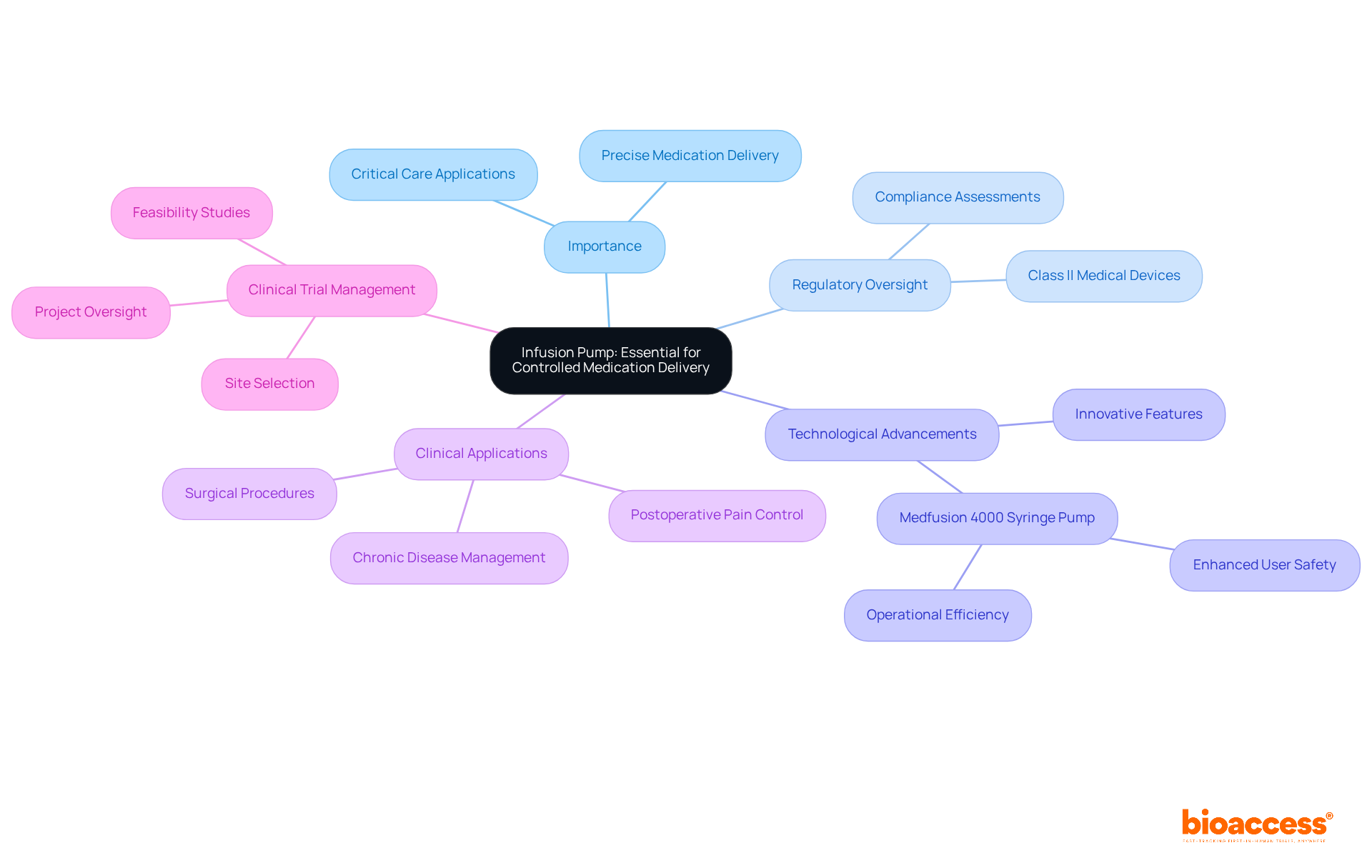
Infusion pumps are indispensable instruments in healthcare, meticulously designed to deliver medications, nutrients, and fluids to patients with precision. As examples of Class II medical devices, they pose a moderate risk and necessitate extensive regulatory oversight, including compliance assessments and setup processes. These pumps are pivotal in diverse medical scenarios, such as surgical procedures and chronic disease management, where accurate dosing is critical.
Recent studies highlight the significance of safety and efficacy in clinical trials for infusion pumps, emphasizing usability to meet stringent regulatory standards. Notably, advancements in infusion pump technology in 2025 have introduced enhanced features that bolster user safety and operational efficiency. The Medfusion 4000 Syringe Pump exemplifies this progress, recognized for its precise medication delivery in critical care environments, including neonatal and pediatric intensive care units.
As the healthcare landscape evolves, the integration of innovative technologies continues to enhance the effectiveness of infusion pumps, ensuring they remain vital tools in patient care. Furthermore, research indicates a high success rate in postoperative pain control, underscoring the practical effectiveness of these devices.
Comprehensive clinical trial management services, encompassing feasibility studies, site selection, and project oversight, are essential in navigating the regulatory landscape, ensuring that products like infusion pumps adhere to necessary safety and efficacy standards.
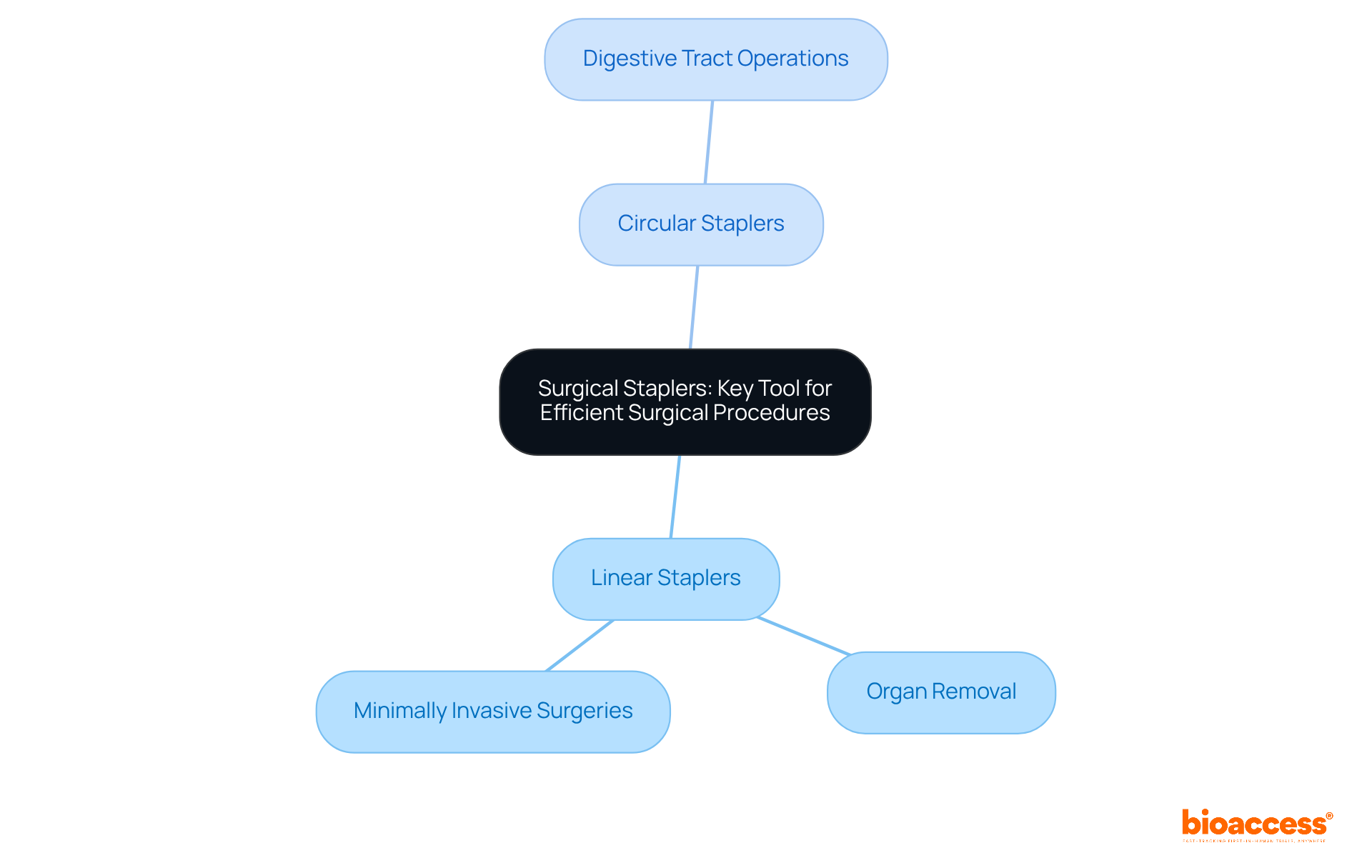
Surgical staplers are indispensable tools in modern surgery, essential for efficiently closing wounds and connecting tissues. They are class II medical devices examples that can be either reusable or disposable, catering to diverse surgical applications. These instruments are primarily divided into two categories:
Given their potential risks if not used correctly, extensive studies on surgical staplers are imperative.
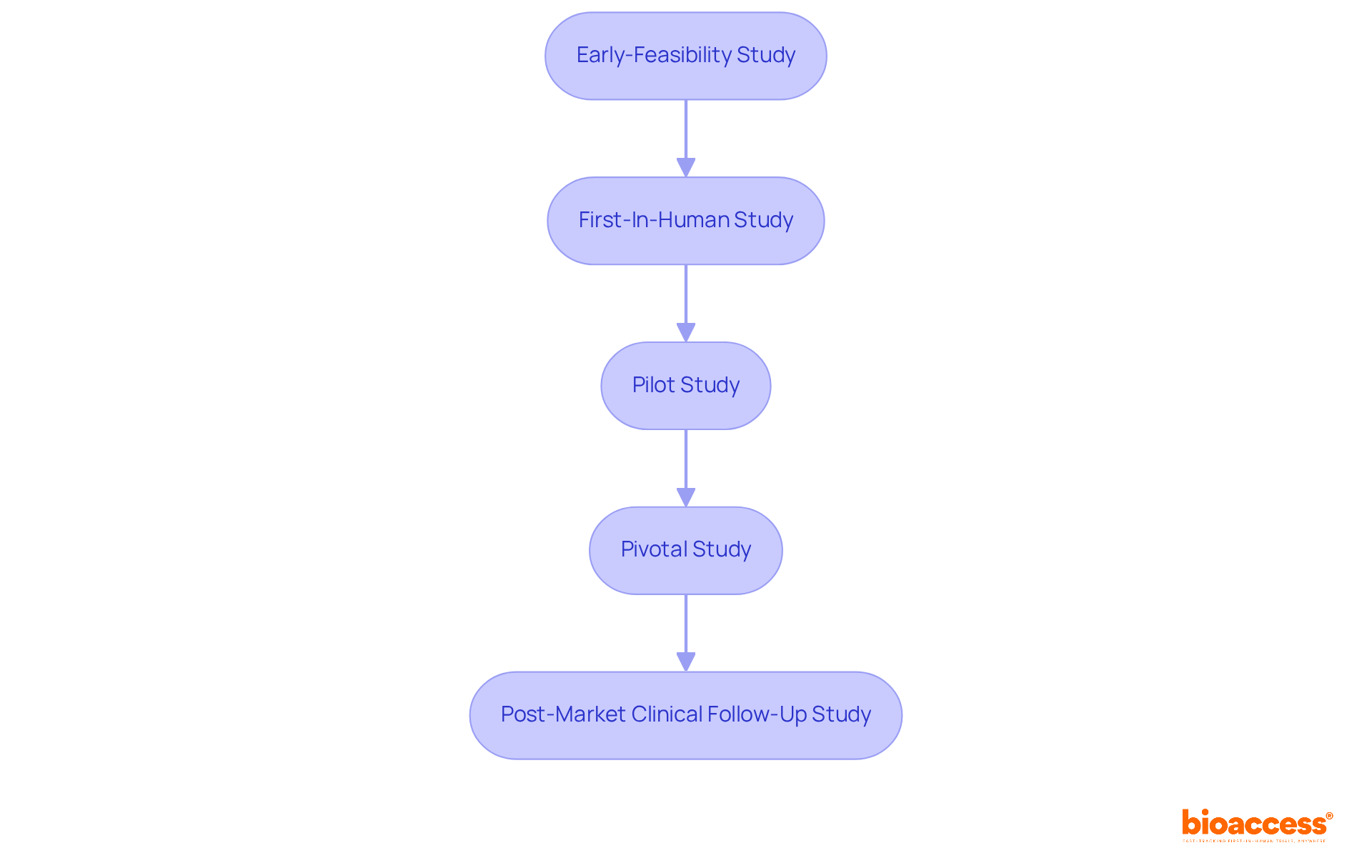
At bioaccess®, we excel in accelerated medical device clinical study services across Latin America, providing comprehensive clinical trial management that includes Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies. This meticulous evaluation is vital for validating the safety and efficacy of surgical staplers, a critical step for securing market approval. Recent innovations in surgical stapler technology in 2025 have significantly enhanced their functionality, resulting in improved precision and reduced surgical times. Surgeons have praised these advancements, noting that contemporary staplers not only streamline procedures but also contribute to enhanced outcomes for patients.
As the landscape of surgical technology continues to evolve, the role of surgical staplers remains pivotal in augmenting surgical efficiency and elevating patient care. Collaboration in clinical research is essential to navigate the challenges within the Medtech landscape, and bioaccess is committed to leading the way.
Catheters, which are flexible tubes utilized to deliver medications and fluids or to drain bodily fluids, are examples of Class II medical devices due to their moderate risk profile. Clinical studies for catheters focus on their design, usability, and safety across various medical applications, including urinary drainage and intravenous therapy.
In this context, bioaccess® offers expert services that connect innovative Medtech, Biopharma, and Radiopharma startups with top-ranked research sites, facilitating accelerated trials for catheters. This approach not only ensures compliance with regulatory standards efficiently but also underscores the importance of patient safety and effective treatment outcomes.
With bioaccess®'s support, startups can confidently advance their research initiatives.
MRI machines represent cutting-edge imaging technology, delivering intricate visuals of the body's internal structures. As class II medical devices examples, they require thorough clinical studies to validate their imaging capabilities and ensure safety. These trials must evaluate the machine's efficacy across diverse diagnostic scenarios, confirming compliance with the stringent standards established by regulatory authorities. The continuous evolution of MRI technology demands persistent research and development, aimed at improving diagnostic accuracy and addressing the challenges within the Medtech landscape.

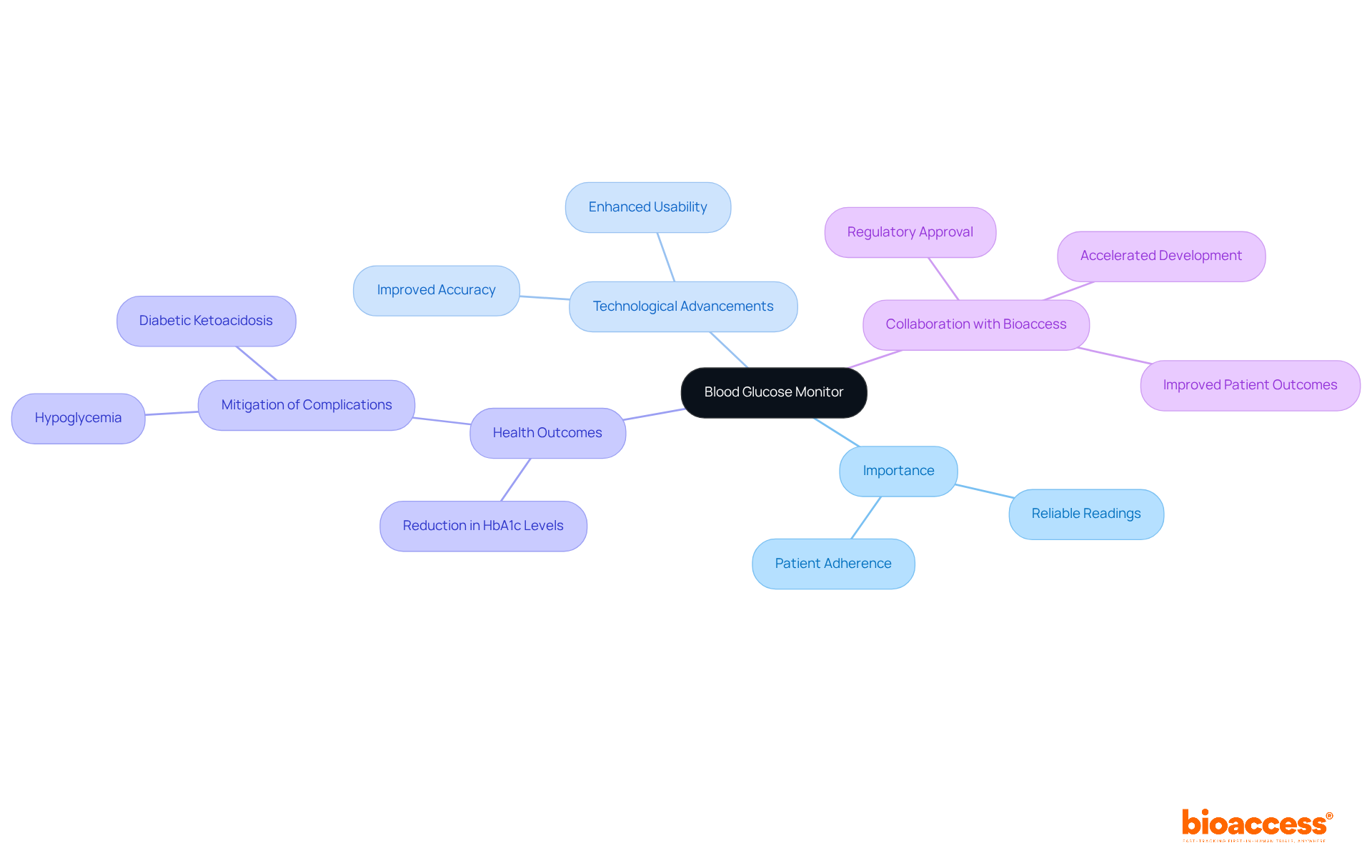
Blood glucose monitors are indispensable tools for effective diabetes management, allowing individuals to accurately track their blood sugar levels. Examples of class II medical devices are classified as medical instruments that are regulated due to their moderate risk. Recent advancements in diabetes management technology have significantly enhanced the accuracy and usability of these devices, which are crucial for ensuring reliable readings. Clinical studies underscore the importance of these factors, as they directly influence individual adherence and health outcomes.
Effective blood glucose monitoring can lead to a substantial reduction in HbA1c levels and mitigate complications such as hypoglycemia and diabetic ketoacidosis. As diabetes management continues to evolve, the integration of advanced blood glucose monitors is vital for enhancing care and achieving successful management outcomes.
Collaborating with bioaccess can accelerate the development and regulatory approval of these products, providing Medtech startups with expedited research services, including efficient patient recruitment and a faster route to market, ultimately improving patient outcomes.
Defibrillators are life-saving instruments employed to restore normal heart rhythms during cardiac emergencies. As class II medical devices examples, they necessitate extensive clinical studies to evaluate their efficacy and safety in real-world scenarios. These trials, which can be expertly managed by bioaccess® in Latin America, must evaluate the device's performance across diverse settings, including hospitals and public spaces. This ensures that timely and effective treatment can be delivered during critical situations.
With bioaccess®'s specialized knowledge and experience in conducting Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies, the pathway to regulatory approval and successful implementation of defibrillators is expedited, ultimately enhancing health outcomes.
Ventilators serve a critical function in delivering respiratory support to patients who cannot breathe adequately on their own. As examples of Class II medical devices, they are subject to rigorous evaluations to ensure their safety and effectiveness. These trials assess ventilator performance in diverse clinical scenarios, including:
Recent advancements in ventilator technology have markedly enhanced outcomes for individuals in critical care settings, improving both safety and efficacy. Adhering to regulatory standards is essential to ensure that these products deliver effective treatment while safeguarding patient health.
Endoscopes serve as pivotal examples of Class II medical devices that are essential for both diagnostic and therapeutic procedures within the body. Their classification necessitates comprehensive medical evaluations to confirm safety and effectiveness, meticulously assessing the endoscope's functionality across diverse healthcare applications. These evaluations guarantee that endoscopes deliver precise diagnostics while minimizing discomfort for patients.
At bioaccess®, we excel in managing clinical trials for medical instruments, including endoscopes, providing a suite of services such as:
Recent advancements in endoscopic technology, particularly the integration of suction tools with single-use scopes, have significantly enhanced procedural efficiency and patient outcomes. As the field progresses, ongoing research and development are vital to further elevate the safety and effectiveness of these indispensable tools in minimally invasive medicine.
Pacemakers serve as implantable instruments that play a crucial role in regulating heart rhythms for individuals experiencing arrhythmias. They are examples of class ii medical devices, which require comprehensive studies to rigorously evaluate their safety and efficacy. Such assessments must scrutinize the pacemaker's performance across diverse populations, ensuring compliance with regulatory standards for cardiac devices.
Organizations like bioaccess® are at the forefront of this effort, providing extensive research management services that include:
These studies are vital for advancing innovative pacemaker technologies. Ongoing research and rigorous clinical trials are indispensable for enhancing patient outcomes and propelling the evolution of pacemaker technology.
The exploration of Class II medical devices underscores their critical role in modern healthcare, highlighting the necessity for rigorous clinical studies to guarantee their safety and efficacy. Devices such as infusion pumps and pacemakers serve distinct purposes, significantly enhancing patient care and treatment outcomes. Technological advancements and streamlined processes provided by organizations like bioaccess® are pivotal in expediting the research and regulatory approval of these essential instruments.
Key insights from the article emphasize the significance of effective clinical trial management in navigating the complexities of regulatory landscapes. The examples presented, including surgical staplers and blood glucose monitors, illustrate how ongoing innovation and meticulous evaluation can lead to improved patient safety and healthcare delivery. Moreover, the collaboration between startups and research entities cultivates an environment where groundbreaking medical technologies can flourish.
In conclusion, as the demand for Class II medical devices continues to escalate, the commitment to enhancing research efficiency and regulatory compliance becomes paramount. Stakeholders in the medical technology field are urged to prioritize collaboration and innovation, ensuring that essential devices reach the market swiftly and safely. By doing so, the healthcare community can better meet the needs of patients and enhance health outcomes across diverse medical settings.
What is bioaccess® and its role in clinical research for Class II medical devices?
bioaccess® specializes in accelerating clinical research for Class II medical devices by navigating regulatory landscapes in Latin America, the Balkans, and Australia. Their services include feasibility and selection of research sites, trial set-up, and meticulous study project management.
How quickly can bioaccess® obtain ethical approvals and enroll participants?
bioaccess® can obtain ethical approvals in approximately 4-6 weeks and achieve participant enrollment 50% faster than in conventional markets.
Why is the efficiency of regulatory processes important for Class II medical devices?
Enhancing regulatory efficiency is crucial for fostering innovation, allowing companies to meet the growing demand for Class II medical devices and ensuring timely access to essential healthcare solutions.
What partnership is bioaccess® involved in to enhance clinical studies in Latin America?
bioaccess® has partnered with Caribbean Health Group to establish Barranquilla as a key location for clinical studies in Latin America, with support from Colombia's Minister of Health.
What are infusion pumps and their significance in healthcare?
Infusion pumps are essential devices designed to deliver medications, nutrients, and fluids to patients with precision. They are classified as Class II medical devices and are critical in various medical scenarios, including surgical procedures and chronic disease management.
What advancements have been made in infusion pump technology recently?
Recent advancements in infusion pump technology, particularly in 2025, have introduced enhanced features that improve user safety and operational efficiency, exemplified by the Medfusion 4000 Syringe Pump, known for its precise medication delivery in critical care settings.
What role do clinical trials play in the development of infusion pumps?
Comprehensive clinical trial management services, including feasibility studies and project oversight, are essential to ensure that infusion pumps meet necessary safety and efficacy standards.
What are surgical staplers and their applications in surgery?
Surgical staplers are key tools used in modern surgery to efficiently close wounds and connect tissues. They can be reusable or disposable and are categorized into linear staplers for organ removal and circular staplers for digestive tract operations.
Why is extensive study important for surgical staplers?
Given the potential risks associated with surgical staplers if used incorrectly, extensive studies are imperative to validate their safety and efficacy, which is essential for securing market approval.
How has surgical stapler technology evolved recently?
Innovations in surgical stapler technology in 2025 have significantly improved their functionality, resulting in enhanced precision and reduced surgical times, contributing to better patient outcomes.
What services does bioaccess® provide for surgical staplers?
bioaccess® offers accelerated clinical study services for surgical staplers, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, to navigate the regulatory landscape effectively.