


The article highlights the transformative impact of various decentralized clinical trial platforms on research, emphasizing their role in enhancing participant engagement, streamlining processes, and improving data management. Platforms such as bioaccess, Medable, and Science 37 leverage innovative technologies and methodologies that effectively address recruitment challenges and regulatory complexities. This strategic approach culminates in faster and more efficient clinical trials, underscoring the importance of these advancements in the Medtech landscape. Collaboration among stakeholders is crucial to navigate these challenges, paving the way for a more effective clinical research environment.
Decentralized clinical trials are rapidly reshaping the landscape of medical research, presenting innovative solutions to long-standing challenges in participant recruitment and data management. As the industry navigates the complexities of traditional trial methods, these platforms promise enhanced accessibility, improved patient engagement, and streamlined processes that have the potential to revolutionize study conduct. However, with numerous platforms emerging, stakeholders face the question: how can they identify the most effective tools to leverage in this evolving environment? This article delves into ten groundbreaking decentralized clinical trial platforms that are not only transforming research but also establishing new standards for efficiency and participant involvement.
bioaccess® leverages its extensive knowledge across Latin America, the Balkans, and Australia to drive decentralized clinical trial platforms with remarkable efficiency. By securing swift regulatory approvals—achievable in just 4-6 weeks—and engaging diverse demographics, bioaccess® significantly accelerates the time to market for innovative medical technologies. This exceptional agility not only streamlines participant recruitment through pre-qualified networks, activating over 50 sites in under eight weeks, but also ensures that studies are conducted expeditiously, ultimately benefiting both researchers and participants.
The comprehensive services offered by bioaccess® include:
The ability to navigate complex regulatory frameworks efficiently is crucial, especially as the FDA's initiatives reflect a commitment to improving drug regulation and healthcare—an essential factor in today's competitive pharmaceutical landscape.
As the average cost per drug for discovery and development approaches approximately $802 million, the efficiency of bioaccess®'s processes becomes increasingly vital in tackling the challenges posed by high costs and complexities in drug development. Collaboration with bioaccess® not only enhances the research process but also positions stakeholders to thrive in the evolving Medtech landscape.

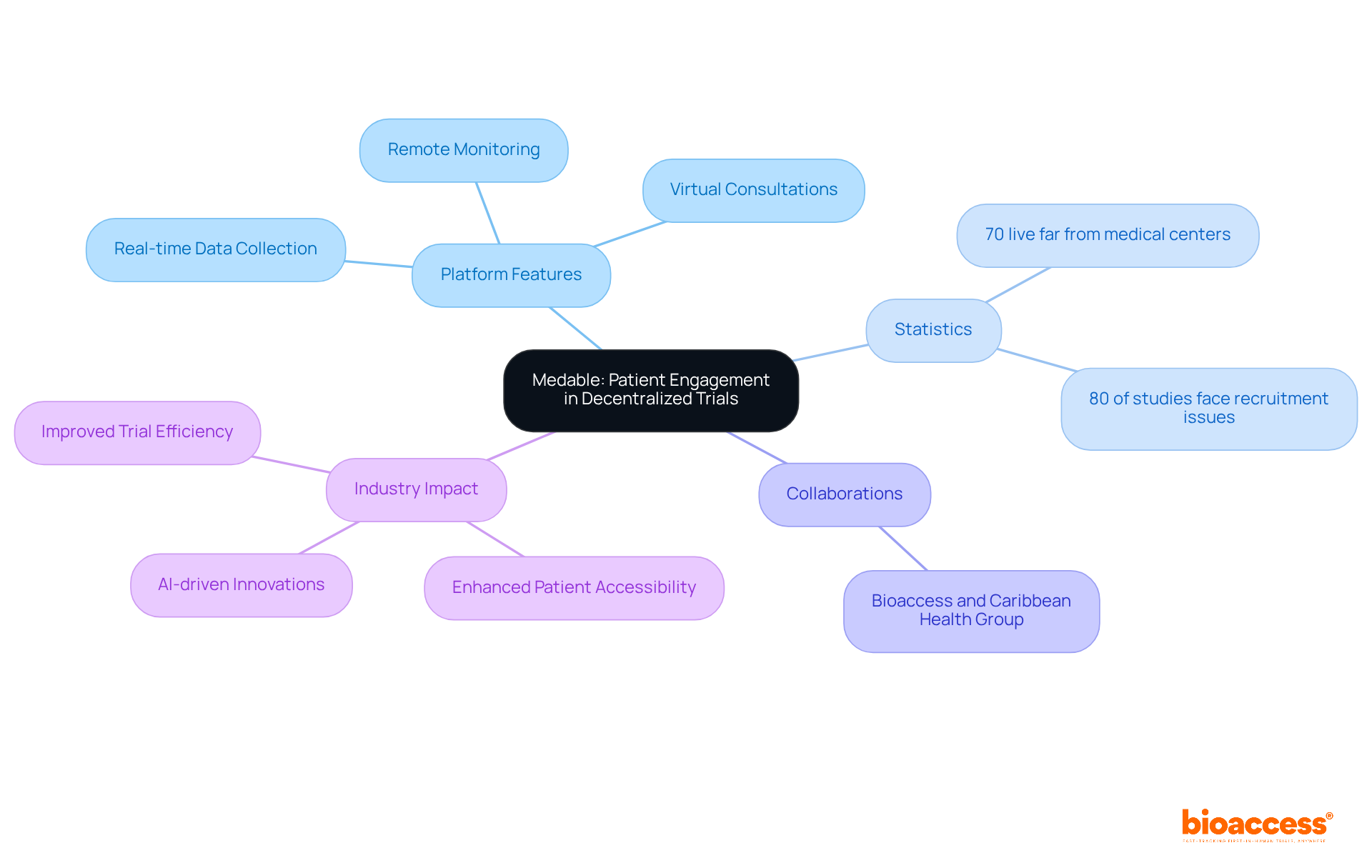
The platform establishes a robust foundation that significantly enhances participant involvement during the clinical study process, particularly for Medtech and Biopharma startups facing recruitment challenges. By integrating tools for remote monitoring, virtual consultations, and real-time data collection, bioaccess empowers patients to participate in studies from the comfort of their homes. This approach not only elevates enrollment rates but also fosters improved retention, culminating in more efficient and effective study completions.
The convenience of remote participation is particularly crucial, given that approximately 70% of the population lives two hours or more from an academic medical center, underscoring the need for accessible study alternatives. Furthermore, the collaboration between Bioaccess and Caribbean Health Group aims to position Barranquilla as a key hub for medical studies in Latin America, with support from Colombia's Minister of Health. This initiative is part of a broader strategy to tackle participant recruitment issues, with innovative solutions leading to a marked reduction in recruitment time and enhanced retention rates.
As the landscape of research evolves, decentralized clinical trial platforms are vital for boosting participant engagement and improving overall study outcomes. Notably, around 80% of clinical studies face delays or cancellations due to recruitment difficulties, emphasizing the urgency of these solutions. Additionally, AI-powered chatbots and virtual assistants are transforming patient interactions, further increasing retention rates during studies.
The FDA's guidance released in May 2023 supports the shift towards decentralized clinical trial platforms, emphasizing their significance in this evolving context. As the global electronic Clinical Outcome Assessments (eCOA) solutions market is projected to grow at a CAGR of 16.1% through 2030, the importance of digital solutions in medical studies continues to rise.
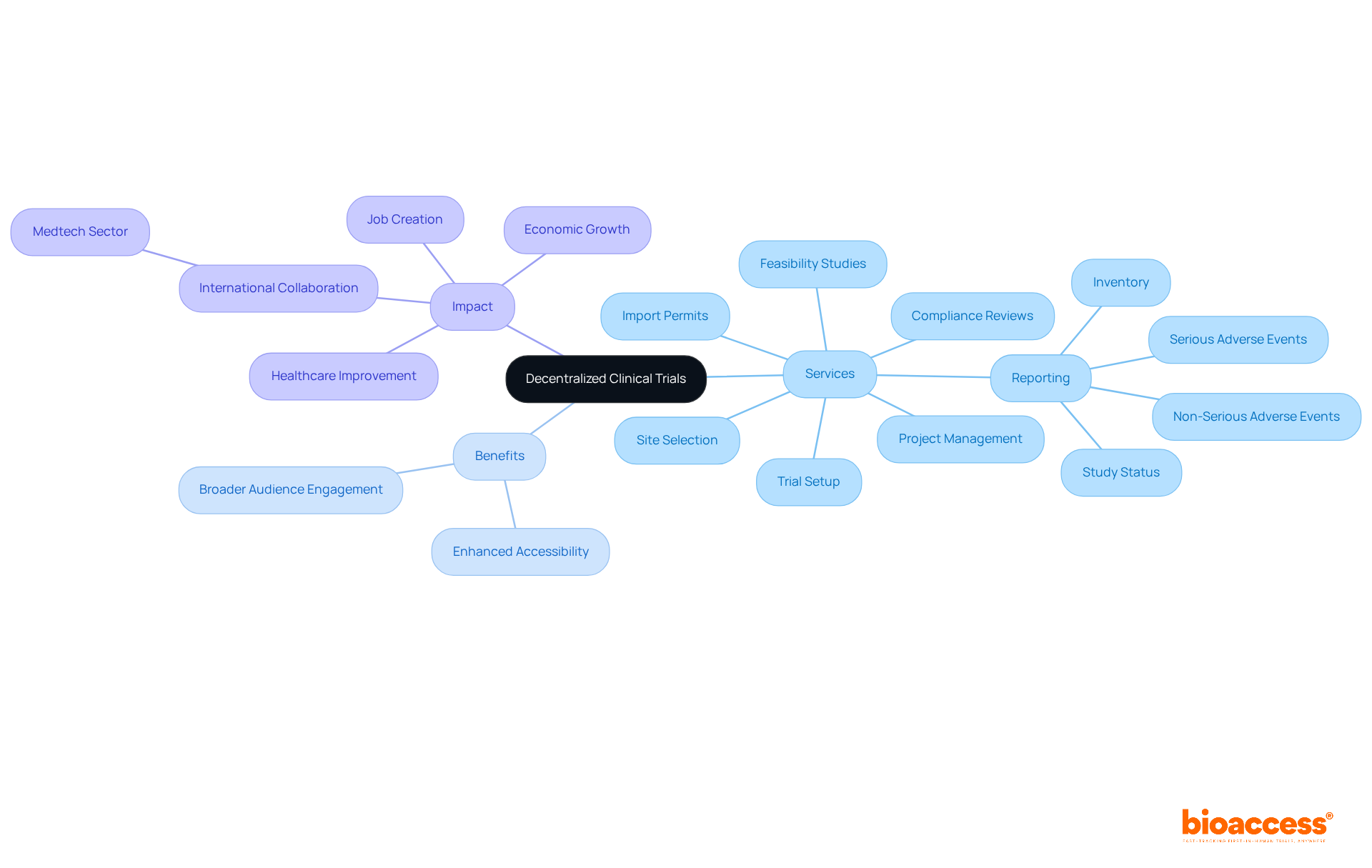
The organization has embraced decentralized clinical trial platforms, enabling individuals to participate in digital clinical studies without the need for in-person site visits. This innovative model significantly enhances accessibility, particularly for patients in remote or underserved areas. By leveraging telemedicine and digital tools, the organization ensures that decentralized clinical trial platforms can engage a broader audience, ultimately resulting in more diverse and representative data. Our comprehensive clinical trial management services encompass:
This holistic approach not only streamlines the trial process but also contributes to local economies through job creation, economic growth, and healthcare improvement, fostering international collaboration in the Medtech sector.
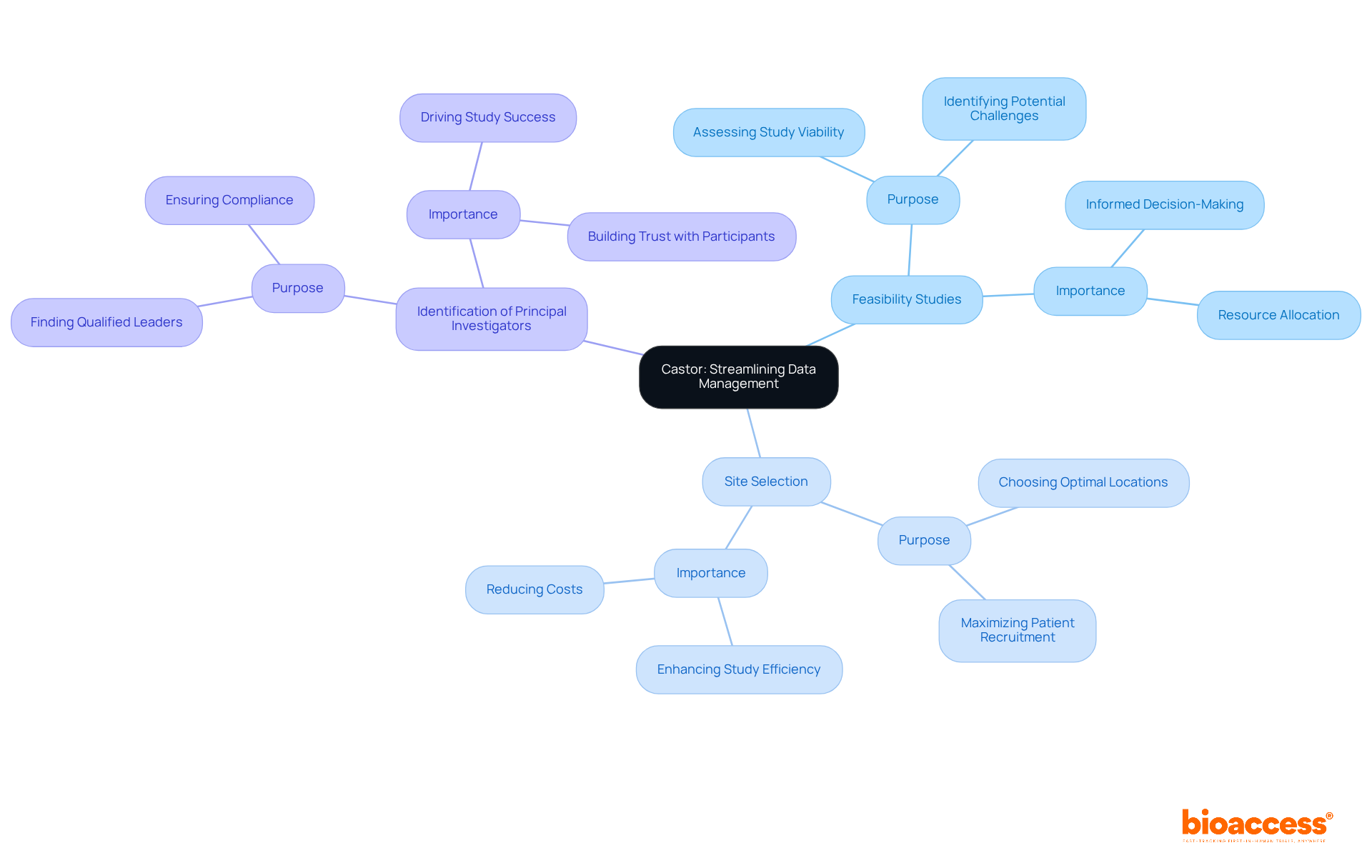
Bioaccess offers a comprehensive suite of services designed to enhance the management of decentralized clinical trial platforms. Our offerings include:
All aimed at ensuring an efficient setup that complies with regulatory standards. We conduct meticulous reviews and provide feedback on study documents to meet country-specific requirements, thereby facilitating smooth approval processes with ethics committees and health ministries. Additionally, we manage import permits and the nationalization of investigational devices, which are essential for fostering international cooperation in medical research.
Our project management and monitoring services guarantee effective study execution, complete with detailed reporting on study status, inventory, and adverse events. As the market for decentralized clinical trial platforms is projected to grow significantly, the necessity for thorough management services, such as those offered by Bioaccess, becomes increasingly apparent. With 94% of research locations adopting decentralized clinical trial platforms, our expertise is crucial for organizations seeking to enhance their clinical study processes and contribute to global health improvement through innovation in medtech.
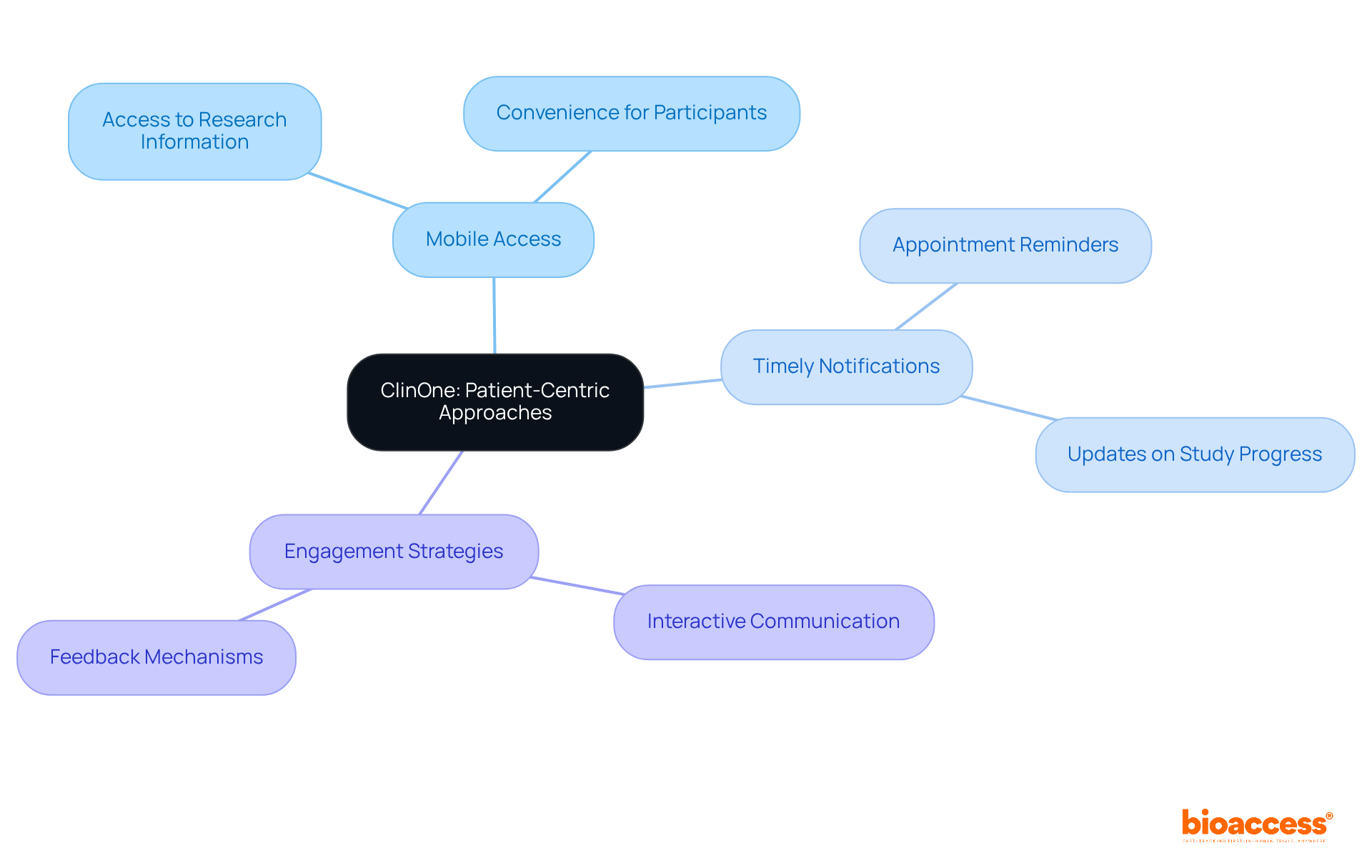
ClinOne enhances individual-focused methodologies in decentralized clinical trial platforms by providing a system that facilitates interaction between individuals and researchers. With features such as mobile access to research information and timely notifications for appointments, ClinOne leverages decentralized clinical trial platforms to ensure that individuals remain engaged and informed throughout the research process. This focus on patient experience is vital for improving retention rates and overall study success.
Furthermore, bioaccess™'s collaboration with Caribbean Health Group aims to position Barranquilla as a key hub for research studies in Latin America, supported by Colombia's Minister of Health. This partnership streamlines the recruitment process and integrates comprehensive research study management services, including:
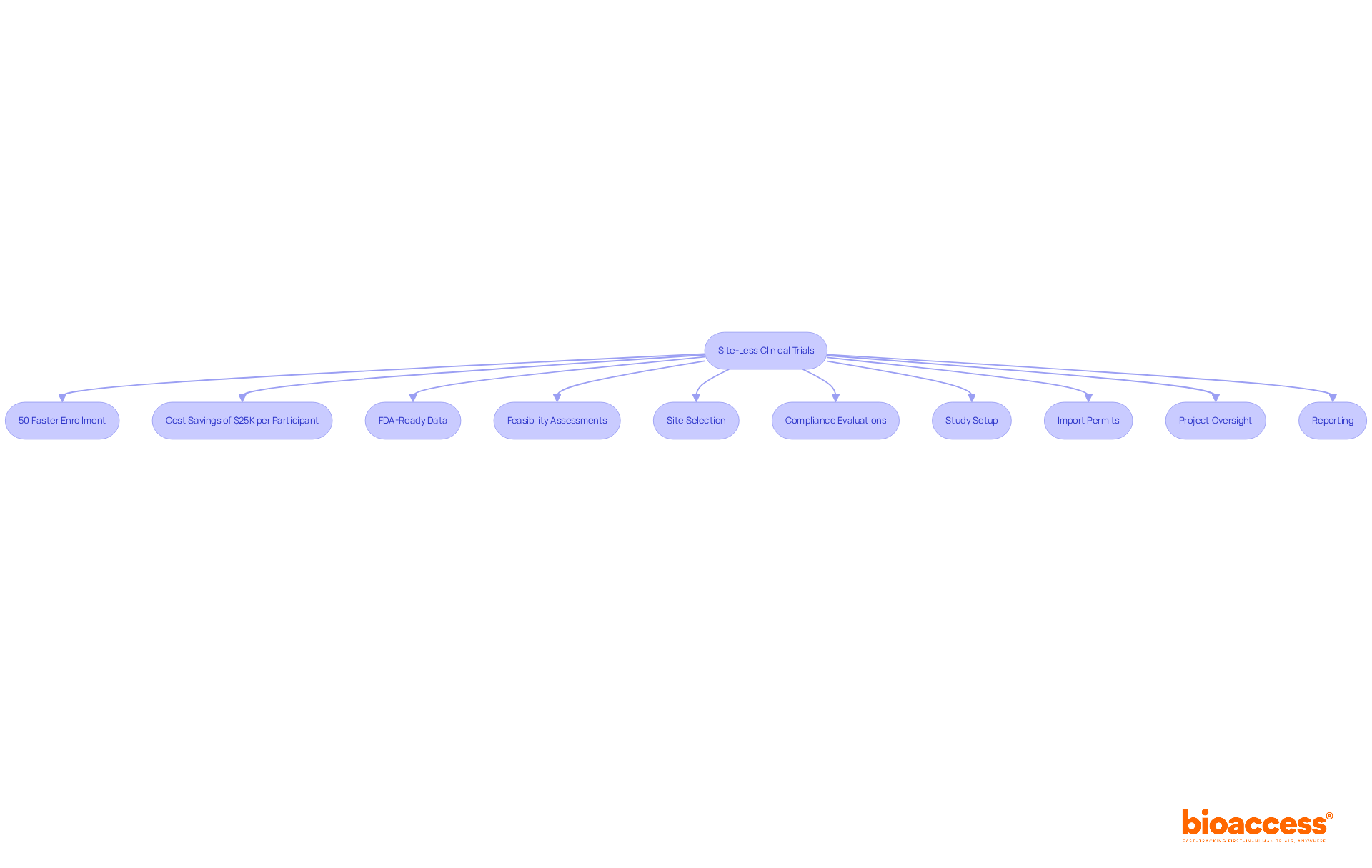
The company is revolutionizing the research landscape with its groundbreaking method, enabling treatment-naive cardiology or neurology groups to enroll 50% faster than conventional Western locations. This site-less model significantly enhances flexibility in participant recruitment and data collection through decentralized clinical trial platforms, resulting in substantial cost savings of $25K per participant with FDA-ready data—effectively eliminating the need for rework and delays.
By optimizing operations and providing comprehensive management services for research studies—including:
the organization greatly enhances the practicality of conducting studies, particularly for innovative therapies.
The platform revolutionizes the research environment by empowering both study locations and individuals through its innovative system designed for seamless collaboration and interaction. Our extensive clinical study management services encompass:
This ensures meticulous handling of every aspect of the process. By offering advanced tools for remote observation and efficient data gathering, bioaccess enables research locations to proficiently oversee their studies while ensuring high levels of participant involvement. This dual focus not only enhances the overall experience but also significantly contributes to improved outcomes.
With nearly 80% of Americans supporting remote monitoring and almost half expressing strong favor towards its integration into medical care, the incorporation of such technologies into decentralized studies is increasingly recognized as a vital component for success. Furthermore, the ability to reduce hospital readmission rates by up to 76%, as reported by the University of Pittsburgh Medical Center, underscores the potential for better management of individuals in research studies.
As we approach 2025, when an estimated 71 million Americans are expected to utilize various forms of remote health monitoring services, the emphasis on collaborative tools in decentralized clinical trial platforms will continue to grow, making such platforms essential for advancing research and enhancing care.

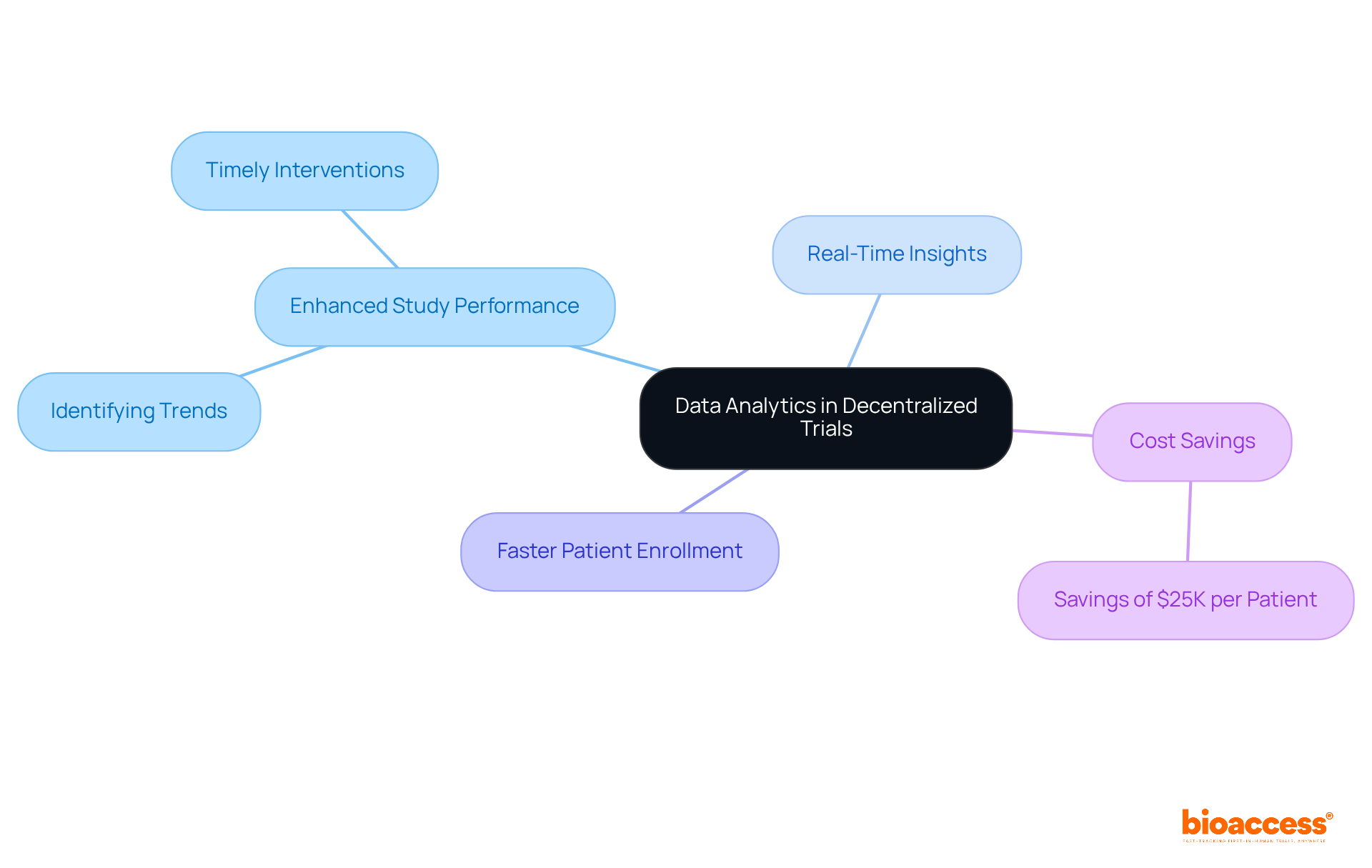
The platform employs sophisticated data analysis to empower researchers in enhancing study performance and making informed decisions. By delivering real-time insights into individual data and study progress, the platform significantly boosts the ability to identify trends and address challenges swiftly. This analytical approach is crucial for the success of decentralized clinical trial platforms, as it facilitates timely interventions and adjustments that ultimately improve outcomes and efficiency.
Organizations leveraging the platform have reported achieving patient enrollment 50% faster than traditional methods, translating to $25K savings per patient with FDA-ready data—no rework, no delays.
Furthermore, the collaboration between Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. This underscores the transformative impact of data-informed decision-making in medical research.
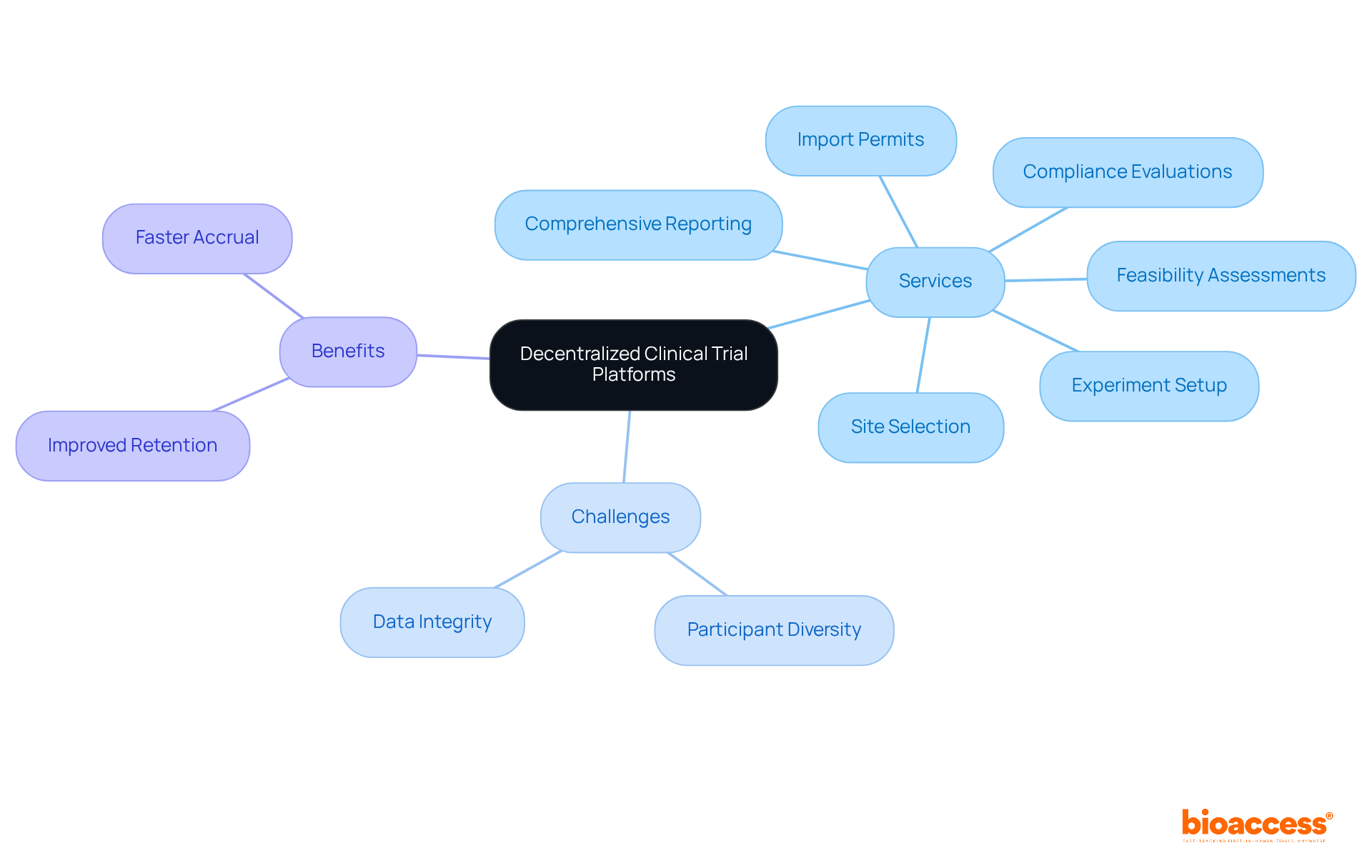
The decentralized clinical trial platforms play a pivotal role in ensuring high-quality data gathering in decentralized studies through their extensive clinical study management services. By enabling feasibility assessments, site selection, compliance evaluations, experiment setup, import permits, and comprehensive reporting on study progress and adverse occurrences, the organization significantly contributes to preserving the integrity of research results. This unwavering commitment to quality is essential for complying with regulatory standards, ensuring that results are both reliable and actionable.
As decentralized clinical trial platforms proliferate, the variability of data can present challenges such as participant diversity and absent data mechanisms. The organization's innovative approach mitigates risks associated with data integrity, ultimately enhancing the trustworthiness of research outcomes.
According to the FDA, decentralized clinical trial platforms (DCTs) are defined as studies where some or all activities occur at locations other than conventional research sites, which underscores the importance of maintaining data integrity in diverse environments. Furthermore, statistics indicate that DCTs can facilitate faster accrual and improved retention of participants, emphasizing the necessity for robust data management strategies to ensure the reliability of collected data.
By leveraging bioaccess's expertise, researchers in the medical field can navigate the complexities of decentralized studies with efficiency.
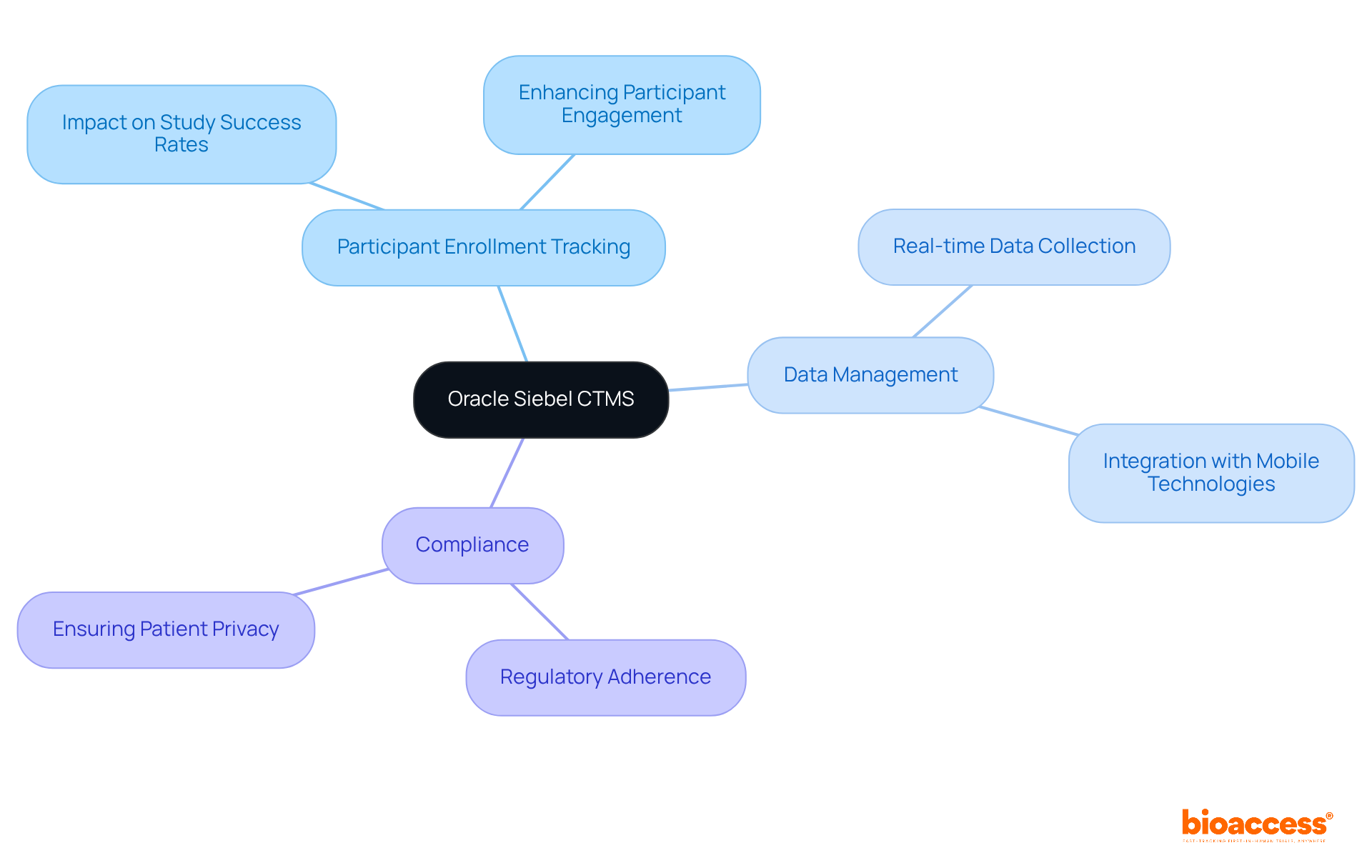
Oracle Siebel CTMS serves as a powerful suite of management solutions designed for decentralized clinical trial platforms, empowering researchers to meticulously oversee every facet of their investigations. Its advanced features enable precise tracking of participant enrollment, comprehensive data management, and stringent compliance adherence—elements that are essential for navigating the complexities inherent in decentralized clinical trial platforms.
As we look to 2025, effective patient enrollment tracking is projected to have a profound impact on study success rates, with research indicating that streamlined processes can significantly enhance participant retention and engagement.
By leveraging Oracle Siebel CTMS, organizations can ensure their trials not only comply with regulatory standards but also achieve optimal outcomes, thereby accelerating the journey toward medical advancements.
Decentralized clinical trial platforms are fundamentally transforming the landscape of medical research, providing innovative solutions that enhance efficiency, participant engagement, and data integrity. By adopting these platforms, researchers can adeptly navigate the complexities of clinical trials, ultimately accelerating drug development and improving patient outcomes.
In examining various platforms, several key insights have surfaced. Notably, the critical role of patient-centric approaches, the integration of advanced technologies for data management, and the significance of accessibility stand out. Companies such as bioaccess, Medable, Science 37, and others are harnessing their unique strengths to overcome traditional recruitment challenges, streamline processes, and promote collaboration within the healthcare ecosystem. The anticipated growth of these platforms highlights their essential contribution to meeting the urgent demands of contemporary medical research.
As the transition towards decentralized trials progresses, stakeholders within the healthcare sector are urged to recognize the transformative potential of these platforms. Embracing decentralized clinical trials not only enhances research efficiency but also lays the groundwork for more inclusive and representative studies, thereby propelling advancements in medical science. Engaging with these innovative solutions is crucial for maintaining competitiveness in an evolving landscape where patient engagement and data integrity are of utmost importance.
What is bioaccess and what does it offer in the context of decentralized clinical trials?
bioaccess is a platform that accelerates decentralized clinical trials by leveraging its knowledge across Latin America, the Balkans, and Australia. It offers services such as feasibility studies, investigator selection, compliance reviews, study setup, import permits, nationalization of investigational devices, project management, and thorough reporting on study status and adverse events.
How quickly can bioaccess secure regulatory approvals for clinical trials?
bioaccess can secure regulatory approvals in just 4-6 weeks.
What advantages does bioaccess provide for participant recruitment in clinical trials?
bioaccess significantly streamlines participant recruitment by utilizing pre-qualified networks, activating over 50 sites in under eight weeks, which accelerates the time to market for innovative medical technologies.
What are the financial implications of drug discovery and development that bioaccess addresses?
The average cost per drug for discovery and development approaches approximately $802 million, making the efficiency of bioaccess's processes vital in tackling the high costs and complexities involved in drug development.
What role does Medable play in decentralized clinical trials?
Medable establishes a comprehensive platform that enhances participant involvement during clinical studies, particularly for Medtech and Biopharma startups, by integrating tools for remote monitoring, virtual consultations, and real-time data collection.
Why is remote participation important in clinical trials?
Remote participation is crucial because approximately 70% of the population lives two hours or more from an academic medical center, highlighting the need for accessible study alternatives that facilitate enrollment and retention.
How does bioaccess collaborate with Caribbean Health Group?
bioaccess collaborates with Caribbean Health Group to position Barranquilla as a key hub for medical studies in Latin America, aiming to tackle participant recruitment issues and reduce recruitment time.
What challenges do clinical studies face regarding recruitment?
Approximately 80% of clinical studies face delays or cancellations due to recruitment difficulties, underscoring the urgency for decentralized clinical trial solutions.
How is technology being utilized to improve participant engagement in clinical trials?
AI-powered chatbots and virtual assistants are transforming patient interactions, further increasing retention rates during studies, and enhancing overall participant engagement.
What recent FDA guidance supports decentralized clinical trial platforms?
The FDA's guidance released in May 2023 emphasizes the significance of decentralized clinical trial platforms in the evolving research landscape.