

This article emphasizes the critical aggregate reports required for success in clinical research, highlighting their role in ensuring regulatory compliance and participant safety. It details specific report types, such as Development Safety Update Reports (DSURs) and Periodic Safety Update Reports (PSURs), which consolidate risk information and facilitate informed decision-making. These reports ultimately enhance the effectiveness and safety of clinical trials, underscoring their indispensable nature in the Medtech landscape.
The landscape of clinical research is increasingly complex, necessitating rigorous documentation to ensure patient safety and regulatory compliance. Aggregate reports are at the forefront of this challenge, functioning as essential tools for synthesizing data from various studies and providing critical insights into the safety and efficacy of new therapies. However, navigating the intricacies of these reports presents significant hurdles for researchers.
How can stakeholders effectively utilize aggregate reporting not only to meet regulatory demands but also to enhance the overall integrity of clinical trials?
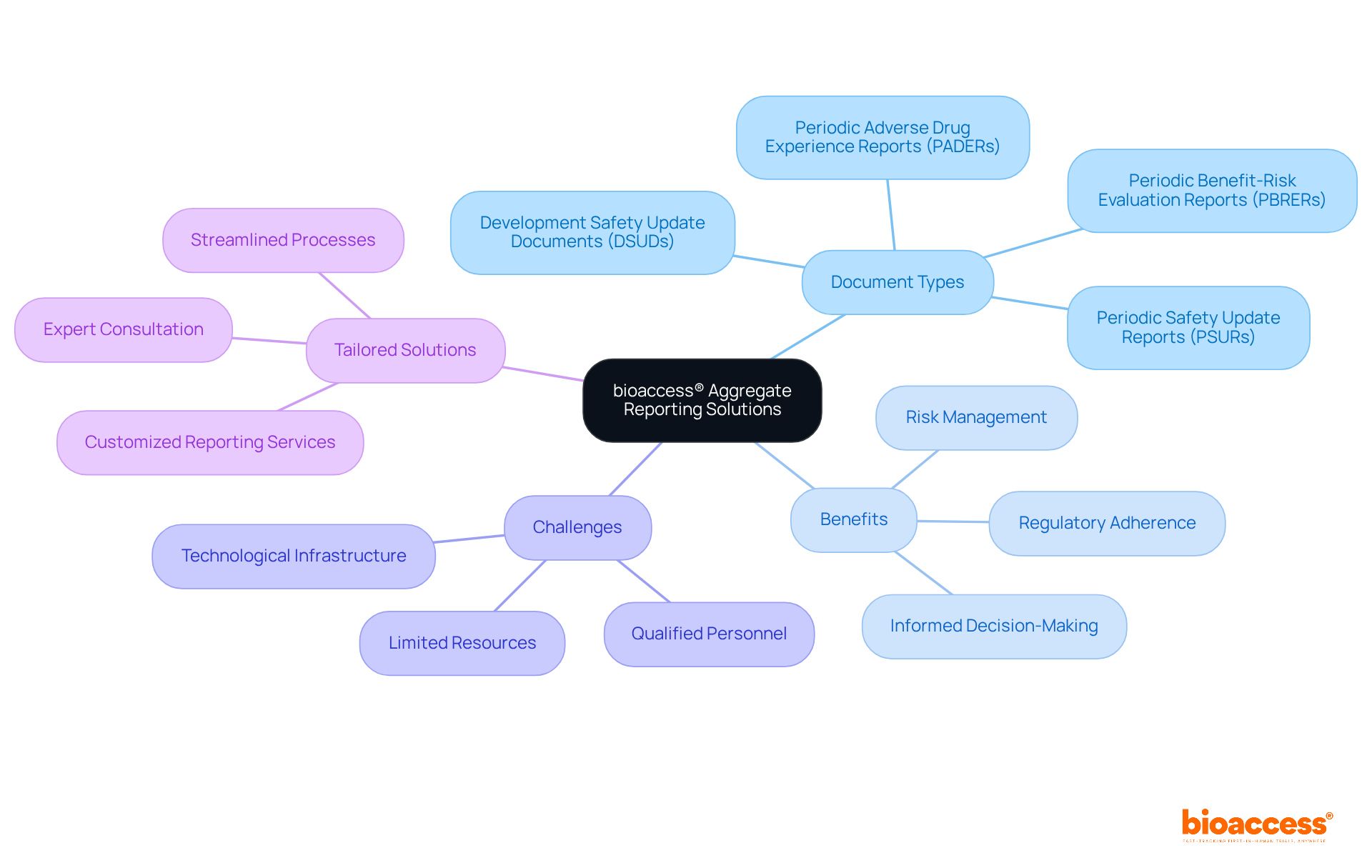
Bioaccess® offers a comprehensive suite of aggregate reports solutions specifically designed for innovators in Medtech, Biopharma, and Radiopharma. These documents are essential for guaranteeing adherence to regulations and protecting the well-being and effectiveness of trials. Aggregate documentation serves as a fundamental element in pharmacovigilance, with essential summaries such as Development Safety Update Documents (DSUDs) and Periodic Safety Update Documents (PSUDs) compiled at specified intervals to gather risk information from diverse sources, including trials and post-marketing monitoring. This systematic approach not only fulfills international regulatory standards but also promotes proactive risk management and strategic decision-making.
With a strong emphasis on quality and accuracy, bioaccess® meticulously compiles each aggregate document, ensuring that stakeholders receive thorough insights essential for informed decision-making during the research process. The significance of these reports is underscored by their examination of adverse events, trends, and recognized concerns, ultimately aiding in the creation of more secure and effective medications. By leveraging their extensive experience and operational knowledge, bioaccess® empowers clients to navigate the complexities of aggregate reports, enhancing patient protection and regulatory adherence in the rapidly evolving landscape of clinical research.
Moreover, it is crucial to acknowledge the challenges associated with collective reporting, such as limited resources, including qualified personnel and technological infrastructure, which can impact the efficiency of data management. This awareness enables bioaccess® to offer tailored solutions that address these challenges while ensuring adherence and compliance. Collaboration in this space is not just beneficial; it is essential for advancing the standards of clinical research and ensuring the safety and efficacy of new therapies.
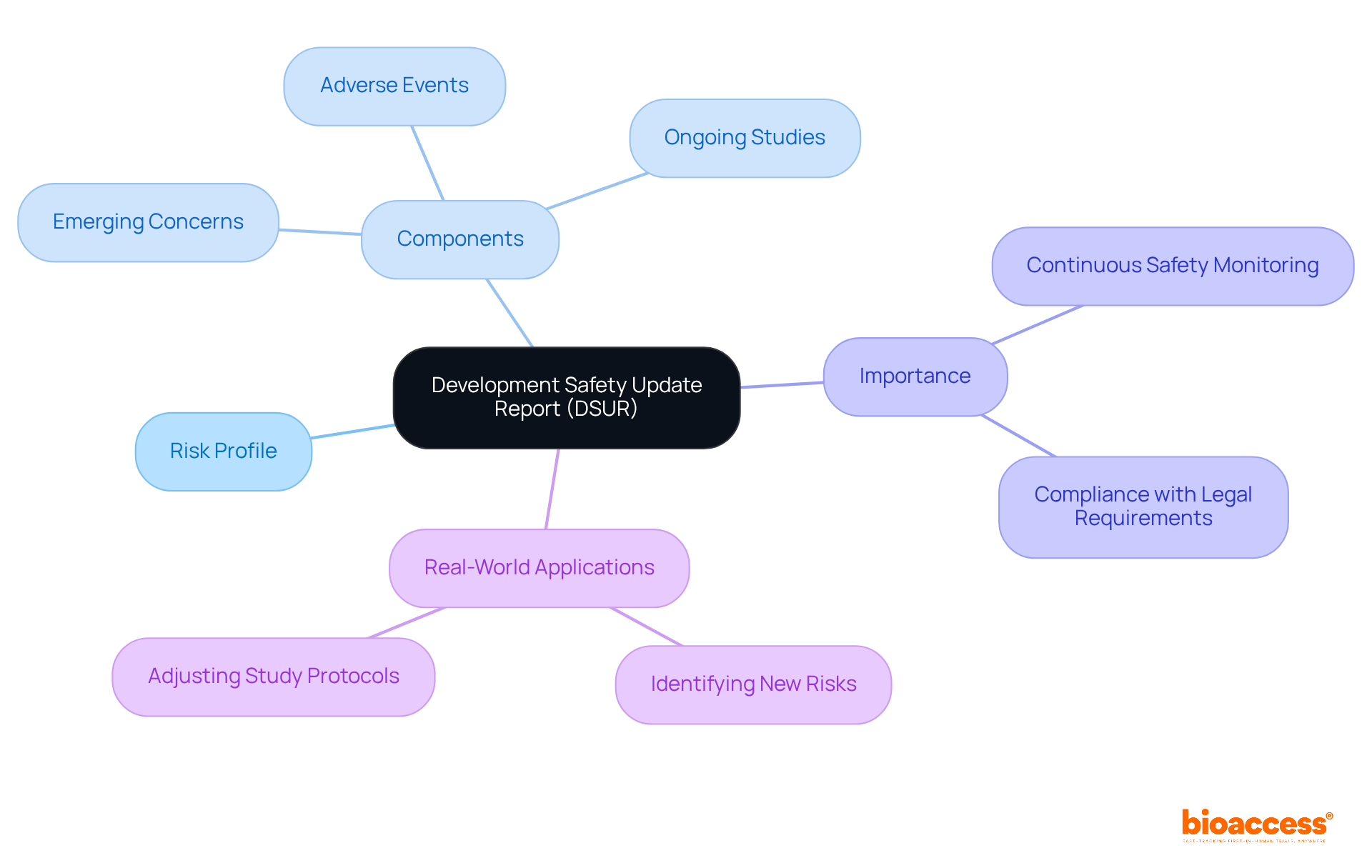
The Development Safety Update Report (DSUR) serves as a vital document in clinical research, offering a thorough overview of the risk profile associated with investigational drugs. Compiled annually, it encompasses information on adverse events, ongoing studies, and emerging concerns regarding participant well-being. This report is indispensable for continuous safety monitoring, as it encapsulates the current understanding and management of identified and potential risks related to the drug. By providing insights into all trials conducted or completed during the review period, including reports on both serious and non-serious adverse events, the DSUR empowers researchers to assess whether new findings align with existing risk knowledge.
Real-world applications of the DSUR underscore its importance in clinical research. For instance, it aids in identifying new risk concerns that may impact the safety of trial participants, facilitating timely adjustments to study protocols. This proactive approach not only enhances participant protection but also ensures compliance with legal requirements. The DSUR is essential for the ongoing evaluation of risk patterns, establishing itself as a crucial resource for research professionals committed to safeguarding participant well-being and advancing medical progress.
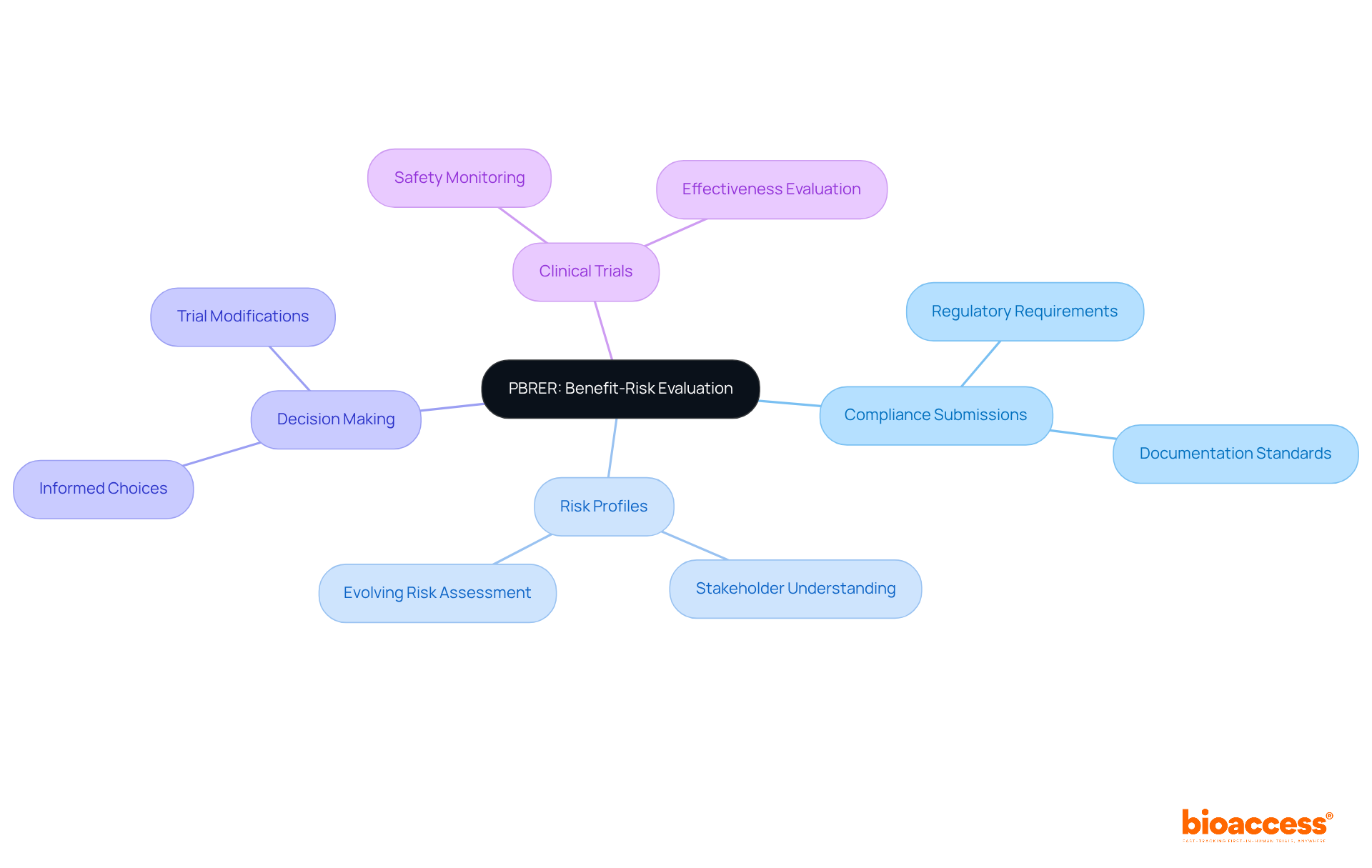
The Periodic Benefit-Risk Evaluation Report (PBRER) serves a pivotal role in providing a comprehensive assessment of the benefits and risks associated with medicinal products throughout their lifecycle. This report is essential for compliance submissions, aiding stakeholders in understanding the evolving risk profiles of drugs. By systematically evaluating the benefit-risk balance, researchers can make informed decisions regarding the continuation or modification of clinical trials, ensuring that clinical research remains both effective and safe.
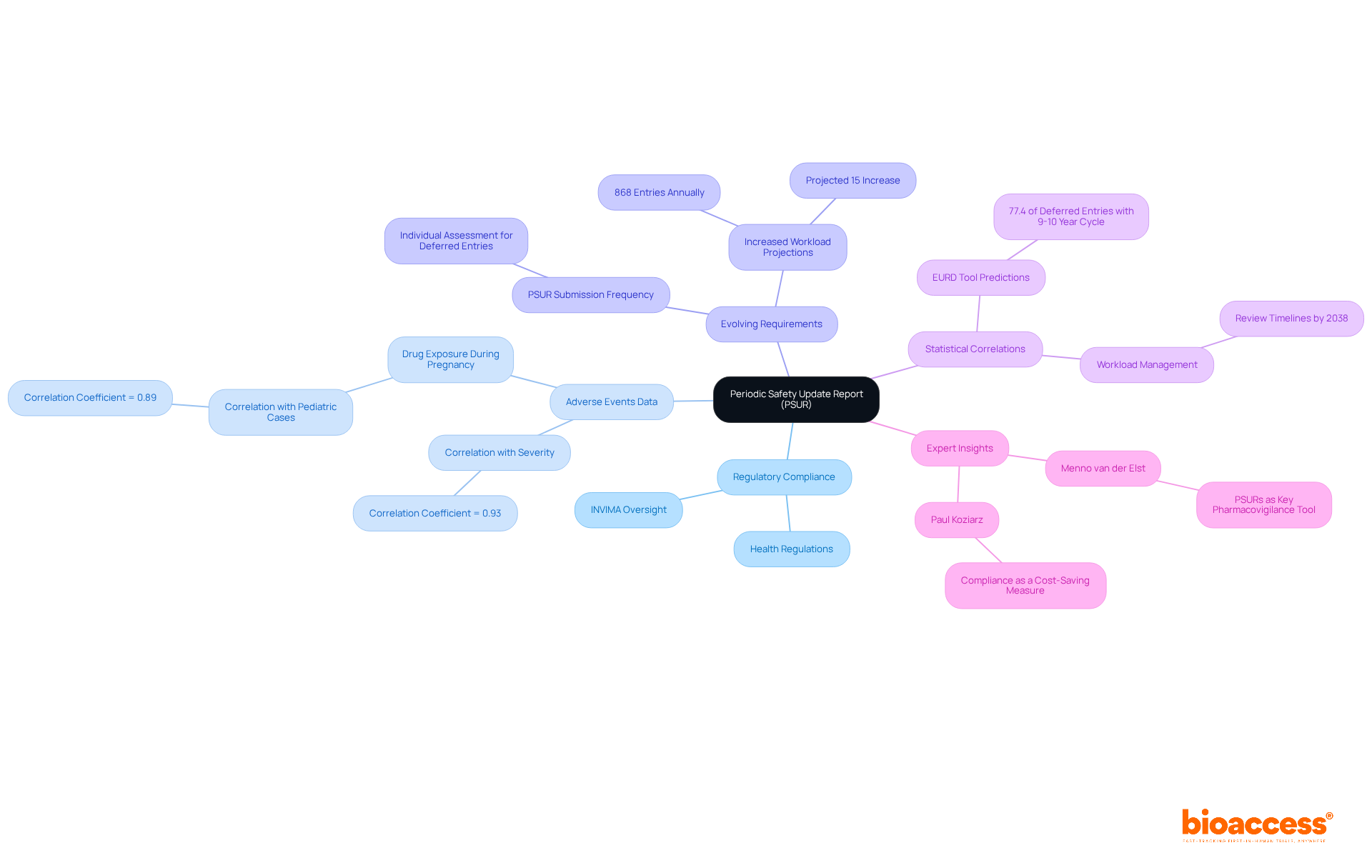
The Periodic Update Report (PSUR) serves as a critical compliance necessity, summarizing the risk profile of a drug at designated intervals. It encompasses comprehensive data on adverse events, risk evaluations, and updates to the product's safety information. As of 2025, the PSUR requirements have evolved, demanding a more rigorous approach to data collection and analysis. Notably, the median workload for PSUR submissions is projected at 868 entries annually, with a potential increase of 15%, enabling the review of all entries by 2038.
Meticulous preparation of PSURs not only ensures adherence to oversight agencies such as INVIMA, the Colombia National Food and Drug Surveillance Institute, but also upholds the integrity of clinical studies. INVIMA plays a vital role in supervising the marketing and production of health products, ensuring compliance with health regulations, and granting medical approval for imports and exports. Recent statistics reveal a strong correlation (correlation coefficient = 0.93) between the number of reported cases and the severity of adverse events, highlighting the importance of timely and accurate PSUR submissions. Real-world instances demonstrate that organizations adhering to PSUR guidelines, particularly those validated by the EURD tool's predictions, have effectively managed compliance submissions, enhancing their credibility and operational efficiency.
The PSUR transcends a mere compliance document; it is an indispensable tool for evaluating the benefit-risk balance of medicinal products post-authorization. As industry expert Menno van der Elst asserts, "Periodic Safety Update Reports (PSURs) are a key pharmacovigilance tool for the continuous evaluation of the benefit-risk balance of a medicinal product in the post-authorisation phase." By prioritizing PSUR compliance, research professionals can significantly bolster the strength of pharmacovigilance systems and the overall safety of medical products, aligning with the standards established by INVIMA and supported by experts like Katherine Ruiz in regulatory affairs for medical devices.
Aggregate reports are essential in clinical research, serving as a vital tool for consolidating data from diverse studies and sources. These reports facilitate a comprehensive evaluation of security and efficacy, enabling researchers to identify trends and make knowledgeable, data-informed choices. For instance, the preparation of Periodic Adverse Drug Experience Reports (PADERs) involves compiling individual case narratives and compliance updates, which together enhance a thorough understanding of a drug's safety profile. This systematic approach not only aids in identifying potential risks but also enhances communication with regulatory authorities and stakeholders.
The significance of data consolidation is underscored by the necessity for prompt submissions; quarterly PADERs must be submitted within 30 days of the data lock point, while annual documents follow within 60 days. Such stringent timelines emphasize the essential function of comprehensive summaries in upholding compliance and guaranteeing patient safety throughout a product's lifecycle. Additionally, the integration of comprehensive trial management services—such as:
ensures that the data gathered is accurate and dependable. This careful administration not only aids in the creation of comprehensive summaries but also enhances the overall success of clinical studies, promoting job creation, economic development, and healthcare advancement in local communities.
Furthermore, the incorporation of cutting-edge technologies, such as Robotic Process Automation and Artificial Intelligence, is transforming how data is handled, optimizing the creation of these documents and minimizing manual mistakes.
In summary, aggregate reports provide a clear view of overall security and enhance proactive risk management, which ultimately aids the regulatory discussion necessary for the successful commercialization of innovative medical products.
Line listings serve as comprehensive tables that detail individual case data concerning adverse events, playing a pivotal role in risk evaluations. These listings empower researchers to conduct meticulous analyses of specific cases, thereby facilitating the identification of patterns and trends that might remain obscured in aggregate reports.
For instance, a study demonstrated that detailed line listings significantly improved the detection of risk signals, leading to more informed decisions regarding study protocols and patient protection measures. By leveraging line listings, clinical researchers enhance their understanding of risk issues, ultimately contributing to more robust clinical trial outcomes and ensuring regulatory compliance through the use of aggregate reports.
As FDA Commissioner Marty Makary pointed out, "Adverse event reporting should be fast, seamless, and transparent," underscoring the essential role that detailed case analysis plays in achieving these objectives. Furthermore, the FDA's initiative to release daily adverse event information from FAERS underscores the importance of line listings in bolstering monitoring and transparency throughout drug development.
The Periodic Adverse Drug Experience Report (PADER) is an essential instrument for monitoring adverse events associated with pharmaceuticals. This comprehensive document details negative drug experiences, enabling researchers to effectively identify potential issues of concern. Regular updates to the PADER are crucial, as they facilitate proactive risk management and ensure patient safety during trials. By systematically tracking adverse events, researchers can improve the overall safety profile of a drug, ultimately leading to enhanced patient outcomes.
The significance of PADER is underscored by its role in fostering transparency and accountability in medical research, reinforcing the commitment to safeguarding patient health. As psychiatrist Michael Rutter aptly states, 'Resilience can be defined as reduced vulnerability to environmental risk experiences, the overcoming of a stress or adversity, or a relatively good outcome despite risk experiences.' This underscores the critical role of PADER in cultivating resilience within research methodologies.
Furthermore, recent statistics indicate that effective monitoring of adverse events can result in a 30% reduction in issues related to well-being during clinical trials, highlighting the imperative nature of PADER in ensuring patient protection. Incorporating case studies, such as the successful application of PADER in a recent biopharmaceutical trial, further illustrates its practical utility and effectiveness in real-world contexts.
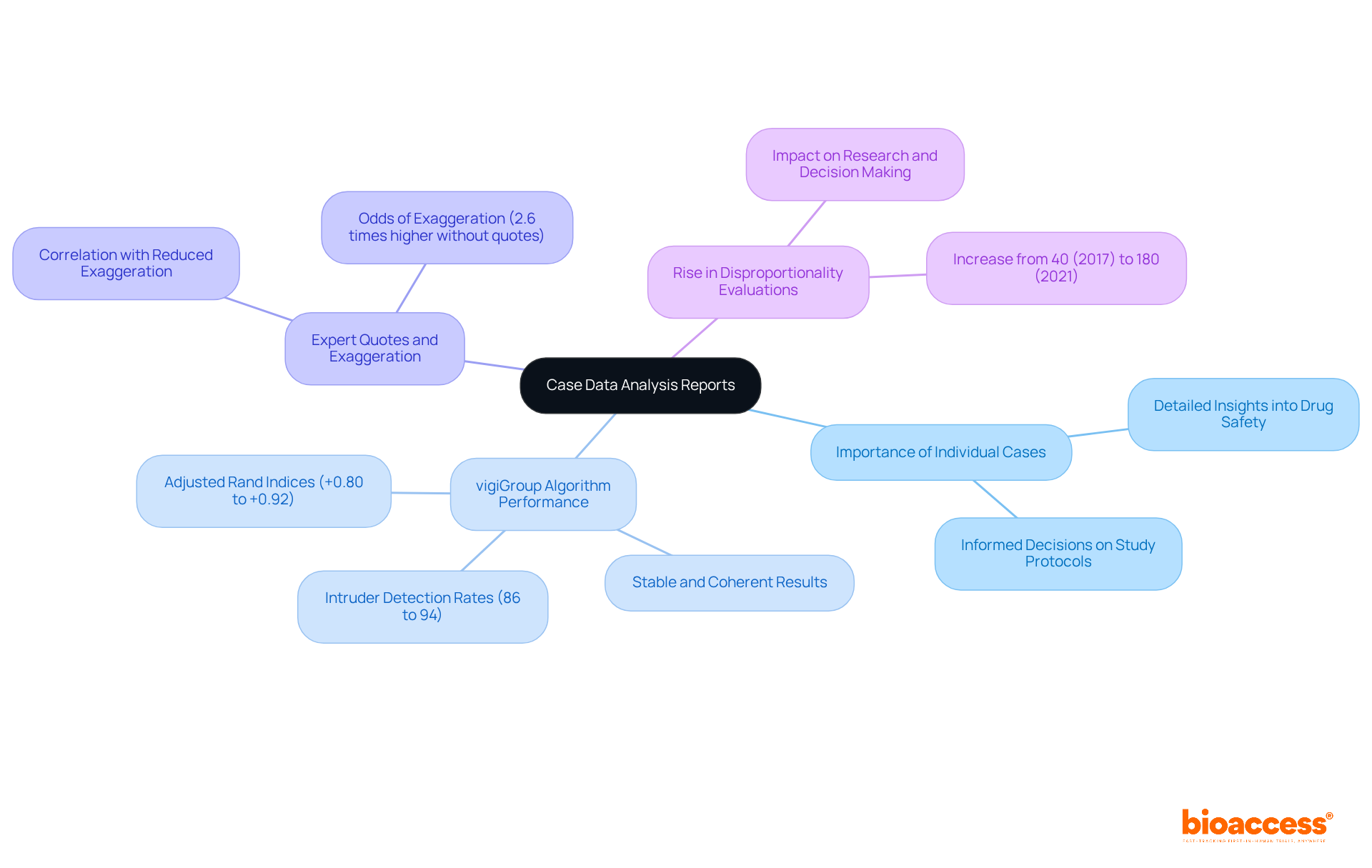
Case data analysis documents are essential in reviewing individual incidents, providing detailed insights into adverse occurrences and their results. These documents are crucial for understanding the intricacies of drug effectiveness and safety on a detailed level. By scrutinizing individual cases, researchers can pinpoint specific risks, enabling informed decisions about study protocols and patient management.
For instance, the vigiGroup algorithm, which utilizes consensus clustering, has demonstrated stable and coherent results in identifying groups of individual case reports related to similar suspected adverse drug reactions, achieving adjusted Rand indices between +0.80 and +0.92. This performance metric enhances the understanding of adverse drug reactions, thereby contributing to better patient protection.
Furthermore, the presence of expert quotes in health news articles has been shown to correlate with reduced exaggeration, with articles lacking external expert quotes being 2.6 times more likely to contain exaggeration. This underscores the significance of reliable sources in accurately communicating information regarding well-being.
Additionally, the quantity of published disproportionality evaluations increased from 40 in 2017 to 180 in 2021, emphasizing the rising importance of thorough monitoring and the role of aggregate reports in the current research environment. This method not only boosts the reliability of results but also promotes a more detailed comprehension of the implications for drug trials, ultimately aiding in better patient protection and informed clinical practices.
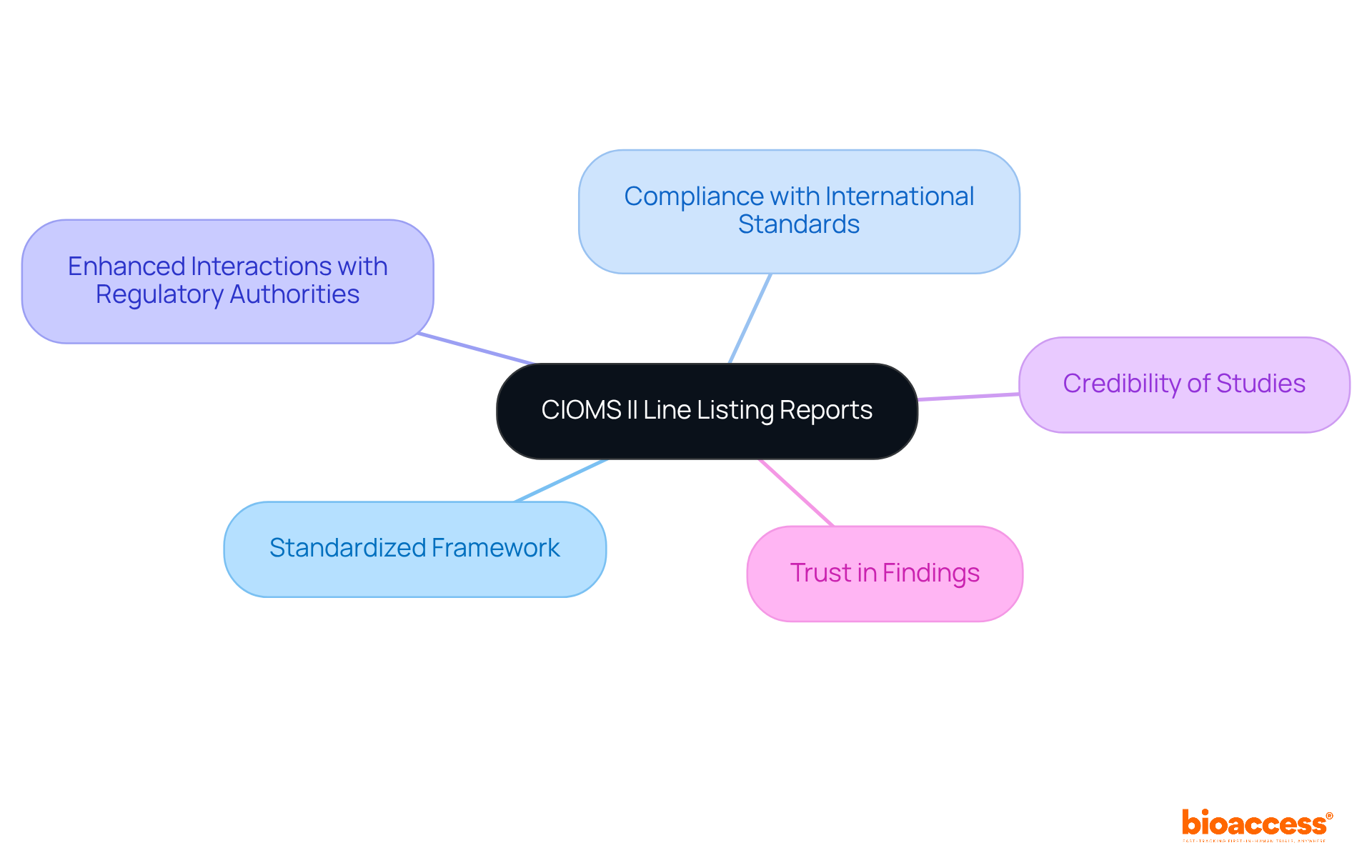
CIOMS II line listing documents serve a critical role in adhering to global safety reporting criteria established by the Council for International Organizations of Medical Sciences (CIOMS). These documents provide a standardized framework for the communication of adverse event information, thereby ensuring compliance with international standards. By following CIOMS II standards, clinical researchers can enhance their interactions with regulatory authorities, bolstering the credibility of their studies and fostering trust in their findings.
Cumulative documents in pharmacovigilance play a crucial role in tracking drug security throughout a product's lifecycle. These documents synthesize information from diverse sources, enabling researchers to identify long-term safety patterns and potential hazards. By consistently updating aggregate reports, clinical researchers can proactively manage safety concerns and ensure compliance with regulatory requirements. This proactive approach not only safeguards patient health but also reinforces the integrity of clinical research processes.
![]()
The importance of aggregate reports in clinical research cannot be overstated; they serve as foundational documents that ensure compliance, enhance patient safety, and facilitate informed decision-making throughout the drug development process. Reports such as Development Safety Update Reports (DSURs), Periodic Benefit-Risk Evaluation Reports (PBRERs), and Periodic Safety Update Reports (PSURs) play a vital role in monitoring the safety and efficacy of medications, ultimately contributing to the successful commercialization of innovative therapies.
Throughout this article, key insights have been presented regarding the various types of aggregate reports and their specific functions. The DSUR provides critical updates on the risk profile of investigational drugs, while the PBRER assesses the benefit-risk balance over time. The PSUR ensures ongoing regulatory compliance by summarizing adverse events and safety evaluations. Additionally, the significance of line listings and case data analysis reports has been highlighted, demonstrating their role in identifying trends and enhancing the understanding of adverse drug reactions.
In light of these discussions, it is essential for stakeholders in clinical research to prioritize the meticulous preparation and submission of aggregate reports. By embracing best practices in aggregate reporting, researchers can not only safeguard patient health but also foster greater transparency and trust with regulatory authorities. As the landscape of clinical research continues to evolve, the commitment to high-quality aggregate reporting will remain a cornerstone of effective pharmacovigilance and patient safety.
What is bioaccess® and what solutions does it offer?
Bioaccess® provides a comprehensive suite of aggregate reporting solutions specifically designed for innovators in Medtech, Biopharma, and Radiopharma, essential for regulatory adherence and ensuring the safety and effectiveness of clinical trials.
What is the purpose of aggregate documentation in clinical research?
Aggregate documentation is fundamental in pharmacovigilance, compiling essential summaries like Development Safety Update Documents (DSUDs) and Periodic Safety Update Documents (PSUDs) to gather risk information from various sources, fulfilling international regulatory standards and promoting proactive risk management.
How does bioaccess® ensure the quality and accuracy of their reports?
Bioaccess® meticulously compiles each aggregate document to provide stakeholders with thorough insights necessary for informed decision-making during the research process, focusing on the examination of adverse events, trends, and recognized concerns.
What challenges are associated with collective reporting in clinical research?
Challenges include limited resources such as qualified personnel and technological infrastructure, which can affect data management efficiency. Bioaccess® addresses these challenges by offering tailored solutions that ensure adherence and compliance.
What is the Development Safety Update Report (DSUR) and its significance?
The DSUR is a vital document in clinical research that provides an overview of the risk profile of investigational drugs, compiled annually to monitor adverse events, ongoing studies, and emerging concerns, thereby facilitating continuous safety monitoring.
How does the DSUR contribute to participant safety in clinical trials?
The DSUR identifies new risk concerns that may affect trial participants, enabling timely adjustments to study protocols, enhancing participant protection, and ensuring compliance with legal requirements.
What is the Periodic Benefit-Risk Evaluation Report (PBRER)?
The PBRER is a comprehensive assessment report that evaluates the benefits and risks of medicinal products throughout their lifecycle, essential for compliance submissions and understanding the evolving risk profiles of drugs.
Why is the PBRER important for clinical research?
The PBRER aids researchers in making informed decisions regarding the continuation or modification of clinical trials by systematically evaluating the benefit-risk balance, ensuring that clinical research remains effective and safe.