


Navigating the complex landscape of biopharma document retention in Serbia poses both a challenge and an opportunity for companies aiming for compliance in clinical research. Understanding local regulations and implementing effective document management practices is not just essential; it’s a cornerstone for maintaining high standards while minimizing the risk of penalties.
How can biopharma organizations ensure they meet regulatory requirements while also enhancing their operational efficiency in this ever-evolving industry? This article delves into ten essential rules designed to empower companies to streamline their documentation processes and safeguard their research initiatives.
bioaccess® leverages its deep understanding of Serbian regulations to streamline the biopharma document retention rules in Serbia for companies. This expertise is crucial in the clinical research landscape, where compliance is paramount. By offering customized solutions that adhere to local regulations, bioaccess® ensures that clients can maintain high standards while focusing on their primary research activities.
The company provides essential guidance on the specific records required and the timelines for retention. This proactive approach not only reduces the risk of non-compliance but also enhances operational efficiency. Furthermore, with over 50 pre-qualified sites launched in under eight weeks, bioaccess® facilitates swift site activation, ensuring that all processes comply with FDA, EMA, and MDR regulations. This commitment to regulatory adherence promotes effective clinical trials in Serbia and beyond, while addressing key challenges in the Medtech landscape through the biopharma document retention rules in Serbia.
In summary, bioaccess® stands as a vital partner for biopharma companies navigating the complexities of clinical research. Their expertise in local regulations and efficient processes empowers clients to concentrate on what truly matters: advancing their research initiatives.
In Serbia, the biopharma document retention rules in Serbia are crucial for clinical research and are primarily governed by the Law on Archival Material and Archival Activities. This law establishes specific retention periods for various document types, ensuring that organizations comply with regulatory expectations. Additionally, the Law on Medicines and Medical Devices outlines essential requirements for clinical trial records, making it imperative for biopharma companies to understand the biopharma document retention rules in Serbia.
Navigating these complexities is essential for reducing the risk of penalties. Companies must be well-versed in the retention timelines and the categories of documents that require preservation. This understanding not only aids in compliance but also enhances operational efficiency in managing clinical trial records.
bioaccess® stands ready to assist Medtech, Biopharma, and Radiopharma startups in effectively navigating these regulations. With expertise in feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, bioaccess empowers clients to accelerate their clinical trials. By ensuring adherence to local laws, they facilitate swift regulatory approval, allowing companies to focus on their core mission.

To maintain the integrity of records, biopharma companies must adopt standardized operating procedures (SOPs) that comply with the biopharma document retention rules in Serbia. Key practices include:
Establishing a centralized document management system is crucial for optimizing record-keeping processes, enabling the monitoring of changes, and ensuring that all necessary files are easily accessible during audits.
Statistics reveal that organizations with robust SOPs experience a significant reduction in regulatory issues, with insufficient SOPs frequently cited during inspections. According to the Biopharma Institute, companies that implement effective SOPs can cut testing expenses by over 30% and enhance product safety, resulting in a 20% boost in customer satisfaction. By prioritizing these practices, pharmaceutical companies can refine their record-keeping processes and ensure compliance with the biopharma document retention rules in Serbia.
As industry expert Murali Menon states, 'The purpose of an SOP is to ensure that personnel performs operations correctly and consistently to achieve a quality outcome through uniform performance.' Furthermore, training programs on SOPs in the GxP regulated sector are available to assist organizations in improving their record-keeping practices.
To safeguard sensitive documentation, biopharma firms must implement robust information security measures. This is not just a regulatory obligation; it’s a strategic imperative for sustaining competitive edge and public confidence. With 83% of organizations lacking fundamental technical protections and user account breaches impacting 74% of organizations operating in the cloud, the urgency for action is clear.
Implementing encryption for electronic records is crucial for protecting information against unauthorized access. Equally important are strict access controls, which limit who can access or alter sensitive documents, thereby reducing the risk of breaches. Regular security audits should be conducted to identify and address vulnerabilities, ensuring that security protocols remain effective against evolving threats.
Moreover, educating personnel on information security protocols is vital. This fosters a culture of adherence and awareness, highlighting the critical importance of protecting sensitive information. Establishing information governance policies and following the ALCOA framework are essential for ensuring compliance with biopharma document retention rules in Serbia as part of Good Documentation Practices in the pharmaceutical industry.
In conclusion, the landscape of clinical research demands that organizations prioritize information security. By taking decisive action now, biopharma firms can not only protect their sensitive data but also enhance their reputation and trustworthiness in the industry.
Implementing electronic documentation systems significantly boosts record-keeping efficiency while adhering to biopharma document retention rules in Serbia. These systems simplify information entry, retrieval, and sharing among team members, making collaboration seamless. Additionally, electronic records can be tailored to automatically meet regulatory requirements, such as audit trails and version control, which are vital for maintaining data integrity and comply with biopharma document retention rules in Serbia.
Companies should seriously consider investing in validated electronic document management systems (EDMS) that adhere to industry standards and regulatory expectations. Notably, paperless validation can lead to a remarkable 50% increase in validation efficiency, while digital validation can cut audit preparation time by up to 90%. Expert insights underscore that these systems are essential for upholding high standards of records, ultimately resulting in quicker, safer, and more efficient product development in the pharmaceutical industry.
To implement these systems effectively, organizations must prioritize training and ensure integration with existing workflows. How prepared is your organization to embrace this shift? The time to act is now.
Instructing personnel on appropriate record-keeping practices is essential for cultivating a strong adherence culture within biopharma companies. Regular training sessions should cover critical topics such as:
Research shows that organizations with a robust adherence culture face fewer regulatory issues and enjoy enhanced operational efficiency. Notably, 72% of pharmaceutical executives report that shortages in digital and analytics skills hinder innovation and adherence, underscoring the necessity for comprehensive training programs.
To reinforce this training, providing resources like manuals and quick reference guides is vital. These tools equip employees with the necessary means to maintain accurate and compliant records. As Devin Mack, a Life Science Consultant, emphasizes, understanding customer needs and establishing proper requirements are crucial for success. This proactive approach not only enhances adherence but also fosters a sense of responsibility among staff, ultimately contributing to the organization's overall success.
Frequent evaluations of record-keeping practices are crucial for ensuring compliance with the biopharma document retention rules in Serbia. These audits should primarily assess the accuracy and completeness of records, adherence to Standard Operating Procedures (SOPs), and the effectiveness of data security measures. By systematically identifying areas for improvement, companies can address regulatory gaps and enhance their practices to comply with biopharma document retention rules in Serbia proactively.
Moreover, meticulous documentation of audit findings is essential. These insights not only inform future training initiatives but also drive continuous process improvements. Statistics reveal that companies with robust audit practices face 30% fewer regulatory issues, underscoring the importance of integrating regular audits into their operational framework. As Laura Dona, an associate at Arnall Golden Gregory, aptly states, "A culture of adherence is essential for pharmaceutical companies to thrive in a regulated environment." This highlights the pivotal role audits play in fostering such a culture.
Creating clear written guidelines for record-keeping practices is essential for ensuring consistency and adherence to biopharma document retention rules in Serbia within pharmaceutical companies. These instructions must detail specific requirements for record-keeping, including formats, retention periods, and security measures, as outlined by the biopharma document retention rules in Serbia. Accessibility is paramount; all staff members should easily access these guidelines, which must be regularly reviewed and updated to align with current regulations and industry best practices.
Insufficient records, especially regarding biopharma document retention rules in Serbia, are a primary reason for regulatory failures, with data indicating that 60% of these failures stem from inadequate record-keeping practices. By establishing clear guidelines, pharmaceutical companies can significantly enhance their adherence efforts, minimize operational disruptions, and foster a culture of accountability. Moreover, organizations are encouraged to adopt a proactive strategy, incorporating digital tools to streamline record-keeping processes and reduce human error. This approach ultimately ensures that all essential information is readily accessible for audits and regulatory reviews.
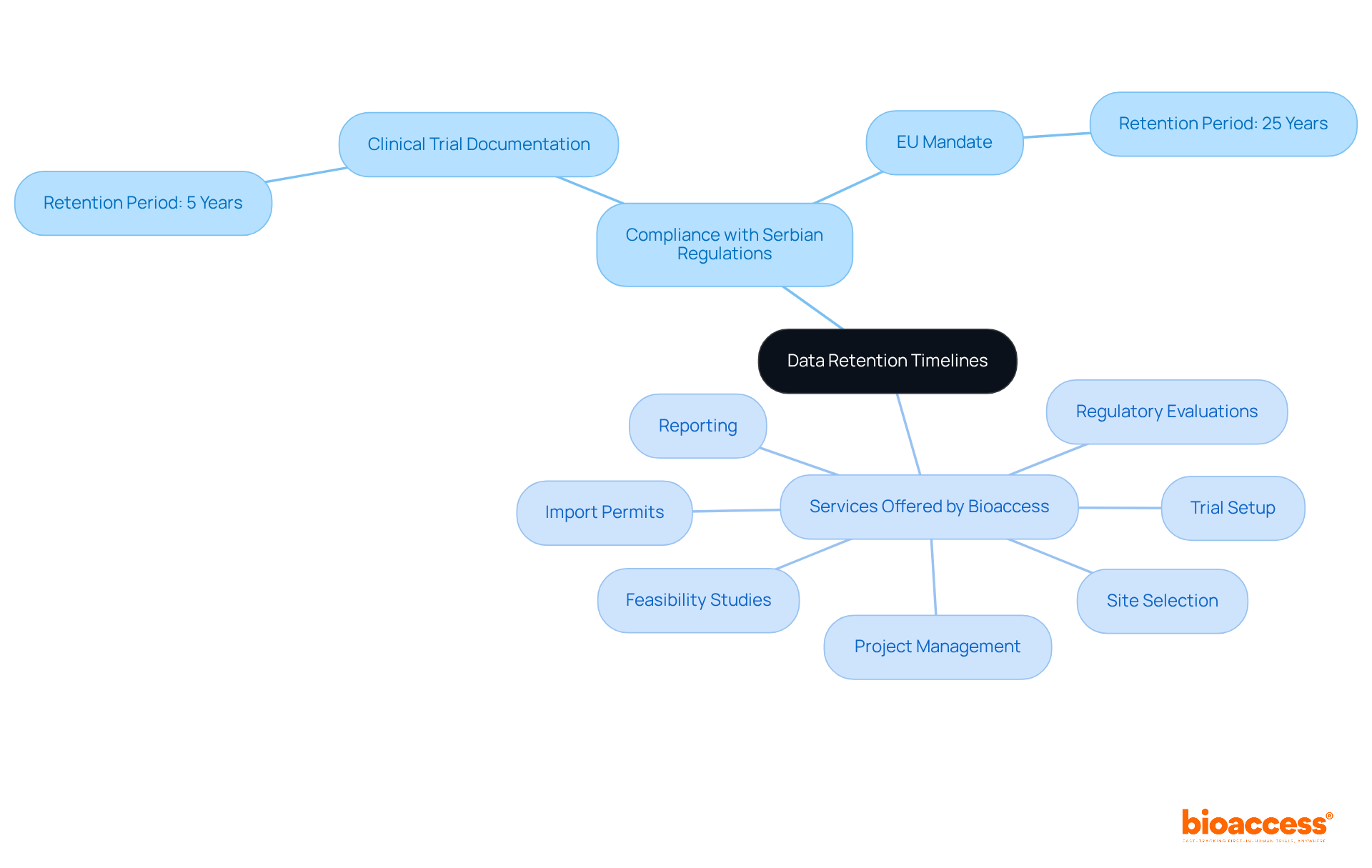
Establishing precise information retention schedules is vital for compliance with the biopharma document retention rules in Serbia. Companies like Bioaccess must craft comprehensive policies that delineate retention periods for various document types, ensuring alignment with biopharma document retention rules in Serbia and local laws. For example, clinical trial documentation typically needs to be retained for a minimum of five years after the study's completion. This compliance not only prepares organizations for audits and inspections but also significantly mitigates the risk of non-compliance.
Moreover, with the European Union's recent mandate requiring clinical trial data to be stored for 25 years, it is essential for Serbian biopharma companies to stay updated on the biopharma document retention rules in Serbia and adapt their retention strategies accordingly. As David Vulcano, Vice President of Research Compliance & Integrity at HCA Healthcare, remarked, "The 25-year record retention requirement for investigators is probably the most significant." By instituting robust document retention policies, organizations can ensure they meet regulatory expectations and uphold the integrity of their clinical research initiatives.
Additionally, Bioaccess offers extensive clinical trial management services, including:
These services are crucial for navigating the complexities of regulatory compliance. Understanding the trend of outsourcing research and development to regions like Chindia provides important context for the competitive landscape, underscoring the significance of adherence in a globalized market.
Implementing information deletion policies is crucial for effectively managing the lifecycle of biopharma documentation while adhering to the biopharma document retention rules in Serbia. These policies must outline the procedures for securely removing documents that are no longer necessary, ensuring compliance with protection regulations. Companies need to establish clear guidelines detailing when and how information should be deleted, including the importance of maintaining records of deletion activities for audit purposes.
By managing data deletion effectively, organizations can significantly reduce the risk of retaining unnecessary information. This not only helps in ensuring compliance with legal requirements but also enhances operational efficiency. Have you considered how your current practices align with these essential policies?
In the ever-evolving landscape of clinical research, the importance of collaboration cannot be overstated. Establishing robust information deletion policies is a vital step toward fostering a culture of compliance and accountability in accordance with the biopharma document retention rules in Serbia.

The significance of adhering to biopharma document retention rules in Serbia is paramount. Organizations within the biopharma sector must prioritize compliance with local regulations to uphold the integrity of their clinical research and avert potential penalties. By grasping and implementing these rules, companies can streamline their operations and concentrate on advancing their research initiatives.
This article has highlighted key strategies, including:
Each of these practices is crucial in fostering a culture of compliance, ultimately enhancing operational efficiency and safeguarding sensitive information.
As the biopharma landscape evolves, companies must remain vigilant in their adherence to document retention regulations. By investing in training, establishing clear guidelines, and actively managing data retention and deletion policies, organizations can mitigate risks and position themselves for success in a competitive market. Embracing these practices is essential for ensuring that biopharma companies thrive while maintaining the highest standards of compliance and integrity in their documentation processes.
What is bioaccess® and how does it assist biopharma companies in Serbia?
bioaccess® is a company that streamlines document retention compliance for biopharma companies in Serbia by leveraging its understanding of local regulations. It provides customized solutions to ensure compliance with document retention rules, allowing clients to focus on their primary research activities.
What specific regulations govern document retention in Serbia for biopharma companies?
Document retention in Serbia for biopharma companies is primarily governed by the Law on Archival Material and Archival Activities, which establishes specific retention periods for various document types. Additionally, the Law on Medicines and Medical Devices outlines requirements for clinical trial records.
How does bioaccess® improve operational efficiency for biopharma companies?
bioaccess® enhances operational efficiency by providing guidance on required records and retention timelines, reducing the risk of non-compliance. Their proactive approach allows companies to streamline their processes and focus on advancing their research initiatives.
What practices should biopharma companies adopt to maintain accurate records?
Biopharma companies should adopt standardized operating procedures (SOPs) that include using indelible ink for handwritten entries, ensuring records are legible and comprehensive, and conducting regular reviews for accuracy. Establishing a centralized document management system is also crucial for optimizing record-keeping.
What benefits do companies experience by implementing effective SOPs?
Companies with robust SOPs experience a significant reduction in regulatory issues and can cut testing expenses by over 30%. They also enhance product safety, leading to a 20% boost in customer satisfaction.
What role does bioaccess® play in facilitating clinical trials in Serbia?
bioaccess® supports Medtech, Biopharma, and Radiopharma startups by assisting with feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring adherence to local laws and facilitating swift regulatory approval.
How can training programs on SOPs benefit organizations in the biopharma sector?
Training programs on SOPs help organizations improve their record-keeping practices by ensuring personnel perform operations correctly and consistently, which is essential for achieving quality outcomes in the GxP regulated sector.