


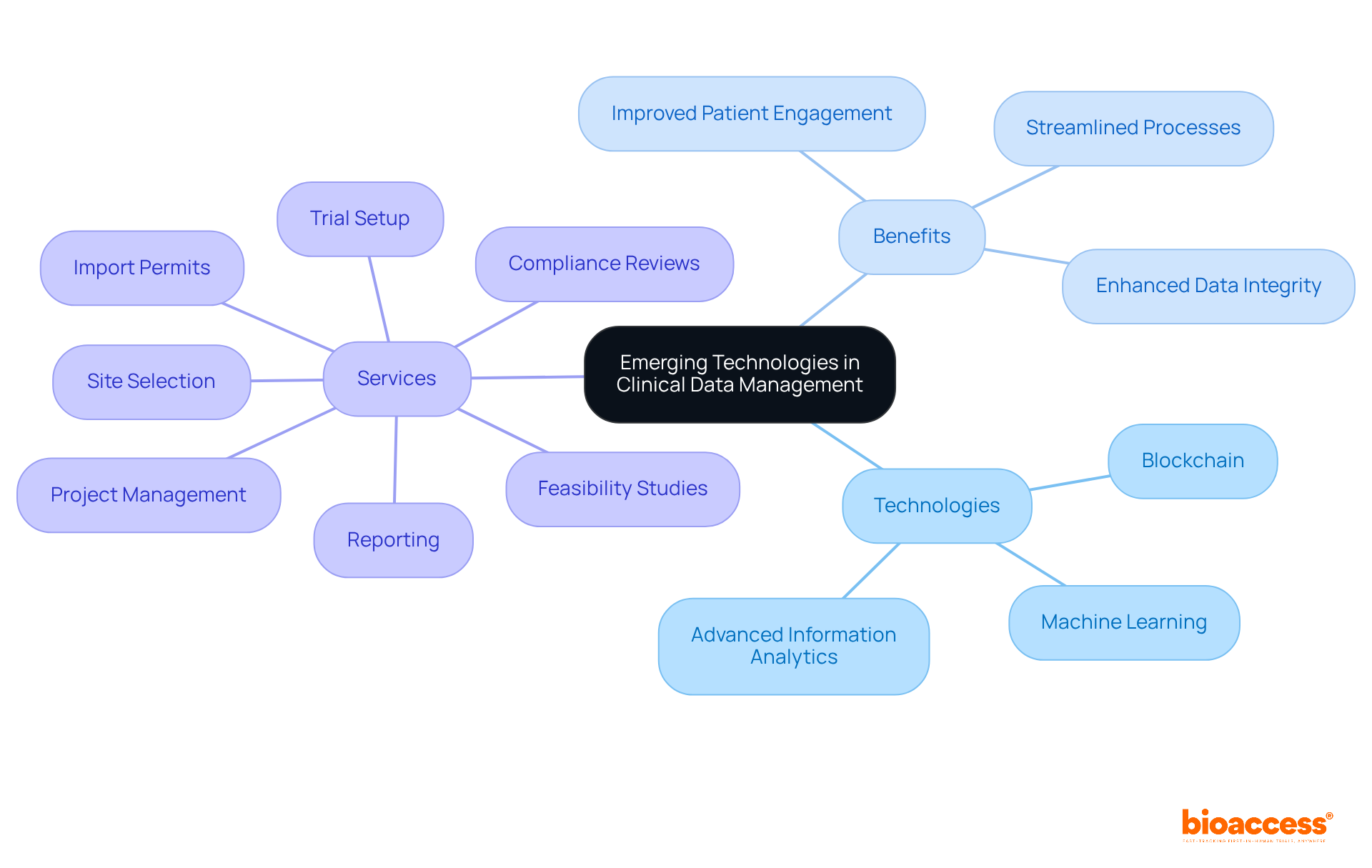
The article titled "10 Essential Clinical Data Management Services for Research Directors" identifies pivotal services that enhance the efficiency and effectiveness of clinical data management for research directors. It underscores essential services such as:
These services collectively streamline the research process and bolster data integrity, ultimately facilitating faster and more cost-effective clinical trials.
In the realm of clinical research, the efficiency and accuracy of data management can decisively influence a study's success. As research directors encounter mounting pressure to deliver results swiftly and cost-effectively, it becomes essential to comprehend the services that streamline clinical data management.
What innovative strategies can be employed to navigate the complexities of modern clinical trials? How can these services enhance patient engagement and data integrity?
This article delves into ten vital clinical data management services that empower research directors to optimize their studies within an ever-evolving landscape.
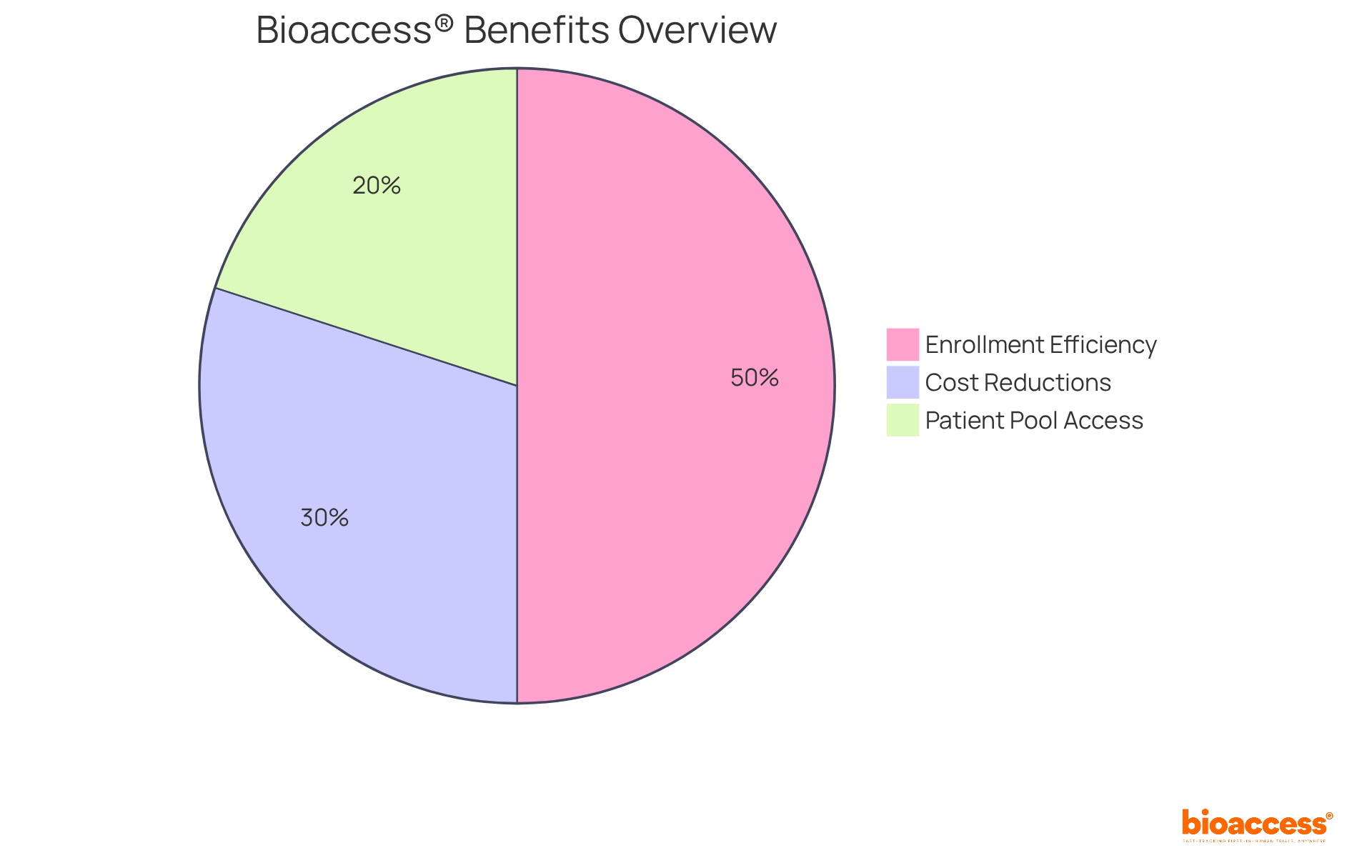
bioaccess® delivers customized healthcare information services that leverage Colombia's competitive advantages, showcasing significant cost reductions exceeding 30% compared to North America and Western Europe. Their regulatory review process ensures ethical approvals in a swift 90-120 days. With access to a diverse patient pool of over 50 million and universal healthcare coverage for 95% of the population, bioaccess® enhances enrollment efficiency by 50%, establishing itself as a preferred partner for Medtech innovators. By focusing on early-phase studies, bioaccess® aids clients in navigating complex regulatory landscapes while maintaining high-quality information integrity throughout the research process.
bioaccess® provides clinical-data-management services as part of a comprehensive suite of healthcare data solutions that seamlessly integrate data gathering, organization, and analysis, enabling research directors to significantly accelerate their studies.
With the ability to enroll treatment-naive cardiology or neurology groups 50% faster than locations in the West, bioaccess® not only streamlines the research process but also provides substantial cost savings of $25K per patient with FDA-ready data—eliminating rework and delays.
Their platform offers real-time insights and analytics, empowering research directors to make informed decisions swiftly.
bioaccess® ensures efficient and effective studies by addressing patient recruitment and compliance challenges through a variety of clinical-data-management services, such as:
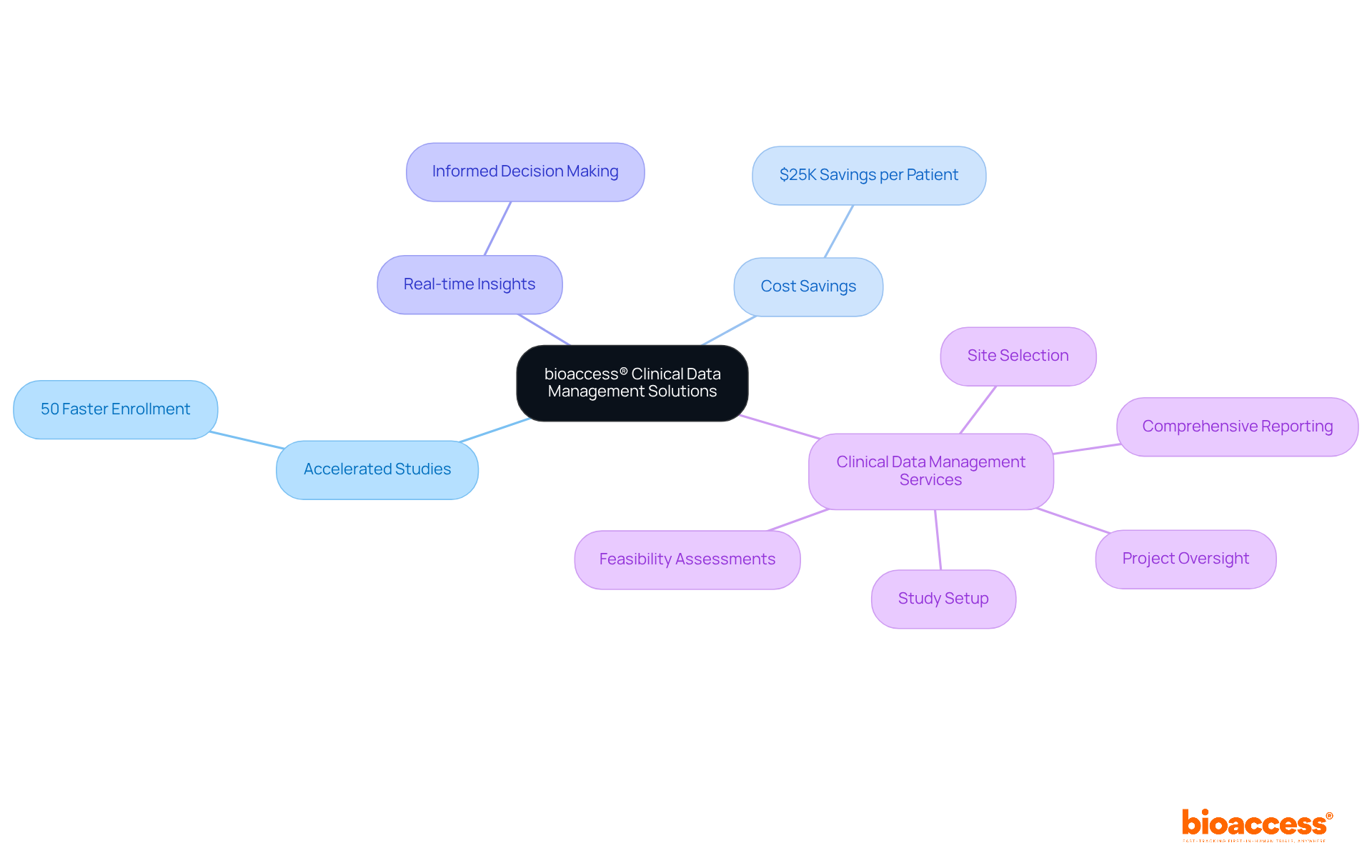
Bioaccess specializes in comprehensive oversight of clinical-data-management services, ensuring that every aspect of research information handling is addressed from study design to final reporting. Their extensive CRO research services in Colombia encompass:
This includes:
By offering seamless integration of services, including clinical-data-management services, project coordination, study oversight, and adherence to regulatory standards, Bioaccess assists research directors in preserving information integrity and expediting site activation throughout the study lifecycle.
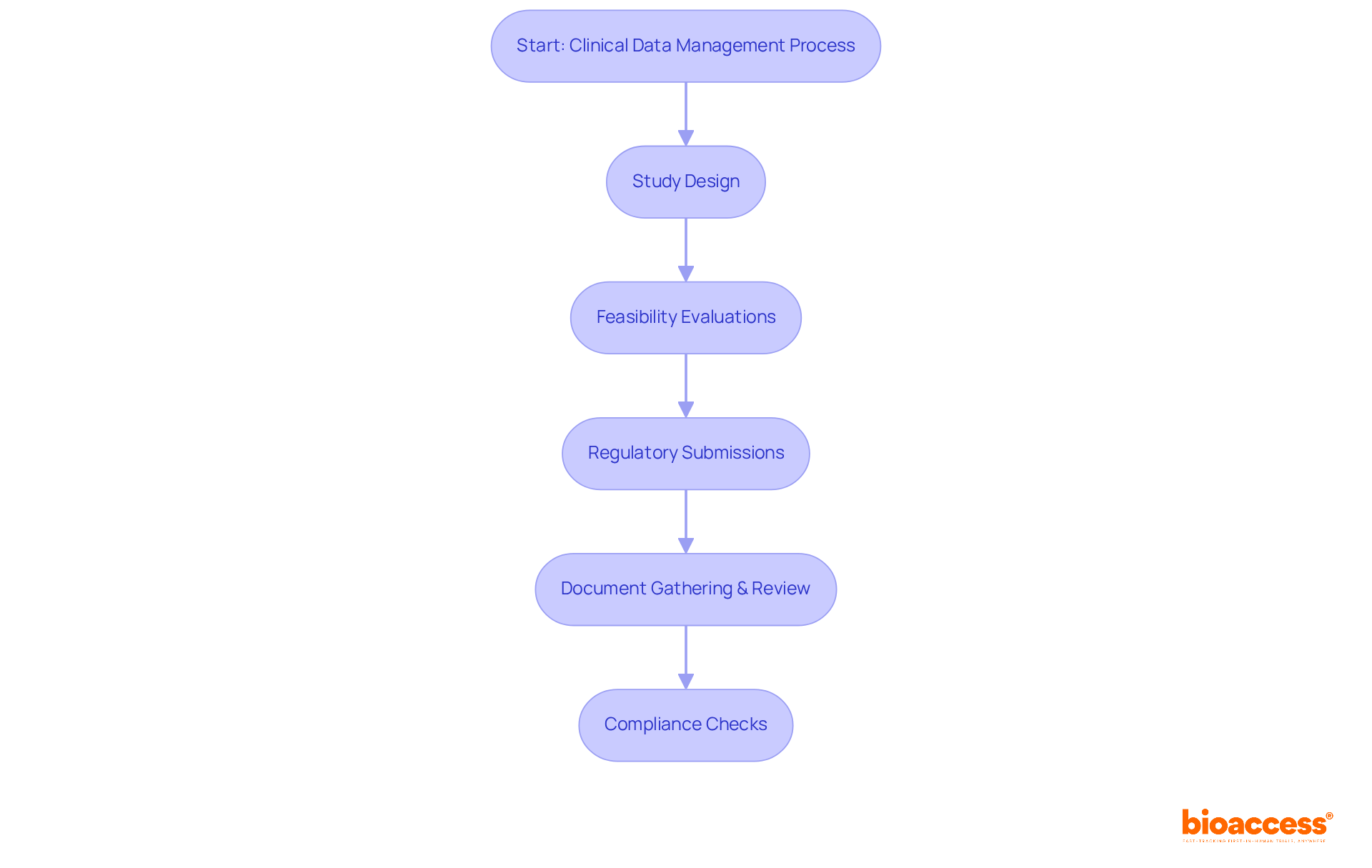
bioaccess® focuses on delivering customized clinical-data-management services tailored to the distinct needs of each clinical study. Their offerings encompass innovative, bespoke information-gathering techniques that significantly enhance efficiency by ensuring that the information is both pertinent and actionable.
For instance, the use of electronic patient-reported outcomes (ePRO) and mobile health applications facilitates real-time information collection directly from participants, thereby boosting engagement and precision. With bioaccess®, studies can achieve patient enrollment 50% faster than traditional methods, leading to substantial cost savings of $25K per patient through FDA-ready data—eliminating rework and delays.
Real-time monitoring capabilities further streamline the process, allowing for timely adjustments based on emerging information trends. As we approach 2025, the emphasis on adaptive data management approaches has become increasingly vital, as organizations strive to navigate the complexities of medical studies effectively. This encompasses addressing the growing intricacies of experimental designs and the need for advanced planning and operational flexibility.
Insights from research directors underscore the importance of these adaptive strategies, which not only enhance operational agility but also promote better patient engagement and inclusion, especially among underrepresented populations. By aligning their clinical-data-management services with the specific objectives of research directors, bioaccess® plays a pivotal role in enhancing the overall success of research studies.
bioaccess provides specialized services for handling medical information, aimed at improving accuracy and optimizing procedures in research. Our comprehensive capabilities include:
By utilizing bioaccess's extensive expertise, research directors can ensure that their clinical trial management processes are efficient, compliant, and capable of delivering high-quality results. This allows them to focus on innovation and regulatory excellence in Latin America, addressing key challenges in the Medtech landscape. Collaboration with bioaccess not only enhances operational efficiency but also fosters an environment of trust and reliability in clinical-data-management services.
Firmaclinicalresearch provides a comprehensive beginner's guide to research information supervision, covering essential concepts such as information gathering, validation, and reporting. This resource is crucial for new research directors seeking to understand the fundamentals of healthcare data oversight. By mastering these foundational components, they can navigate the complexities of research studies more effectively and make informed decisions throughout the investigation process.
Additionally, bioaccess offers extensive clinical-data-management services, which include:
These services ensure that research leaders are thoroughly equipped to oversee studies efficiently, adhere to regulatory standards, and enhance study outcomes.
Lindus Health underscores the critical importance of information protection and confidentiality in research studies. Their services are characterized by robust information protection protocols, strict adherence to regulatory standards, and comprehensive risk mitigation strategies. By prioritizing information protection, Lindus Health empowers research leaders to safeguard confidential details and uphold the integrity of their studies. This commitment not only enhances the reliability of research outcomes but also fosters trust among stakeholders, making Lindus Health an invaluable partner in the clinical research landscape through its clinical-data-management services.
bioaccess offers a suite of innovative solutions designed to enhance oversight of medical information in research studies. Among the key features are:
By harnessing these capabilities, clinical trials can achieve enhanced efficiency and accuracy in data handling. This ultimately drives global health improvement through international collaboration and innovation in Medtech, emphasizing the importance of working together to address key challenges in the clinical research landscape.

In the evolving landscape of clinical information handling, bioaccess® stands at the forefront, delivering expedited clinical research services alongside clinical-data-management services tailored for Medtech, Biopharma, and Radiopharma startups. The transformation is driven by key trends such as the increasing integration of artificial intelligence and data analytics, which enhance efficiency and foster patient-centric methodologies.
By leveraging bioaccess®'s expertise in:
research directors can adeptly navigate these emerging trends. Furthermore, bioaccess® connects startups with premier research facilities across Latin America, Eastern Europe, and Australia, ensuring quicker study results and improved patient outcomes in a competitive environment.
Emerging technologies such as blockchain, machine learning, and advanced information analytics are revolutionizing healthcare information oversight. These innovations not only enhance data integrity but also streamline processes and improve patient engagement.
With bioaccess®, research directors can achieve patient enrollment 50% faster than traditional Western sites, translating to significant cost savings of $25K per patient through FDA-ready data—effectively eliminating rework and delays.
By embracing these technologies and leveraging clinical-data-management services—such as:
research directors can significantly enhance the efficiency and effectiveness of their clinical trials. This paves the way for future advancements in medical research, underscoring the critical importance of collaboration and proactive engagement in the evolving landscape of clinical research.
The landscape of clinical data management is evolving rapidly, emphasizing tailored services that enhance efficiency and accuracy for research directors. By leveraging innovative solutions and specialized expertise, organizations like bioaccess® are redefining the standards of clinical data management, ensuring that studies are conducted with integrity and speed. This commitment to excellence is crucial in navigating the complexities of modern medical research.
Key insights from the article highlight the importance of comprehensive clinical data management services. From feasibility assessments and site selection to compliance reviews and project oversight, these services facilitate smoother study processes and lead to significant cost savings and improved patient engagement. The integration of emerging technologies further amplifies these benefits, paving the way for more effective and patient-centric research methodologies.
As the clinical research landscape continues to transform, embracing these advanced data management strategies is essential. Research directors should prioritize collaboration with experienced partners to enhance their studies' success and drive innovation in the Medtech field. By doing so, they will not only meet today's challenges but also position themselves for future advancements in healthcare research.
What services does bioaccess® provide for Medtech innovators?
bioaccess® offers customized healthcare information services, including clinical data management, regulatory review, and oversight of clinical studies, helping Medtech innovators navigate complex regulatory landscapes.
How does bioaccess® ensure cost efficiency in clinical data management?
bioaccess® leverages Colombia's competitive advantages, providing cost reductions exceeding 30% compared to North America and Western Europe, along with substantial savings of $25K per patient.
What is the typical timeline for regulatory approvals with bioaccess®?
bioaccess® ensures ethical approvals are obtained within a swift timeframe of 90-120 days.
How does bioaccess® enhance patient enrollment for clinical studies?
With access to a diverse patient pool of over 50 million and universal healthcare coverage for 95% of the population, bioaccess® improves enrollment efficiency by 50%.
What are some specific clinical data management services offered by bioaccess®?
bioaccess® provides feasibility assessments, site selection, study setup, project oversight, and comprehensive reporting as part of their clinical data management services.
How does bioaccess® support the research process for cardiology and neurology groups?
bioaccess® can enroll treatment-naive cardiology or neurology groups 50% faster than locations in the West, streamlining the research process.
What features does bioaccess® offer to enhance decision-making for research directors?
Their platform provides real-time insights and analytics, enabling research directors to make informed decisions swiftly.
How does bioaccess® ensure compliance with regulatory standards?
bioaccess® conducts feasibility evaluations, regulatory submissions, and ensures compliance with local regulations by gathering and reviewing study documents and submitting them to ethics committees.