


Navigating the landscape of clinical trials in Serbia presents a unique set of challenges, particularly in understanding the requirements that govern their execution. For sponsors and researchers, grasping the essential elements of clinical trial agreements is not just beneficial; it is imperative for ensuring compliance and success. As the regulatory environment evolves, questions arise about how to effectively streamline processes while maintaining ethical standards.
What are the key requirements that can make or break a clinical trial in Serbia?
How can organizations leverage local expertise to enhance their research outcomes?
These inquiries are crucial for anyone involved in clinical research.
bioaccess® plays a pivotal role in simplifying the clinical trial agreement requirements in Serbia, thanks to its deep understanding of local regulations and operational complexities. By meticulously preparing all necessary documentation, the organization accelerates the approval process, ensuring a seamless experience for sponsors. This strategic connection between sponsors and local regulatory bodies effectively minimizes delays and enhances the overall efficiency of research studies.
For Medtech and Biopharma innovators, this approach is particularly advantageous as they seek to expedite their research and development timelines. With Serbia's regulatory framework allowing most research study applications to gain approval within an average of 60 days, it is essential to consider the clinical trial agreement requirements in Serbia for the involvement of local representatives like bioaccess®. It maximizes success rates and navigates the complexities of conducting research in the region.
Consider the challenges you face in clinical research. Collaborating with bioaccess® not only streamlines the process but also positions your projects for success in a competitive landscape. The expertise and local knowledge they bring can make a significant difference in your research outcomes.
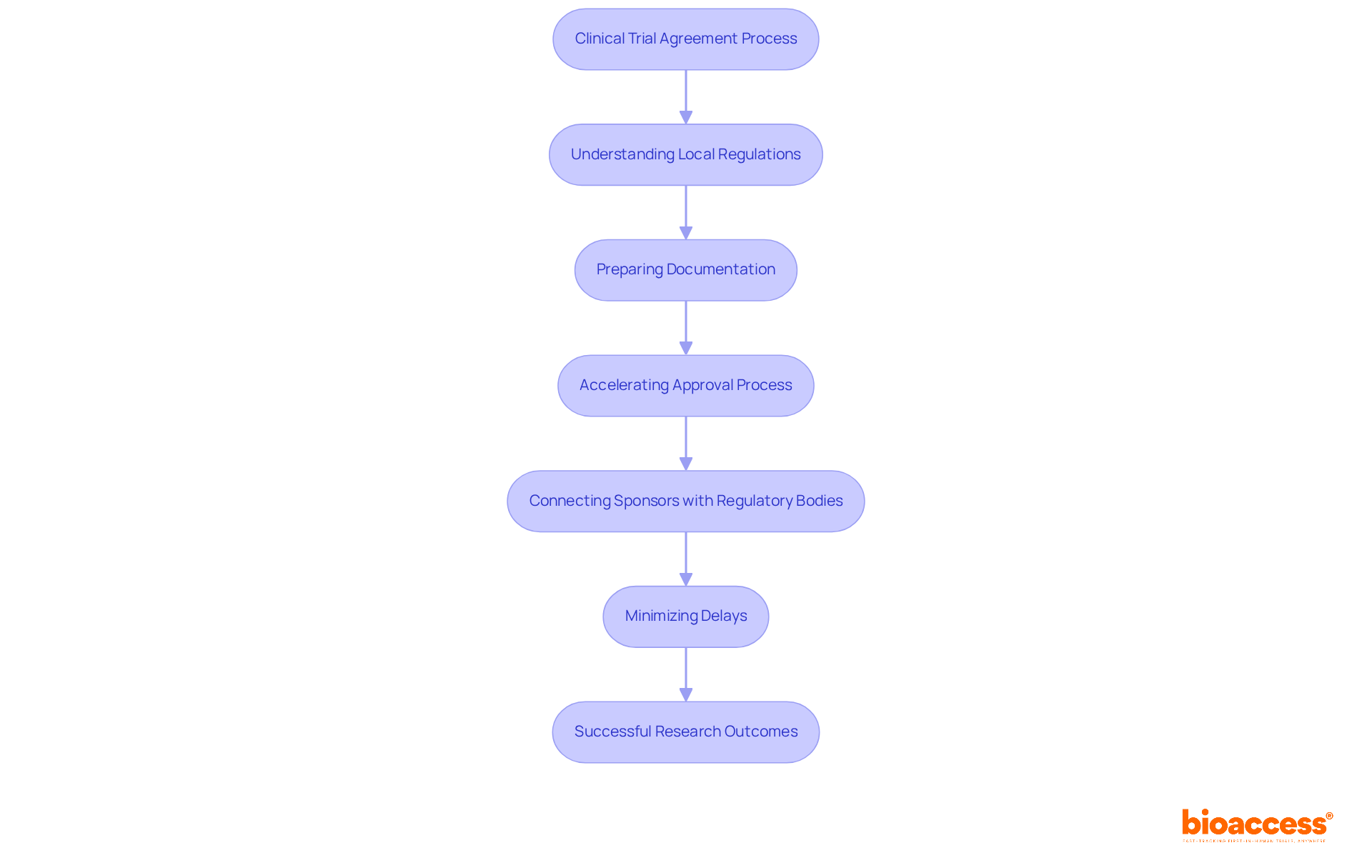
In Serbia, appointing a local representative is not just a formality; it’s a crucial requirement for sponsors based outside the country due to the clinical trial agreement requirements in Serbia. This representative acts as a regulatory proxy, ensuring that all local laws and regulations are meticulously followed. Their responsibilities include:
Navigating the complexities of the Serbian regulatory environment and adhering to the clinical trial agreement requirements in Serbia can be daunting without a local representative, significantly increasing the risk of delays in both initiation and execution.
Recent updates reveal that studies with local representatives often secure authorization within 80 days. This statistic underscores their vital role in streamlining the approval process and enhancing efficiency. Furthermore, starting January 2025, marketing authorization holders will be required to utilize the IRIS platform for managing Post-Authorization Safety Studies (PASS). This shift further highlights the necessity of local expertise in regulatory compliance.
Strategic local representation not only fosters adherence to moral standards but also significantly boosts the overall success of medical studies by ensuring compliance with clinical trial agreement requirements in Serbia. As you consider your own challenges in clinical research, reflect on how a local representative could mitigate risks and enhance your study's efficiency. The time to act is now-ensure compliance and success by engaging local expertise.
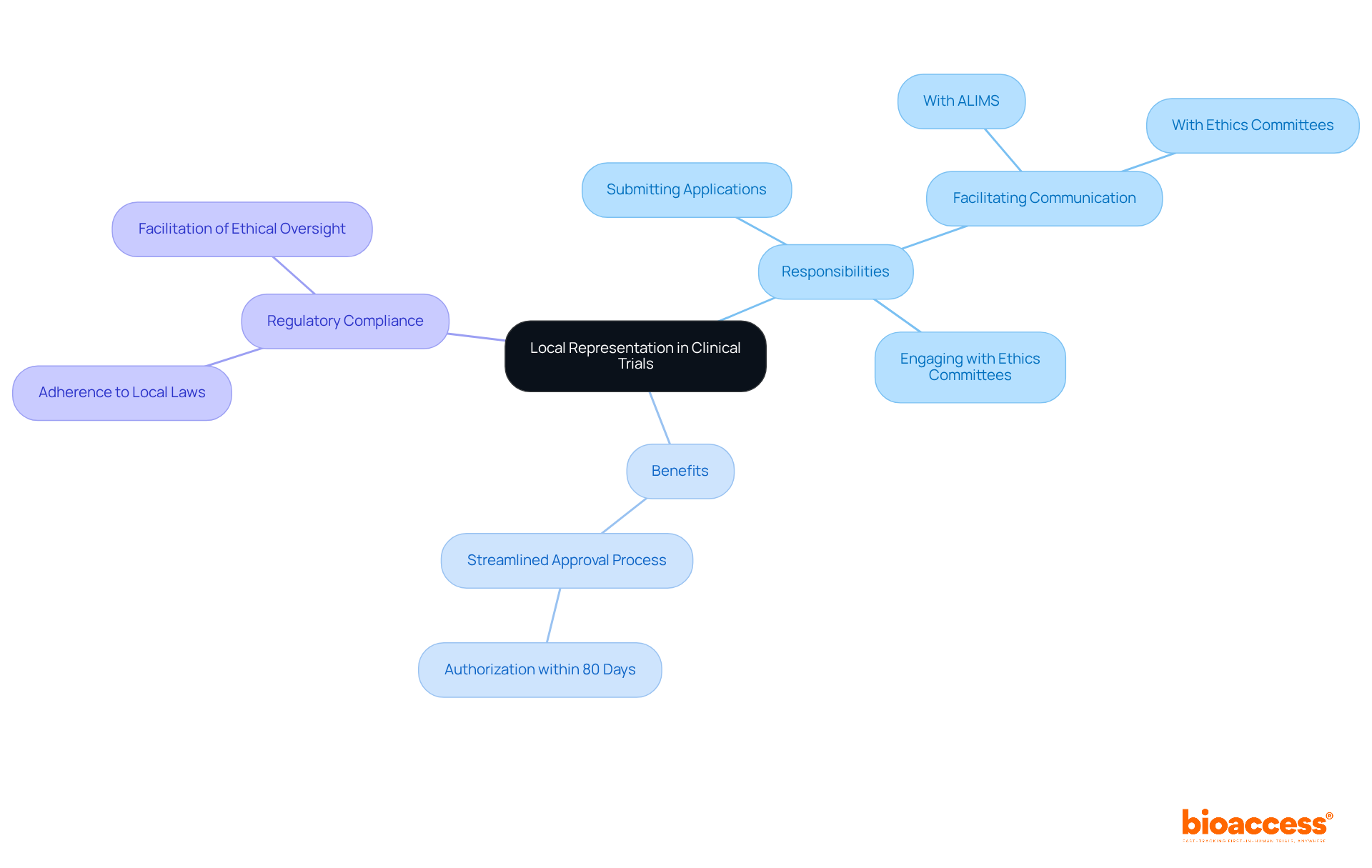
Serbia's research guidelines are meticulously crafted to uphold moral standards throughout investigations, aligning closely with international benchmarks like the Declaration of Helsinki and Good Clinical Practice (GCP). These regulations mandate that all research studies prioritize subject safety, informed consent, and data integrity. Researchers are tasked with ensuring their studies not only comply with local laws but also exemplify the highest moral standards. The active involvement of ethics boards is crucial, as they oversee research trials to guarantee adherence to these standards and protect participant rights.
With an impressive average of 90 clinical studies for medications and medical devices approved each year, Serbia demonstrates a robust framework for responsible clinical research. Notably, most studies receive authorization within 80 days, with protocols often approved in as little as 30 days, and occasionally within three weeks, as highlighted by Rory Gallagher from the regulatory affairs department. This unwavering commitment to ethical compliance fosters trust and integrity within the medical community.
Moreover, bioaccess® enhances this framework by providing expedited regulatory approval in just 6-8 weeks, significantly reducing the time required to initiate studies. By connecting innovative Medtech and Biopharma startups with leading research sites, bioaccess® facilitates quicker patient enrollment, effectively addressing common recruitment challenges faced in early-stage studies. This collaboration not only streamlines the process but also ensures that ethical standards are upheld throughout the research, reinforcing the integrity of medical research in Serbia.
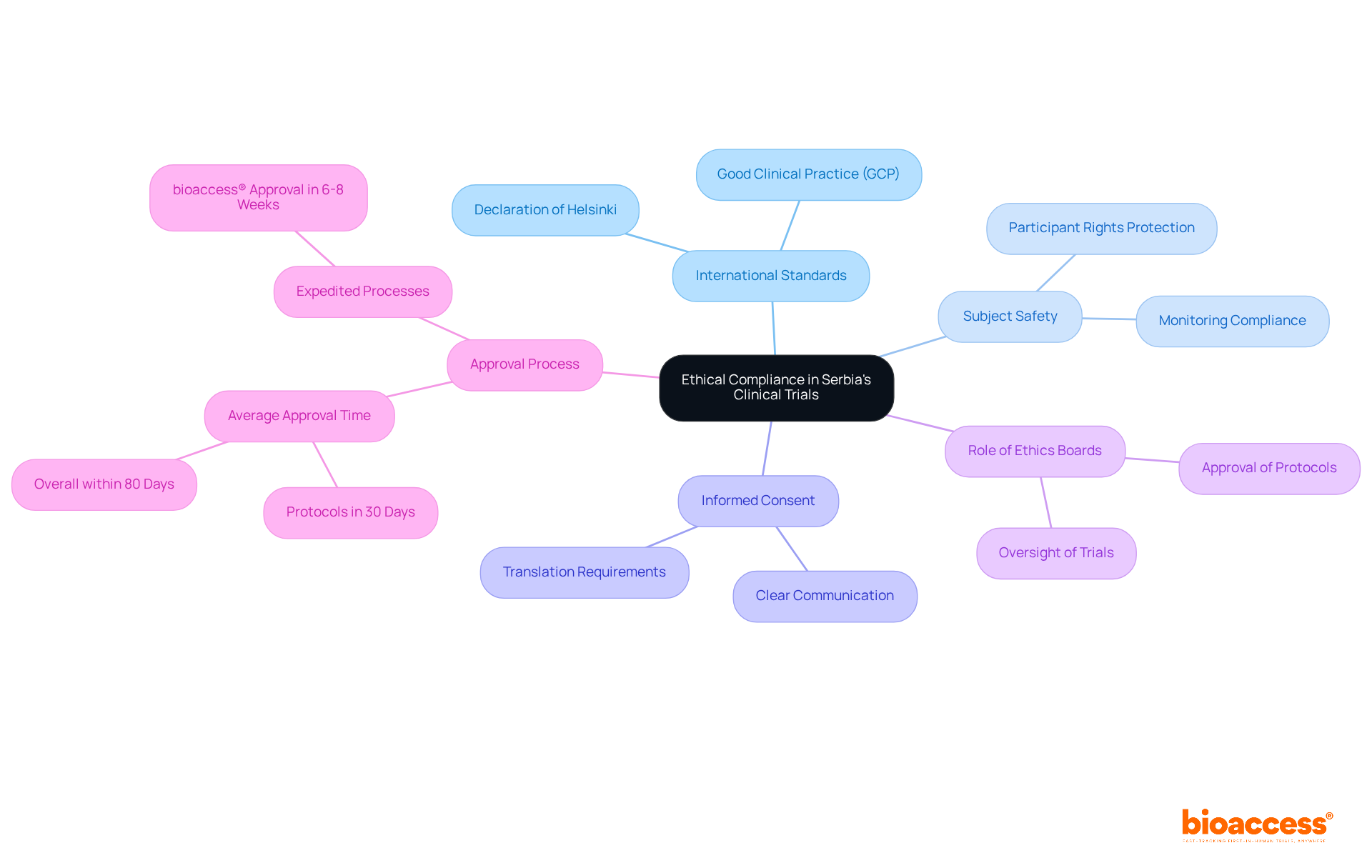
Informed consent stands as a cornerstone of the clinical research process in Serbia. Researchers are mandated to furnish potential subjects with comprehensive information about the study's purpose, procedures, risks, and benefits. This information must be conveyed clearly and accessibly, empowering individuals to make informed decisions about their participation. Moreover, meticulous documentation of the informed consent procedure is essential to uphold individuals' rights and autonomy throughout the study.
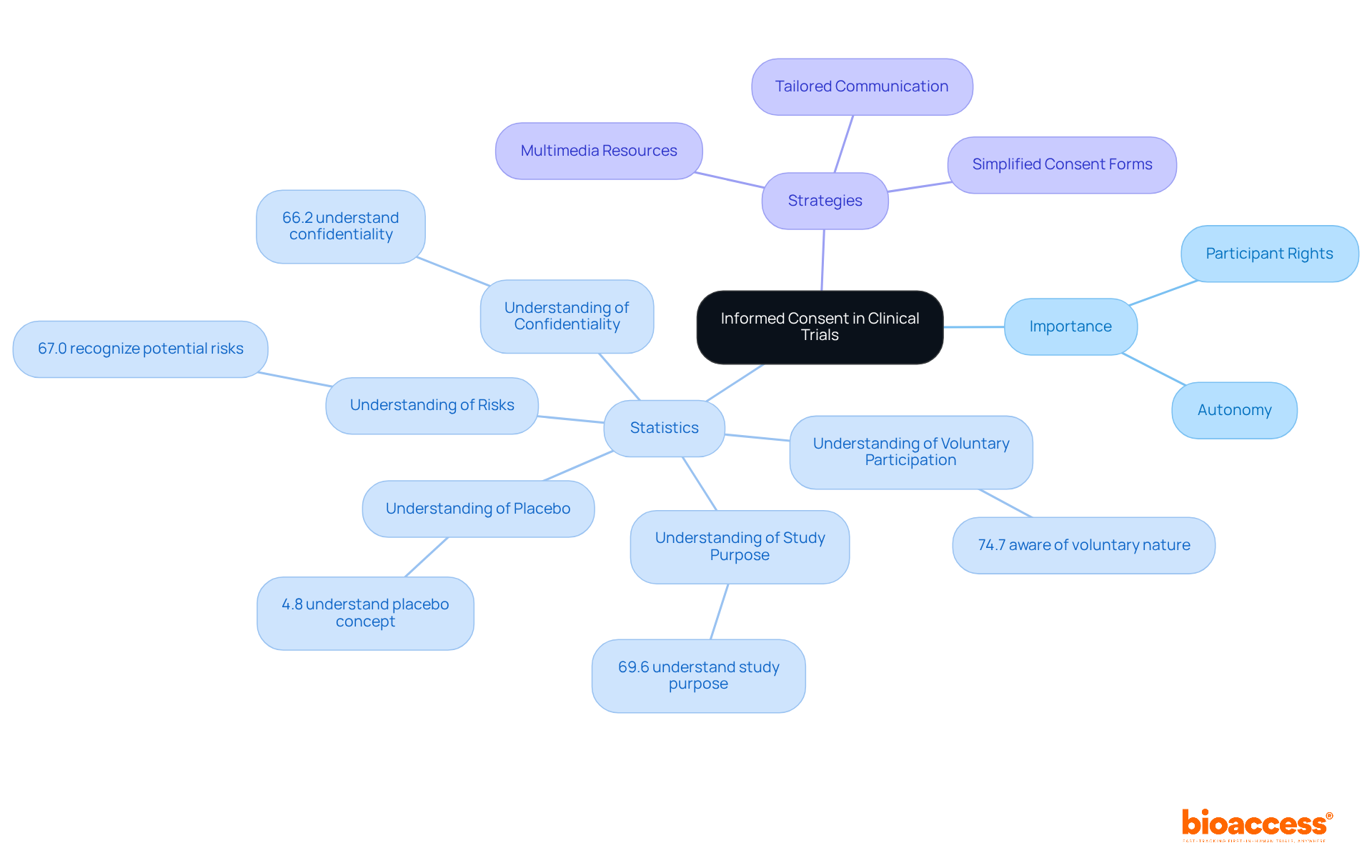
Recent statistics reveal a concerning trend: awareness of informed consent elements among study subjects has not significantly improved over the past 30 years. Only 74.7% of individuals understand the voluntary nature of their involvement, while just 67.0% recognize potential risks and side effects. This data underscores the urgent need for enhanced educational initiatives and simplified consent forms to bolster comprehension.
Effective strategies for informed consent in Serbian studies include:
By prioritizing knowledge and comprehension among subjects, researchers can foster a more principled and transparent research environment in Serbia.
In Serbia, ethics committees are pivotal in the research process, meticulously reviewing and approving protocols to ensure adherence to ethical standards and the protection of volunteer welfare. These committees assess the scientific validity of proposed studies, the robustness of the informed consent process, and the potential risks for participants. Given the challenges faced by Medtech, Biopharma, and Radiopharma startups in accessing healthcare providers and recruiting eligible patients, the significance of ethics committees is amplified. Researchers must present their protocols to the relevant ethics committee for approval, adhering to the clinical trial agreement requirements in Serbia, before initiating any research activities. This step is essential not only for compliance with the clinical trial agreement requirements in Serbia but also for maintaining the integrity of the research.
Comprehensive trial management services, such as those provided by bioaccess, are crucial in navigating these complexities effectively. Services include:
Recent trends indicate that the review process can be remarkably swift, with many protocols receiving approval within 30 days, and some even as quickly as three weeks. This efficiency underscores the commitment of Serbian ethics committees to support medical research while upholding high ethical standards.
Successful interactions with these committees are vital, ensuring that studies are designed to contribute meaningfully to scientific knowledge while safeguarding the rights and safety of participants. As you consider your own challenges in clinical research, think about how collaboration with ethics committees and comprehensive trial management services can enhance your research efforts.
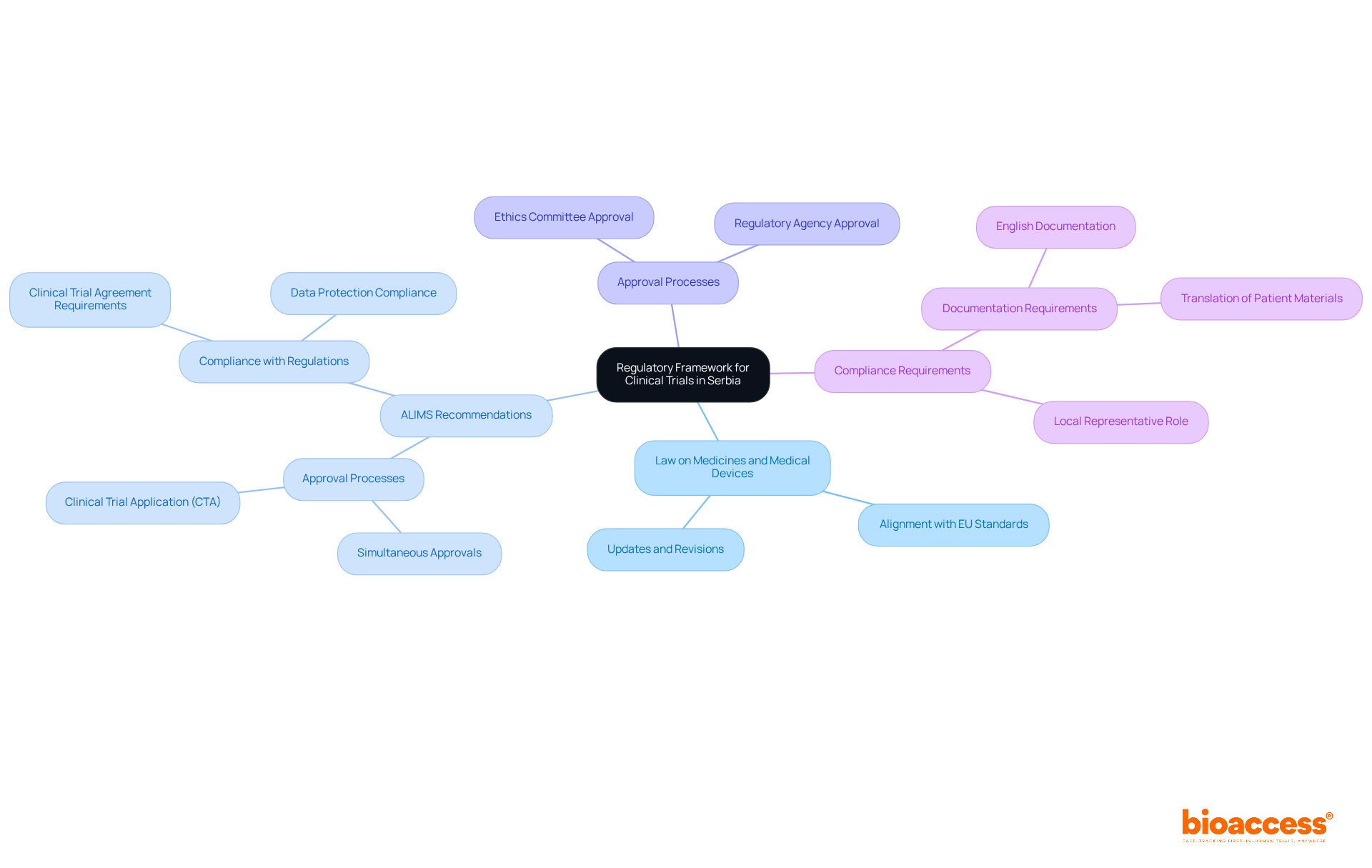
The regulatory framework for clinical studies in Serbia is primarily governed by the Law on Medicines and Medical Devices, which has seen significant updates to align with EU standards. This framework, bolstered by recommendations from the Agency for Medicines and Medical Devices of Serbia (ALIMS), delineates the processes for obtaining necessary approvals, conducting studies, and reporting results. Researchers must skillfully navigate this complex landscape to ensure compliance with all legal requirements, including the clinical trial agreement requirements in Serbia and the submission of a Clinical Trial Application (CTA).
Importantly, simultaneous approvals from ALIMS and the relevant ethics committee are crucial for fulfilling the clinical trial agreement requirements in Serbia before initiating any study. Adherence rates to Serbian research legislation have shown improvement, reflecting the effectiveness of these regulations in fostering a robust research environment. Specialist insights underscore that understanding the nuances of this regulatory structure is vital for successful study execution, particularly as Serbia continues to enhance its reputation as an attractive location for medical research.
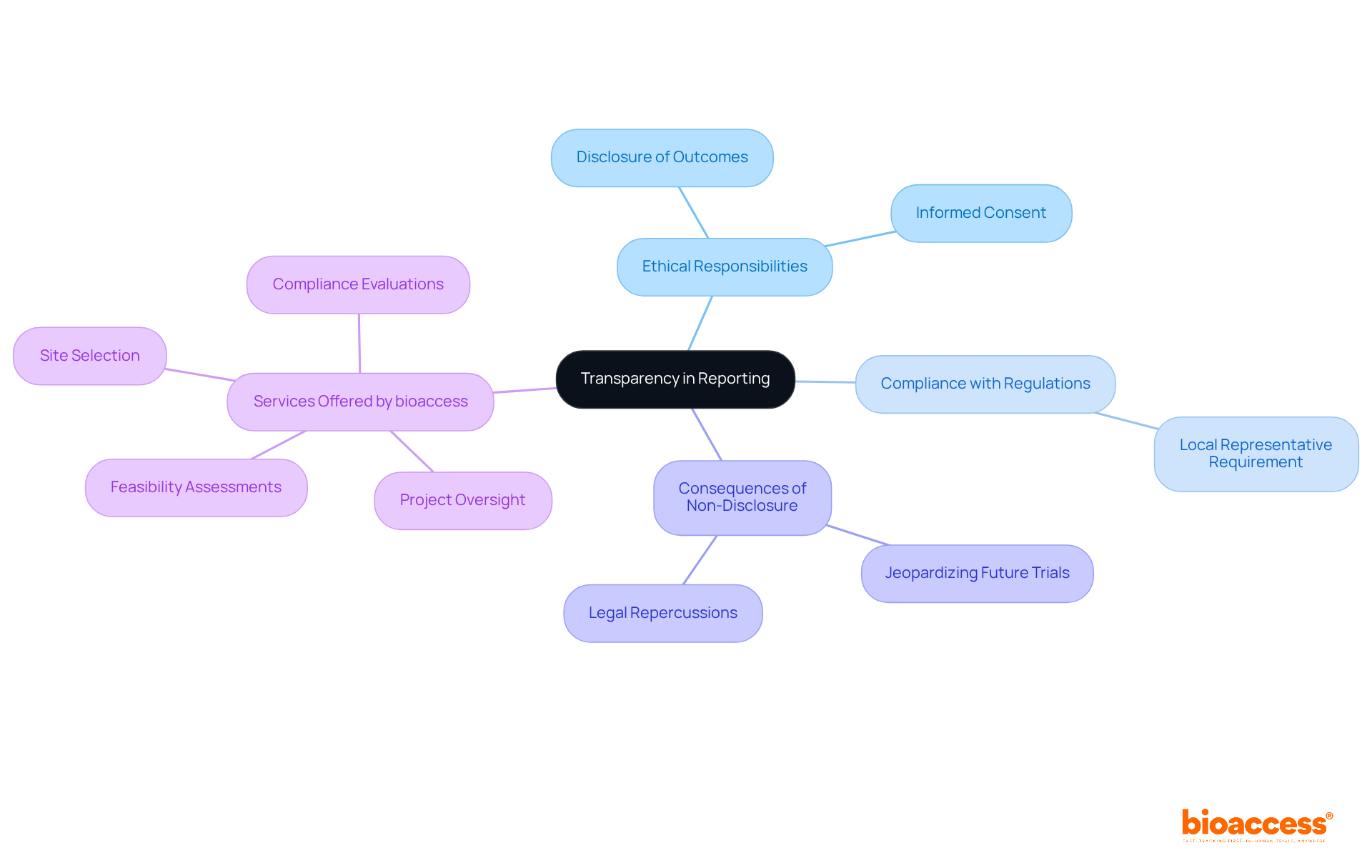
In Serbia, researchers conducting clinical studies bear a crucial ethical responsibility to report their findings transparently. This obligation encompasses the disclosure of both positive and negative outcomes, as well as any adverse events that may arise during the study. Such transparency is essential; it fosters trust among contributors and the public while bolstering the integrity of the scientific community. A qualitative study highlighted that investigators recognize the importance of reporting results to honor informed consent and uphold participant trust.
Moreover, the duty to disclose results is vital for adhering to moral standards in medical research. Failure to report can jeopardize informed consent for future trials, raising significant ethical concerns. Compliance with local regulations, specifically the clinical trial agreement requirements in Serbia established by the Medicines and Medical Devices Agency (ALIMS), is fundamental for promoting accountability in medical research. By embracing these ethical practices, researchers contribute to a culture of transparency, increasingly recognized as a cornerstone of responsible medical research in Serbia.
Significantly, only 39% of studies registered in ClinicalTrials.gov disclosed outcomes by early October 2021, underscoring the urgent need for improved reporting practices. Furthermore, foreign sponsors are required to appoint a Local Representative to ensure compliance with the clinical trial agreement requirements in Serbia, further emphasizing the importance of transparency and responsibility in research studies.
bioaccess offers comprehensive study management services, including:
These services are crucial for upholding ethical standards and ensuring thorough review and feedback on research documents.
Continuous moral supervision is essential to meet the clinical trial agreement requirements in Serbia. Ethics committees play a pivotal role in monitoring adherence to approved protocols throughout the study's duration. This responsibility includes routine evaluations of study progress, participant safety, and compliance with ethical standards. Are researchers prepared for audits and inspections by ethics committees and regulatory bodies? It's crucial to ensure that studies adhere to all clinical trial agreement requirements in Serbia, as well as all moral and legal standards.
Bioaccess offers comprehensive research management services that address these challenges head-on. From feasibility studies and site selection to compliance evaluations, setup, import permits, project oversight, and reporting, bioaccess ensures that every aspect of the study is conducted ethically and efficiently. This commitment not only enhances the integrity of clinical research but also fosters trust among stakeholders.
In the evolving Medtech landscape, collaboration is key. By partnering with experts like bioaccess, researchers can navigate the complexities of clinical studies with confidence. The importance of maintaining high ethical standards cannot be overstated, as it directly impacts participant safety and the validity of research outcomes. As we move forward, consider how your organization can benefit from these essential services.
Following the conclusion of a clinical study, researchers bear significant ethical obligations towards the individuals involved. This includes offering access to any advantageous treatments that were part of the research. Such post-trial access is not merely a courtesy; it is crucial for ensuring that individuals continue to receive appropriate care and support. Furthermore, researchers must convey the findings of the study to those involved, fostering transparency and trust in the research process. These responsibilities are essential for upholding moral standards and ensuring participant well-being long after the study's duration.

Continuous education for researchers is crucial for upholding moral principles in research studies. This encompasses comprehensive training in Good Clinical Practice (GCP), ethical considerations, and regulatory compliance. Researchers must remain informed about the latest advancements in research ethics and regulations to conduct their studies responsibly. Regularly updated training programs are vital to reflect changes in laws and best practices, fostering a culture of ethical research within the clinical trial community.
As Albert Szent-Györgyi aptly stated, research involves seeing what others have seen and thinking what nobody else has thought. This perspective underscores the significance of continuous learning and adaptation in the field. By prioritizing ongoing education, researchers not only enhance their expertise but also contribute to the integrity of clinical research as a whole.
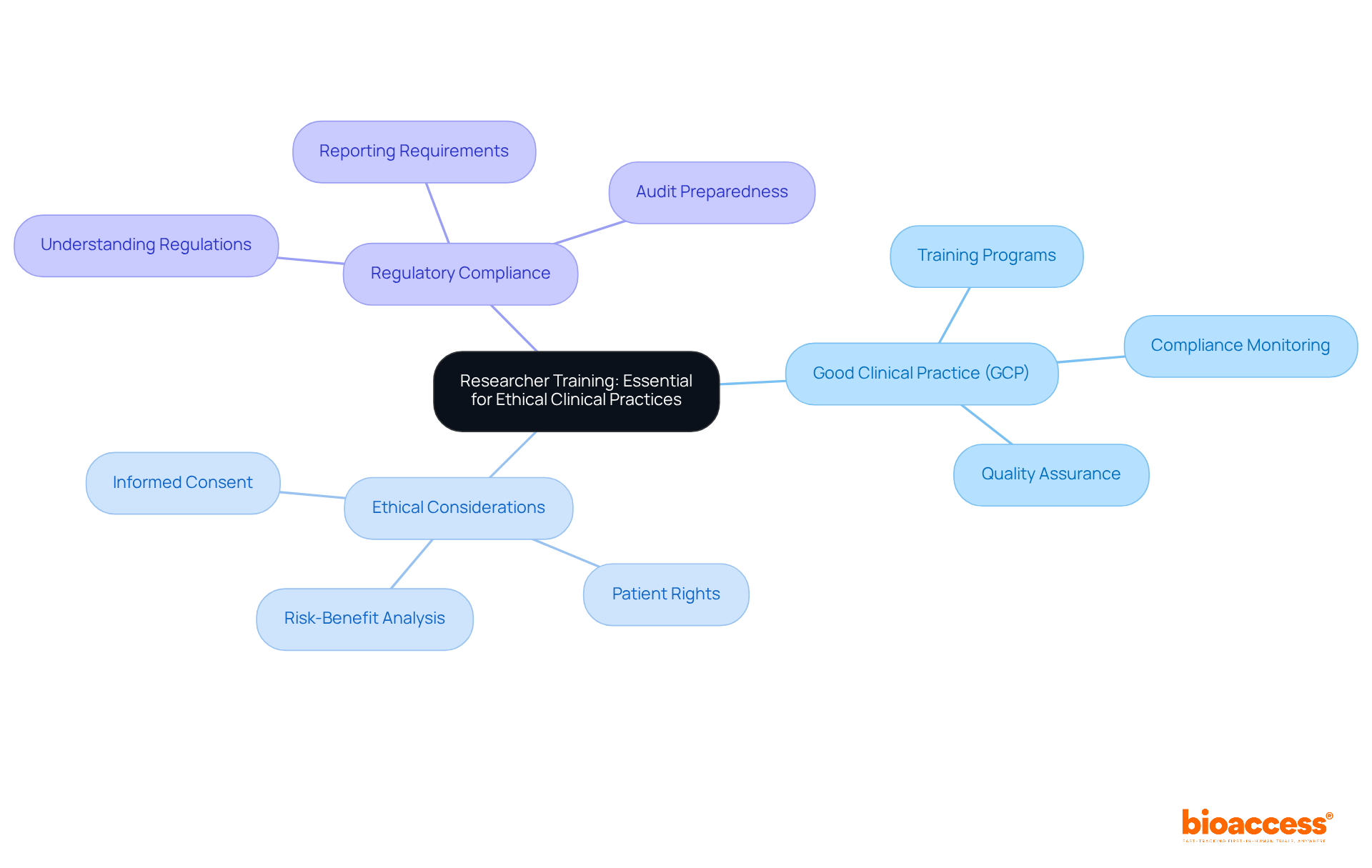
The landscape of clinical trials in Serbia is defined by essential requirements that ensure ethical compliance, regulatory adherence, and operational efficiency. Understanding these clinical trial agreement requirements is crucial for sponsors and researchers who wish to navigate the complexities of conducting studies in the region. By leveraging the expertise of local representatives like bioaccess®, sponsors can significantly enhance their chances of success, streamline the approval process, and uphold the highest ethical standards.
Key insights emphasize the importance of:
Local representatives play a vital role, facilitating communication with regulatory bodies to ensure compliance with Serbian laws and expedite the approval process. Furthermore, adherence to ethical guidelines, as outlined by ethics committees, fosters trust and integrity in research. Informed consent remains a cornerstone of participant rights and safety, reinforcing the ethical framework of clinical trials.
Ultimately, a commitment to ethical practices and regulatory compliance enriches the research environment in Serbia, positioning it as an attractive destination for innovative studies. For researchers and sponsors alike, engaging with local expertise and prioritizing ethical standards can lead to more successful outcomes and a responsible approach to clinical research. Embracing these principles is essential for advancing medical science and ensuring the well-being of participants throughout the research process.
What is bioaccess® and what role does it play in clinical trials in Serbia?
bioaccess® simplifies the clinical trial agreement requirements in Serbia by preparing necessary documentation and accelerating the approval process. It connects sponsors with local regulatory bodies to minimize delays and enhance research efficiency.
Why is local representation important for clinical trials in Serbia?
Local representation is crucial for sponsors outside Serbia as it ensures compliance with local laws and regulations. A local representative acts as a regulatory proxy, facilitating communication with regulatory agencies and ethics committees, which helps avoid delays in study initiation and execution.
What are the responsibilities of a local representative in Serbia?
A local representative is responsible for submitting research study applications, facilitating communication with the Medicines and Medical Devices Agency (ALIMS), and engaging with ethics committees to ensure compliance with local regulations.
How long does it typically take to gain approval for research studies in Serbia?
Most research study applications in Serbia gain approval within an average of 60 days, and studies with local representatives often secure authorization within 80 days.
What changes are expected in January 2025 regarding regulatory compliance in Serbia?
Starting January 2025, marketing authorization holders will be required to use the IRIS platform for managing Post-Authorization Safety Studies (PASS), emphasizing the need for local expertise in regulatory compliance.
What ethical standards are upheld in Serbia's clinical trial guidelines?
Serbia’s clinical trial guidelines prioritize subject safety, informed consent, and data integrity, aligning with international benchmarks such as the Declaration of Helsinki and Good Clinical Practice (GCP).
How does bioaccess® contribute to ethical compliance in clinical research?
bioaccess® enhances ethical compliance by providing expedited regulatory approval, facilitating quicker patient enrollment, and ensuring that ethical standards are upheld throughout the research process.
What is the average number of clinical studies approved in Serbia each year?
Serbia approves an average of 90 clinical studies for medications and medical devices each year, demonstrating a robust framework for responsible clinical research.