


The article titled "10 Essential Clinical Trial Protocol Examples for Research Leaders" serves as a critical resource for research leaders in the clinical field. It highlights the essential components and examples of clinical trial protocols, which are vital for designing effective studies.
Structured protocols are emphasized for their role in ensuring participant safety, data integrity, and regulatory compliance. By illustrating these points with real-world examples, the article also brings to light the common challenges faced during protocol development, reinforcing the necessity for well-crafted protocols in clinical research.
The landscape of clinical trials is evolving rapidly, driven by the need for efficient methodologies and robust protocols that ensure safety and integrity in research. As the demand for innovative medical technologies grows, understanding the essential components of clinical trial protocols becomes crucial for research leaders aiming to navigate this complex environment. However, with increasing complexity in study designs and regulatory requirements, how can organizations effectively streamline their processes to enhance participant recruitment and ensure compliance?
This article explores ten essential clinical trial protocol examples that not only highlight best practices but also provide insights into overcoming common challenges faced by research teams today.
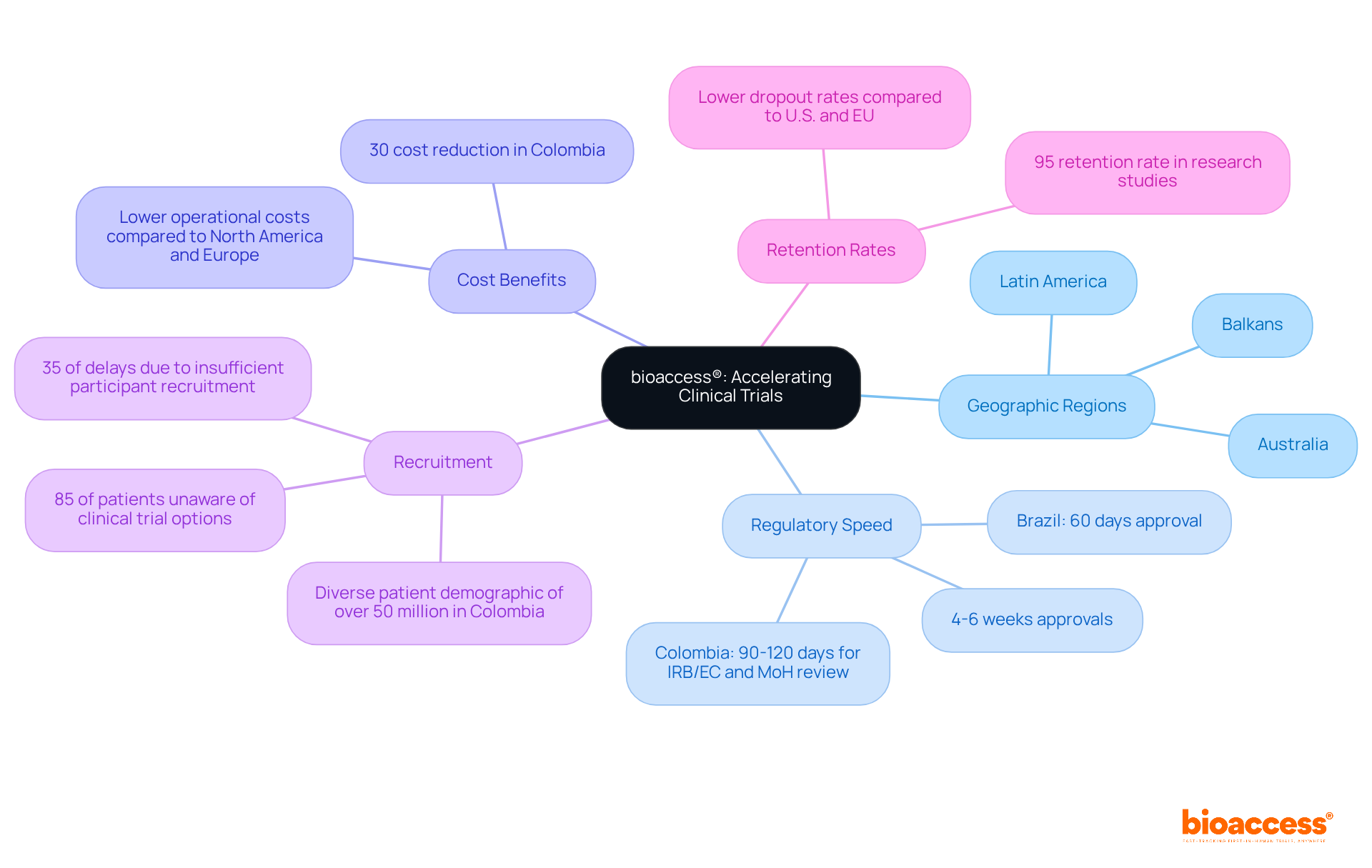
bioaccess® strategically positions itself across Latin America, the Balkans, and Australia, significantly enhancing research protocols for Medtech innovations. By leveraging the rapid regulatory processes inherent in these regions, bioaccess® achieves ethical approvals in an impressive 4-6 weeks. This expedited timeline accelerates the journey to market for new medical technologies, empowering Medtech companies to swiftly adapt to evolving market demands and regulatory landscapes.
In Colombia, research studies benefit from cost reductions exceeding 30% compared to North America and Western Europe, alongside a swift IRB/EC and MoH (INVIMA) review process that typically takes only 90-120 days. Furthermore, the R&D tax incentives available in Colombia, including a 100% tax deduction for investments in science and technology, significantly enhance the competitive edge for Medtech firms. The ability to conduct experiments effectively results in a notable competitive advantage, particularly as 35% of delays in research studies arise from insufficient participant recruitment.
Moreover, Colombia's diverse patient demographic of over 50 million, with 95% covered by universal healthcare, bolsters recruitment initiatives, leading to an impressive 95% retention rate in research studies. This statistic underscores the effectiveness of patient engagement strategies. As Brazil is projected to lead Latin America with nearly 10,000 registered medical studies by 2025, the importance of regulatory speed in achieving successful outcomes in Medtech cannot be overstated. As one specialist aptly noted, 'The pace of medical studies is determined by how many days it requires for the studies to succeed,' highlighting the essential nature of these advantages.
To maximize these benefits, Medtech firms should consider how they can integrate bioaccess®'s features into their own research strategies.
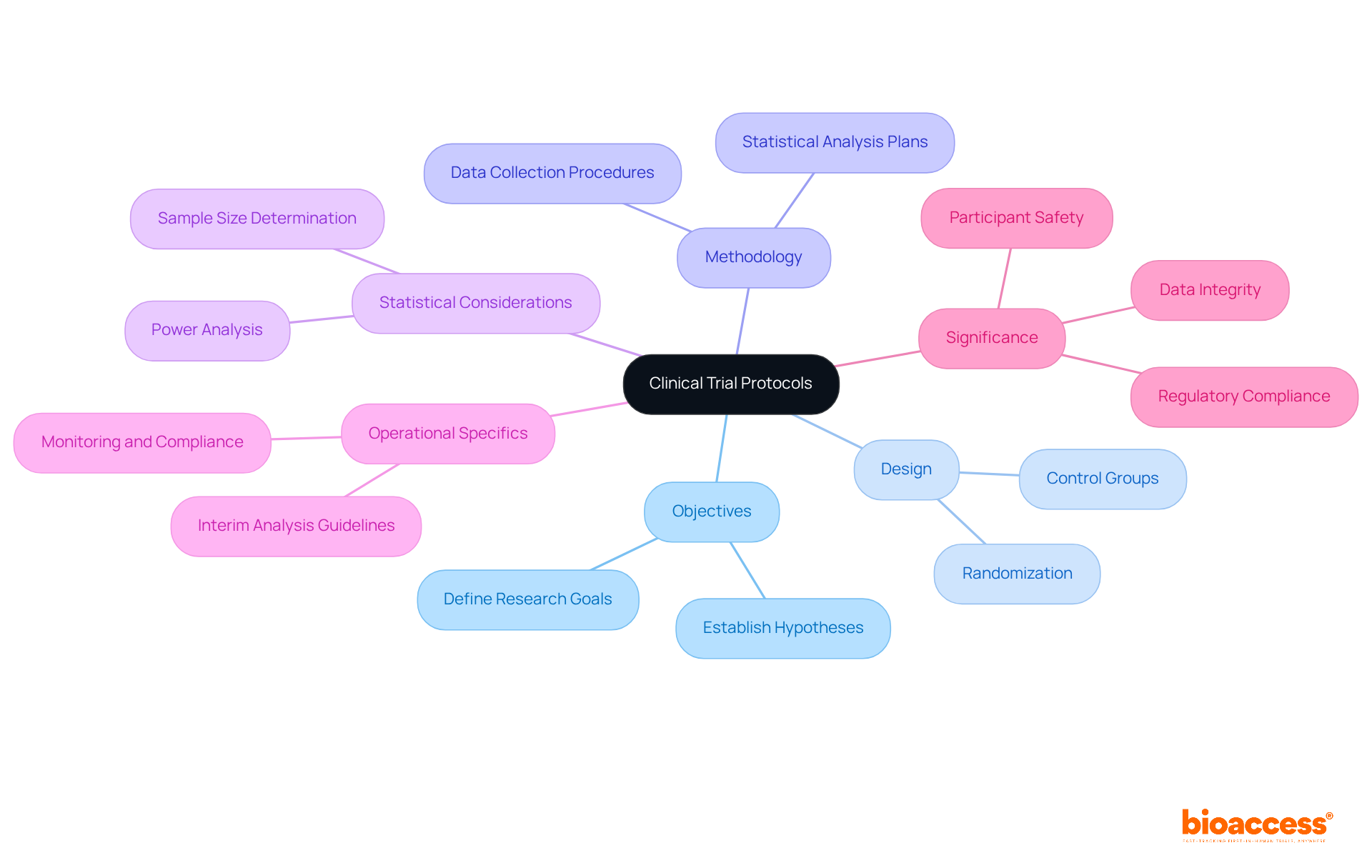
A clinical trial protocol example serves as a detailed document that outlines the objectives, design, methodology, statistical considerations, and operational specifics of a clinical trial. It serves as a clinical trial protocol example, acting as a comprehensive blueprint for executing the study and ensuring that all team members are synchronized with the study's goals and procedures.
The significance of a carefully designed clinical trial protocol example cannot be overstated; it not only safeguards participant safety but also enhances data integrity and ensures regulatory compliance. This, in turn, bolsters the credibility of the research findings.
Statistics indicate that an inadequate clinical trial protocol example is a major factor in research study failures, underscoring the necessity of thorough procedure development. For instance, clearly outlined procedures have been shown to enhance participant safety by establishing explicit guidelines for managing adverse occurrences and ensuring that ethical standards are upheld.
By prioritizing procedure integrity, researchers can significantly mitigate risks and elevate the overall quality of medical studies.
Key components of a clinical trial protocol include:
Including these components not only improves the quality of medical studies but also aligns with current trends that underscore the significance of having a clinical trial protocol example, which emphasizes clear objectives and ethical adherence in healthcare research. Additionally, Bioaccess® offers an accelerated regulatory approval process, achieving approvals in 6-8 weeks compared to the typical 6-12 months in the US/EU, and facilitates patient enrollment in cardiology and neurology cohorts 50% faster than Western sites. This capability tackles frequent patient recruitment issues encountered by Medtech and biopharma startups, ensuring effective management from feasibility assessments, site selection, compliance reviews, to project monitoring and reporting.

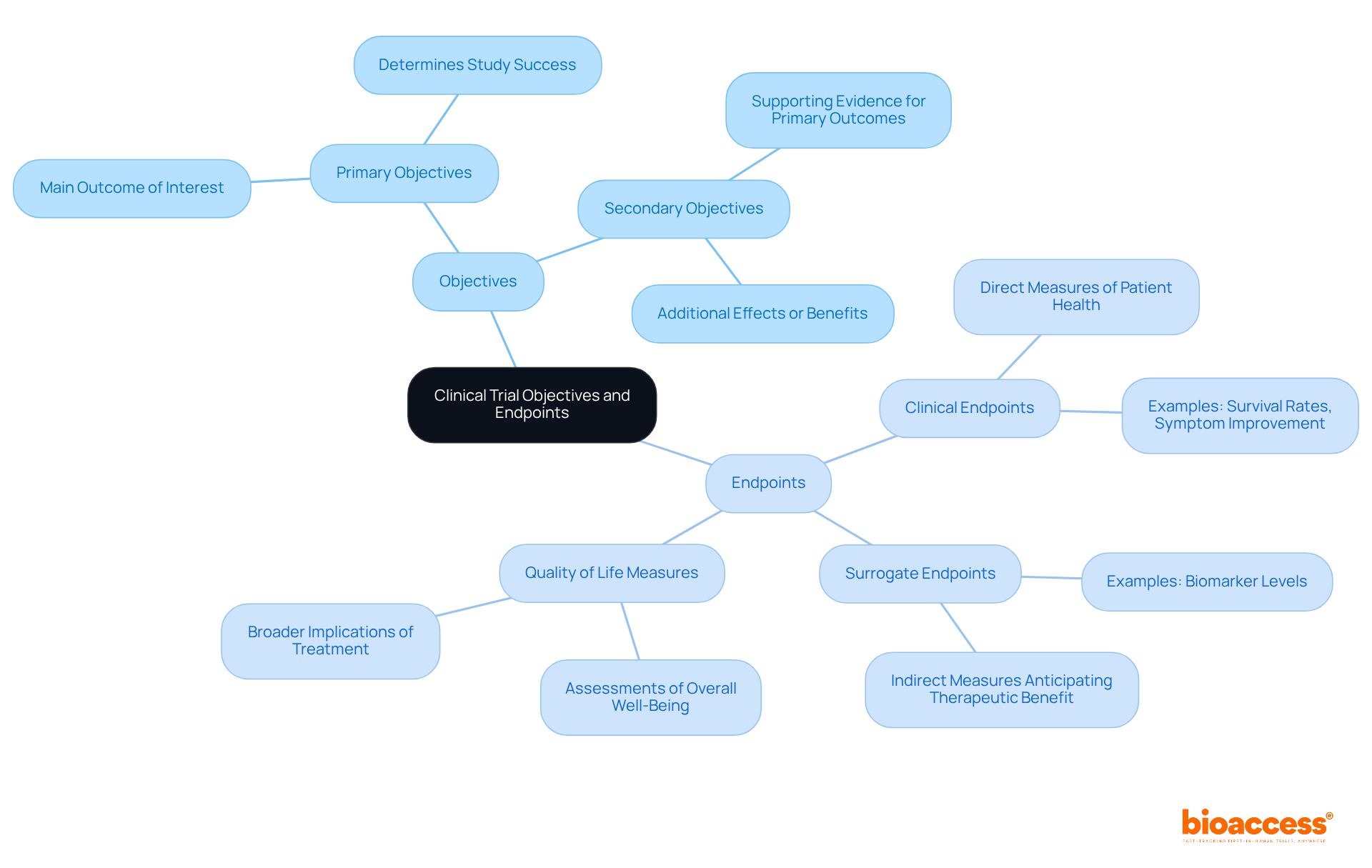
Goals in a clinical trial protocol example are essential, as they outline the specific questions the research intends to address, divided into primary and secondary objectives. Primary objectives concentrate on the main outcome of interest, determining the study's success, while secondary objectives explore additional effects or benefits of the intervention, providing a more comprehensive understanding of its impact.
Endpoints are the specific outcomes measured to assess the effectiveness of the intervention. They can be classified into several types:
Recent trends suggest a notable rise in the complexity of clinical study objectives and endpoints, with the number of endpoints in study protocols growing by 86 percent from 2001 to 2015. This complexity necessitates a clear hierarchy of endpoints to ensure proper statistical analysis and regulatory acceptance, as emphasized by the FDA's 2022 Multiple Endpoints guidance.
For instance, in a randomized controlled study assessing the effectiveness of an antidepressant, the primary outcome might be measured using the Hamilton Rating Scale for Depression, while secondary outcomes could include anxiety levels and quality of life assessments. Such a structured approach not only enhances the clarity of the study but also supports the regulatory approval process by providing compelling evidence of the intervention's effectiveness.
In summary, a clinical trial protocol example should clearly define primary and secondary objectives, along with well-structured endpoints, as these are essential for the success of medical studies. This ensures that the research question is effectively addressed and that the findings are robust and interpretable.
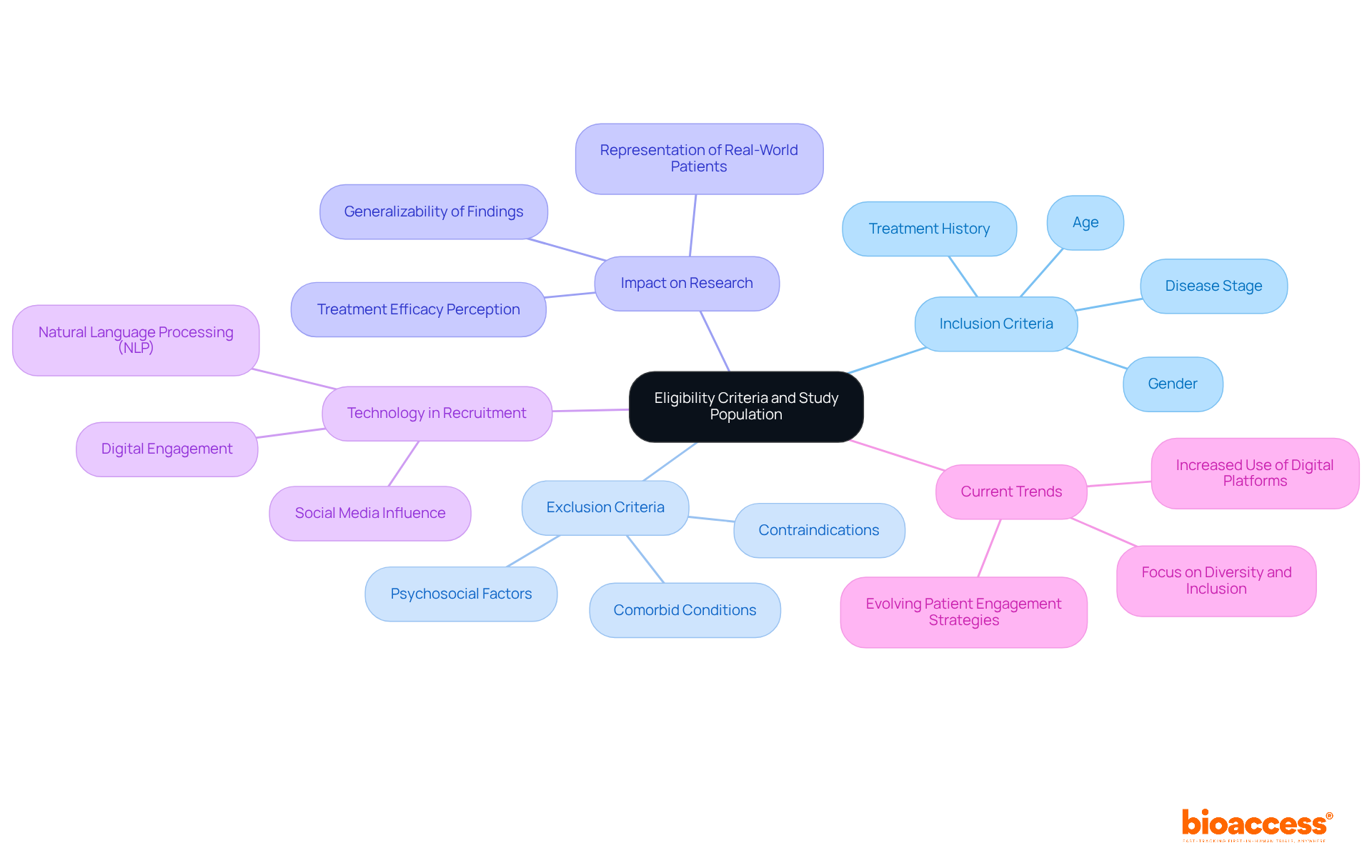
Eligibility criteria are pivotal in determining participant eligibility for a clinical trial protocol example, which encompasses both inclusion and exclusion criteria. Inclusion criteria specify the essential characteristics participants must possess, such as age, gender, disease stage, and treatment history. Conversely, exclusion criteria pinpoint factors that disqualify possible participants, including comorbid conditions or contraindications to the intervention.
Clearly specified eligibility criteria are essential in a clinical trial protocol example for ensuring that the participant group corresponds with the research question, thus improving the reliability of results. For instance, an examination of chronic pain studies indicated that rigorous exclusion criteria frequently resulted in insufficient representation of real-world patients, potentially exaggerating treatment effectiveness and influencing perceived treatment success. This underscores the need for a balanced approach to inclusion and exclusion criteria to enhance the generalizability of findings to broader patient populations.
The significance of thoughtfully evaluating participant groups is further highlighted by recent patterns in medical research recruitment. During the COVID-19 pandemic, digital engagement surged, with social media emerging as a significant source for potential participants; notably, 15% of participants learned about studies through these platforms. This evolution necessitates that researchers modify their eligibility standards to accurately represent the varied demographics of the patient population, ensuring that studies are inclusive and representative.
A clinical trial protocol example can demonstrate effective participant selection in research protocols through the incorporation of natural language processing (NLP) technologies, which have enhanced patient-study matching processes. For example, IBM's Watson system reportedly boosted breast cancer study enrollments by 80%, demonstrating the effectiveness of utilizing technology to refine eligibility criteria and improve recruitment strategies.
Moreover, it is crucial to recognize that the frequently referenced statistic indicating that only 5% of eligible patients engage in research studies is based on erroneous data, highlighting the necessity for more inclusive eligibility standards. As V. Salmasi observed, there is an urgent demand for additional research exploring exclusion criteria in chronic pain investigations, which further emphasizes the persistent difficulties in establishing eligibility criteria.
In summary, the careful definition of eligibility criteria not only shapes the research population but also significantly influences the outcomes and relevance of research findings.
Common challenges in clinical trial protocol development include:
Complexity of Protocols: Overly complicated protocols can lead to confusion among study staff and participants, significantly impacting data quality. A 2023 report emphasized that over 50% of data problems arise from complexity in procedures, underscoring the necessity for clarity in design. At bioaccess, we emphasize the importance of clear and concise clinical trial protocol example development to mitigate these issues.
Regulatory Compliance: Navigating the regulatory landscape is often daunting due to frequent updates and changes in requirements. Non-compliance can result in hefty financial penalties and trial delays, making it essential to stay informed and adaptable. Bioaccess provides expert review and feedback on study documents to ensure compliance with country requirements, helping to mitigate these risks and streamline the approval process.
Stakeholder Alignment: Ensuring that all stakeholders—including sponsors, investigators, and regulatory bodies—are aligned on study details can be challenging. A well-designed framework promotes clarity and alignment, optimizing timelines and enhancing the chances of regulatory success. Our extensive clinical trial management services, which include a clinical trial protocol example, encompass feasibility assessments and site selection, both of which are vital for attaining this alignment.
Resource Constraints: Limited resources can impede the comprehensive development and implementation of procedures, leading to delays and heightened expenses. Establishing a structured, cross-functional review process can mitigate these issues by ensuring comprehensive input from all relevant parties. Bioaccess emphasizes project management and monitoring to streamline this process effectively, ensuring that resources are utilized efficiently.
Participant Recruitment: Defining eligibility criteria too narrowly can limit recruitment efforts, delaying study timelines. Engaging patient advisors during protocol development can enhance patient-centric design, improving enrollment and retention rates. Research suggests that recruitment difficulties represent 32% of total research project expenses, highlighting the significance of strategic planning in this domain. By utilizing our knowledge in regulatory matters, especially through experts like Katherine Ruiz, we strive to change lives in Latin America with advanced medtech and effective research studies.

Regulatory compliance in a clinical trial protocol example is essential for ensuring that investigations adhere to laws, guidelines, and ethical standards established by governing bodies such as the FDA and EMA. Key components include:
Good Clinical Practice (GCP): Adhering to GCP is vital for conducting trials ethically and safeguarding participant rights. Recent analyses indicate that GCP compliance for interventional research averages 92.2%, while observational research lags at 79.5%. This disparity underscores the need for improved training and resources for investigators, particularly in regions with diverse participant demographics, such as the UAE, where many participants may not be native English or Arabic speakers. Bioaccess provides extensive clinical trial management services, which include a clinical trial protocol example that covers feasibility assessments and site selection to ensure that trials are conducted in accordance with GCP.
Informed Consent: Obtaining voluntary consent from participants is crucial. However, research has indicated that the readability of informed consent forms (ICFs) often falls short, with 100% of ICFs from observational research classified as 'difficult' for readability. The typical reading level for ICFs in observational research is at a 12th-grade level, making understanding difficult for many participants. Enhancing the clarity of these documents is essential for ethical compliance and participant understanding. Bioaccess offers review and feedback on research documents to meet country requirements, ensuring that ICFs are accessible to all participants.
Any modifications to the clinical trial protocol example must be meticulously documented and approved by relevant regulatory authorities. This ensures that all changes are transparent and maintain the integrity of the study. Bioaccess aids in study setup and approval procedures, including providing a clinical trial protocol example for ethics committee and health ministry endorsements, to promote this compliance.
The significance of GCP compliance in a clinical trial protocol example cannot be exaggerated, as it directly affects study results and the overall integrity of medical research. By prioritizing compliance and improving the readability of ICFs, researchers can foster greater trust and understanding among participants, ultimately advancing medical knowledge and innovation. Katherine Ruiz, a specialist in regulatory matters for medical devices and in vitro diagnostics in Colombia, highlights the importance of these compliance measures in improving the quality and dependability of research studies.
Data collection and management in clinical trials encompass several essential practices that ensure the integrity and reliability of research findings:
Data Collection Methods: Employing diverse techniques such as electronic data capture (EDC), case report forms (CRFs), and patient-reported outcomes (PROs) facilitates efficient data gathering. These methods streamline the process and enhance participant engagement and data accuracy.
Data Quality Assurance: Establishing robust processes to guarantee data accuracy, completeness, and consistency is critical throughout the study. This includes implementing regular audits and validation checks to identify and rectify discrepancies, thereby maintaining high data integrity.
Data Monitoring: Continuous monitoring of data is vital for identifying inconsistencies and ensuring adherence to the study protocol. Regular evaluations assist in quickly tackling any problems that may occur, thus protecting the integrity of the experiment.
Statistical Analysis: Utilizing appropriate statistical methods is crucial for analyzing the collected data. This ensures that conclusions drawn about the intervention's effectiveness are valid and reliable. Understanding key statistical concepts, such as p-values and confidence intervals, is essential for interpreting results accurately and communicating findings effectively.
Incorporating these practices enhances the quality of research data and supports informed decision-making, ultimately contributing to the advancement of medical studies.
Real-world examples of clinical trial protocols illustrate the critical role of structured methodologies in achieving successful outcomes:
CureVac's COVID-19 Vaccine Trial: This Phase 2b/3 randomized, observer-blinded study assessed the efficacy and safety of CVnCoV in adults. The framework serves as a clinical trial protocol example, meticulously crafted to detail objectives, endpoints, and statistical analysis plans, contributing to its robust design and execution.
DNDi's Chagas Disease Trial: In this Phase 2 trial, the focus was on evaluating oral Benznidazole for chronic indeterminate Chagas disease. The guidelines emphasized the importance of a clinical trial protocol example that features transparent eligibility standards and clearly articulated outcome metrics, guaranteeing that the research effectively tackled its inquiries.
ReGelTec's Early Feasibility Assessment on HYDRAFIL™: Conducted in Barranquilla, Colombia, this assessment successfully treated eleven patients with chronic low back pain. The organized procedure featured remote supervision through Zoom, showcasing creative methods in medical study management. This case exemplifies how a clinical trial protocol example with detailed methodologies can lead to effective patient outcomes and compliance with regulatory standards. Furthermore, bioaccess's collaboration in this study highlights its dedication to improving trial services in Colombia.
NIH's Clinical Trial Protocol Example: The National Institutes of Health provides a clinical trial protocol example that serves as a standardized template and valuable resource for researchers. It contains educational material and examples, directing the creation of compliant and effective procedures, thereby improving the quality of medical research.
These instances highlight the significance of thorough guidelines in medical research, as they not only aid in meeting regulatory standards but also enhance the dependability of results. Data shows that well-organized procedures can greatly improve patient participation and retention, tackling the persistent issue of insufficient enrollment in research studies, which impacts almost 37 percent of investigations. By gaining insights from these effective procedures, researchers can more effectively manage the intricacies of study design and implementation.
Frequently asked questions about clinical trial protocols include:
What is a clinical trial protocol? A clinical trial protocol example is a detailed document that outlines the study's objectives, design, methodology, and operational procedures, serving as a roadmap for the research.
Why are guidelines important? Protocols are essential for ensuring that experiments are conducted consistently and ethically. They safeguard participant safety and uphold data integrity, which is essential for the credibility of the research outcomes. Thorough clinical study management services, including feasibility assessments and compliance evaluations provided by bioaccess, guarantee that all elements of the study are carefully managed to uphold adherence to guidelines.
How frequently can guidelines be modified? Protocols can be amended as necessary, but all modifications must be meticulously documented and receive approval from regulatory authorities to maintain compliance and integrity. Bioaccess provides trial setup and project management services to streamline these processes and ensure timely amendments.
What occurs if a procedure is not adhered to? Non-compliance with the guidelines can lead to significant data integrity issues, regulatory penalties, and potential harm to participants. This highlights the necessity of strict adherence to uphold the ethical standards of medical research. Efficient project oversight and tracking, as part of bioaccess's service provisions, play a vital role in ensuring compliance with guidelines.
The occurrence of revisions to the clinical trial protocol example has significantly risen, with research showing that 76% of clinical experiments now necessitate at least one modification. This rise is particularly pronounced in oncology, where 90% of trials necessitate changes due to evolving scientific understanding and regulatory requirements. Notably, 77% of amendments were deemed unavoidable due to regulatory agency requests and changes to study strategy. The typical expense of these changes varies from $141,000 to $535,000, emphasizing the financial consequences of managing procedures. Additionally, studies indicate that around 23% of these changes could be prevented through improved initial design and stakeholder involvement, highlighting the significance of comprehensive planning and communication in the development process. Furthermore, the average duration from recognizing the need for changes to final oversight approval is 260 days, during which research sites function under various version guidelines for approximately 215 days, demonstrating the operational challenges involved in managing amendments. To enhance protocol adherence, organizations should prioritize effective initial protocol design and engage stakeholders early in the process.
The significance of well-structured clinical trial protocols cannot be overstated; they serve as the backbone of successful medical research. By meticulously outlining objectives, methodologies, and ethical considerations, these protocols ensure participant safety while enhancing data integrity and regulatory compliance. In a landscape where the speed and efficiency of clinical trials are paramount—especially in regions like Latin America—the advantages of a robust protocol design become even more crucial for Medtech innovations.
Key insights discussed throughout the article highlight essential components of clinical trial protocols, including:
The role of organizations like bioaccess® in expediting regulatory processes and improving participant recruitment further emphasizes the need for strategic planning in clinical trial design. Real-world examples illustrate how these principles are effectively applied, showcasing the tangible benefits of adhering to best practices in protocol development.
In conclusion, the advancement of medical research hinges on the ability to create and implement effective clinical trial protocols. As the industry continues to evolve, researchers are encouraged to prioritize comprehensive protocol design that incorporates stakeholder input and innovative strategies. By doing so, they can enhance the quality of their studies, improve participant engagement, and ultimately drive forward the future of healthcare solutions. Embracing these practices not only fosters trust in medical research but also paves the way for groundbreaking discoveries that can change lives.
What is bioaccess® and its role in clinical trials for Medtech innovations?
bioaccess® is a strategic organization that enhances research protocols for Medtech innovations across Latin America, the Balkans, and Australia. It leverages rapid regulatory processes in these regions to achieve ethical approvals in just 4-6 weeks, thereby accelerating the journey to market for new medical technologies.
How does conducting research in Colombia benefit Medtech companies?
Research studies in Colombia offer cost reductions exceeding 30% compared to North America and Western Europe, and the IRB/EC and MoH (INVIMA) review process typically takes only 90-120 days. Additionally, there are R&D tax incentives, including a 100% tax deduction for investments in science and technology.
What is the significance of participant recruitment in clinical trials?
Participant recruitment is crucial as 35% of delays in research studies arise from insufficient recruitment. Colombia's diverse patient demographic and universal healthcare coverage contribute to effective recruitment and a high retention rate of 95% in research studies.
What are the key components of a clinical trial protocol?
Key components include: - Title: A concise title reflecting the study's focus. - Objectives: Specific aims using SMART criteria. - Study Design: Detailed description of the study type. - Eligibility Criteria: Clear inclusion and exclusion criteria for participant selection. - Outcome Measures: Defined primary and secondary endpoints. - Statistical Considerations: Methods for data analysis and sample size calculations. - Ethical Considerations: Compliance with ethical standards and participant safety measures.
Why is a well-designed clinical trial protocol important?
A carefully designed clinical trial protocol safeguards participant safety, enhances data integrity, and ensures regulatory compliance, which bolsters the credibility of research findings. Inadequate protocols are a major factor in research study failures.
How does bioaccess® improve the clinical trial process for Medtech firms?
Bioaccess® offers an accelerated regulatory approval process, achieving approvals in 6-8 weeks compared to the typical 6-12 months in the US/EU, and facilitates patient enrollment in cardiology and neurology cohorts 50% faster than Western sites, addressing common patient recruitment issues.
What is the projected trend for medical studies in Brazil?
Brazil is projected to lead Latin America with nearly 10,000 registered medical studies by 2025, highlighting the importance of regulatory speed in achieving successful outcomes in Medtech.