


The article titled "10 Essential Clinical Trial Services for Research Directors" underscores the vital services that empower research directors to conduct clinical trials with efficiency and effectiveness. It delineates ten key offerings, including:
These elements collectively enhance the speed and quality of clinical research, illustrating bioaccess®'s pivotal role as a partner in navigating the complexities of medical studies.
The landscape of clinical trials is rapidly evolving, driven by the need for faster, more efficient research that meets the demands of a growing global market. For research directors, understanding the essential services that can streamline processes and enhance study outcomes is crucial. This article delves into ten indispensable clinical trial services that not only accelerate research timelines but also ensure compliance, patient engagement, and data integrity. As the industry faces increasing complexity and regulatory challenges, how can directors navigate these waters to optimize their clinical studies and achieve successful results?
bioaccess® distinguishes itself in the research sphere by providing unparalleled global-first flexibility, empowering Medtech, Biopharma, and Radiopharma innovators to accelerate their studies. With ethical approvals achieved in an impressive 4-6 weeks and patient enrollment processes that are 50% faster than traditional markets, bioaccess® effectively bridges innovation with market readiness. This rapid pace is realized through the regulatory efficiency of Latin America, the diverse patient demographics in the Balkans, and streamlined pathways in Australia, making bioaccess® an essential ally for study directors focused on expediting medical evaluations. Furthermore, bioaccess® has activated over 50 pre-qualified locations in under 8 weeks, enhancing its Clinical Trial Services to support scientific investigation initiatives.
In collaboration with Caribbean Health Group, bioaccess® is transforming Barranquilla into a premier destination for medical research in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances the region's appeal for medical research but also demonstrates bioaccess®'s commitment to fostering local partnerships that drive success in research studies.
In a rapidly expanding market projected to reach USD 37.23 billion by 2025, the services offered by bioaccess® are increasingly vital. As Katherine Ruiz, a Compliance Affairs specialist, highlights, "Navigating the local legal environment can be intricate, but possessing local knowledge can significantly enhance the speed of obtaining permissions." This insight underscores the significance of bioaccess®'s expertise in facilitating swift regulatory processes. Moreover, case studies illustrate bioaccess®'s effective role in accelerating medical research, underscoring the tangible impact of their services on research outcomes.
bioaccess employs a comprehensive strategy for clinical study management, providing end-to-end solutions that encompass every phase of the process. Their extensive services include feasibility assessments, regulatory compliance, site management, and project oversight, which encompasses review and feedback on study documents, as well as setup, initiation, and approval. This comprehensive service model not only streamlines operations but also significantly enhances the quality and reliability of results.
Key features of their offerings include:
As research study management progresses, bioaccess remains at the forefront, adapting to current trends and ensuring that their clients benefit from the latest innovations in the field. Notably, their collaboration with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia exemplifies their capability to facilitate regulatory access and accelerate site activation in Latin America. Furthermore, their partnership with GlobalCare Clinical Trials has led to substantial enhancements in ambulatory services for research, achieving over a 50% decrease in recruitment duration and 95% retention rates. As Bina Ahmed, MD, FACC, emphasizes, "Timely release and distribution of precise outcomes from significant research studies is essential," reflecting the importance of their thorough approach.

bioaccess is recognized for its ability to manage intricate clinical research requirements, offering a comprehensive range of services tailored to the unique challenges of each project. Our expertise encompasses the entire process of advancing medical device evaluations, from feasibility assessments and site selection to regulatory compliance and project oversight, ensuring that all logistical elements are seamlessly integrated. This capability is particularly beneficial for project leaders overseeing trials that demand complex coordination and specialized knowledge.
The importance of a comprehensive approach in medical research cannot be overstated, as it directly impacts the success of studies. The increasing complexity of medical research, driven by personalized therapies and smaller patient populations, necessitates efficient oversight to ensure that the appropriate investigational devices are delivered to the correct sites at optimal times.
Moreover, Colombia offers competitive advantages for first-in-human studies, including cost efficiency, regulatory speed, high-quality healthcare, and robust patient recruitment strategies, supported by R&D tax incentives. Addressing challenges such as regulatory hurdles, competition, and recruitment issues is vital for the successful execution of clinical trials. As the research landscape evolves, bioaccess positions itself as a leader in providing comprehensive study management services that adeptly tackle these challenges, ensuring the success of medical device development initiatives.

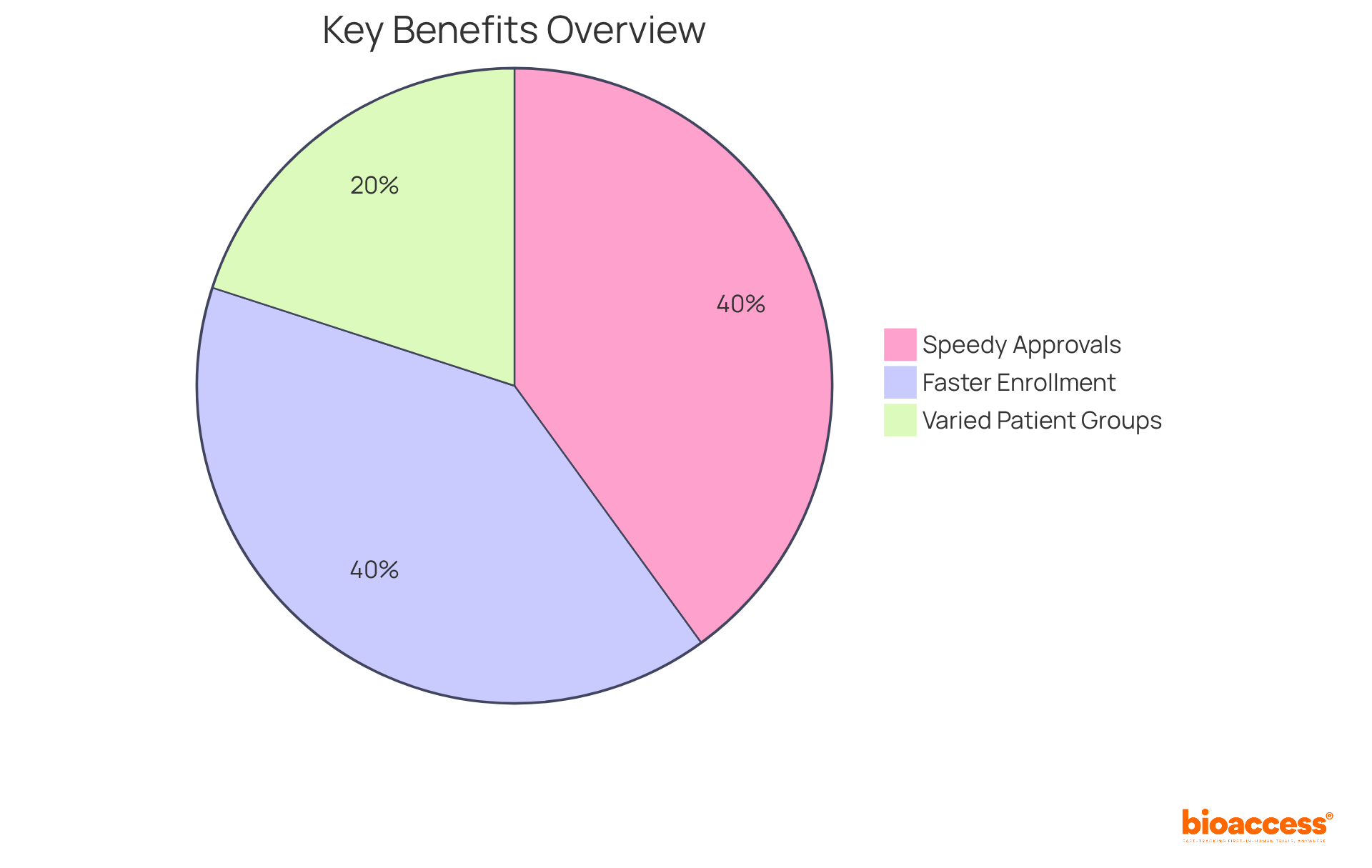
Bioaccess excels in providing Clinical Trial Services that prioritize strict adherence to Good Clinical Practice (GCP) guidelines. This unwavering commitment ensures that all trials not only comply with international standards but also safeguard participant safety and uphold data integrity. By concentrating on GCP adherence, bioaccess effectively reduces risks linked to non-compliance, thereby improving the credibility of study outcomes. This reliability establishes Clinical Trial Services as a dependable ally for study directors.
The importance of GCP compliance cannot be overstated; it directly impacts trial results and the overall success of medical studies. As we approach 2025, remaining informed about GCP guidelines is crucial for all parties involved in the medical study environment. bioaccess's proactive strategies for risk management involve identifying and mitigating compliance risks, thereby protecting the integrity of the investigation process. Continuous education and training for staff are integral to maintaining high compliance standards within clinical trial services, fostering a culture of excellence in clinical research. This comprehensive approach not only enhances compliance but also positions bioaccess as a leader in the field.
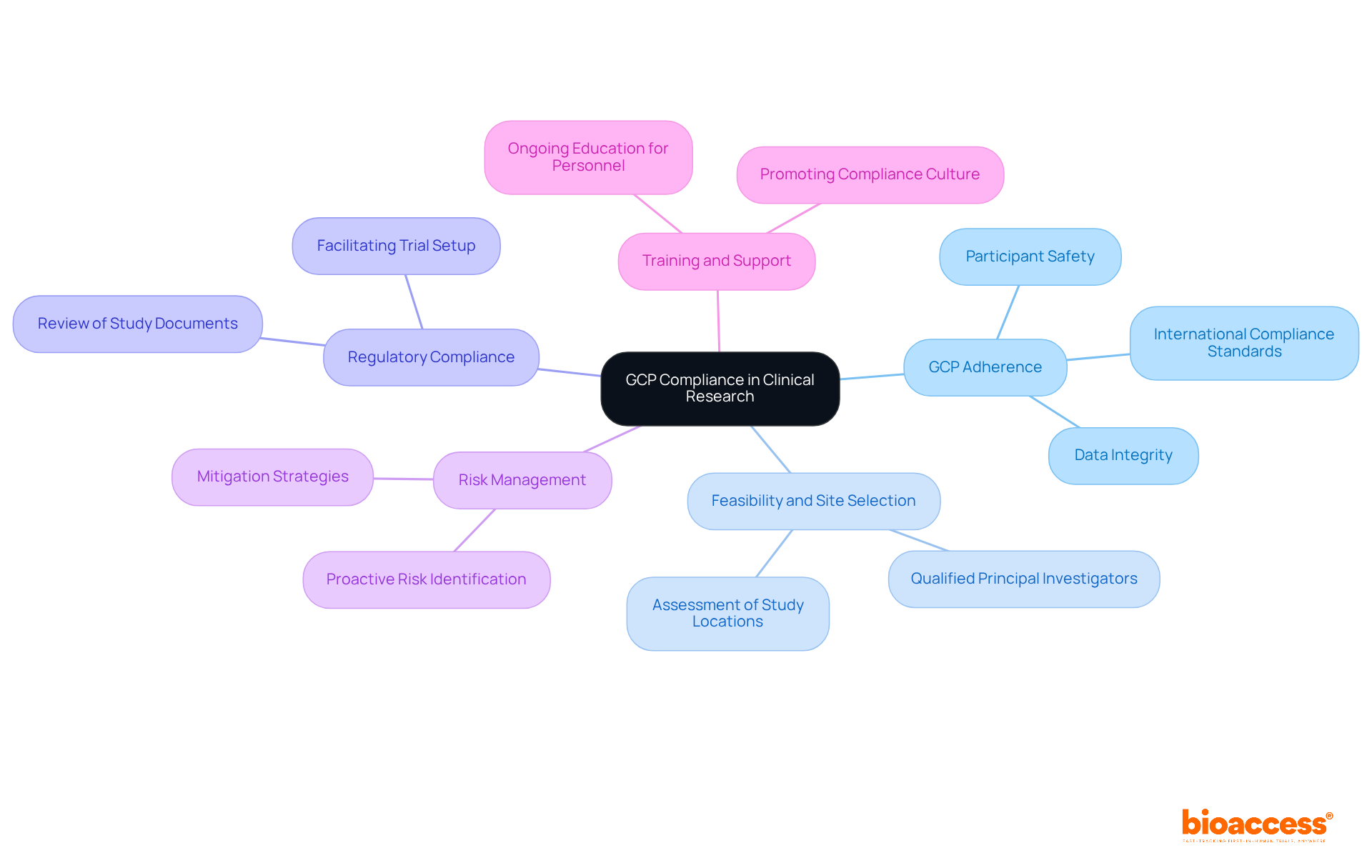
Clinical Trials Arena provides valuable insights into the latest advancements in patient-focused study designs. By concentrating on the needs and experiences of participants, these innovations aim to enhance recruitment, retention, and overall satisfaction. Research directors can leverage these insights to refine their study designs, ensuring they are not only scientifically robust but also aligned with patient expectations.
Accelerated Regulatory Approval: Bioaccess® employs a sprint approach that secures regulatory approval in just 6-8 weeks, significantly quicker than the typical 6-12 months observed in the US and EU. This expedited timeline facilitates faster patient enrollment, particularly in treatment-naive cardiology and neurology cohorts, which can be enrolled 50% faster than in Western sites.
Enhanced Engagement: Strategies to bolster participant involvement and satisfaction are supported by collaborations such as that of bioaccess™ and Caribbean Health Group, which aim to establish Barranquilla as a premier destination for medical studies in Latin America, with the backing of Colombia's Minister of Health.
Flexible Protocols: Adaptable study designs address patient needs, tackling challenges in site participation and patient eligibility, especially for Medtech and Biopharma startups.
Real-World Evidence: Integrating patient experiences into study outcomes is exemplified by initiatives like GlobalCare Clinical Trial Services collaborating with bioaccess™ to enhance research ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
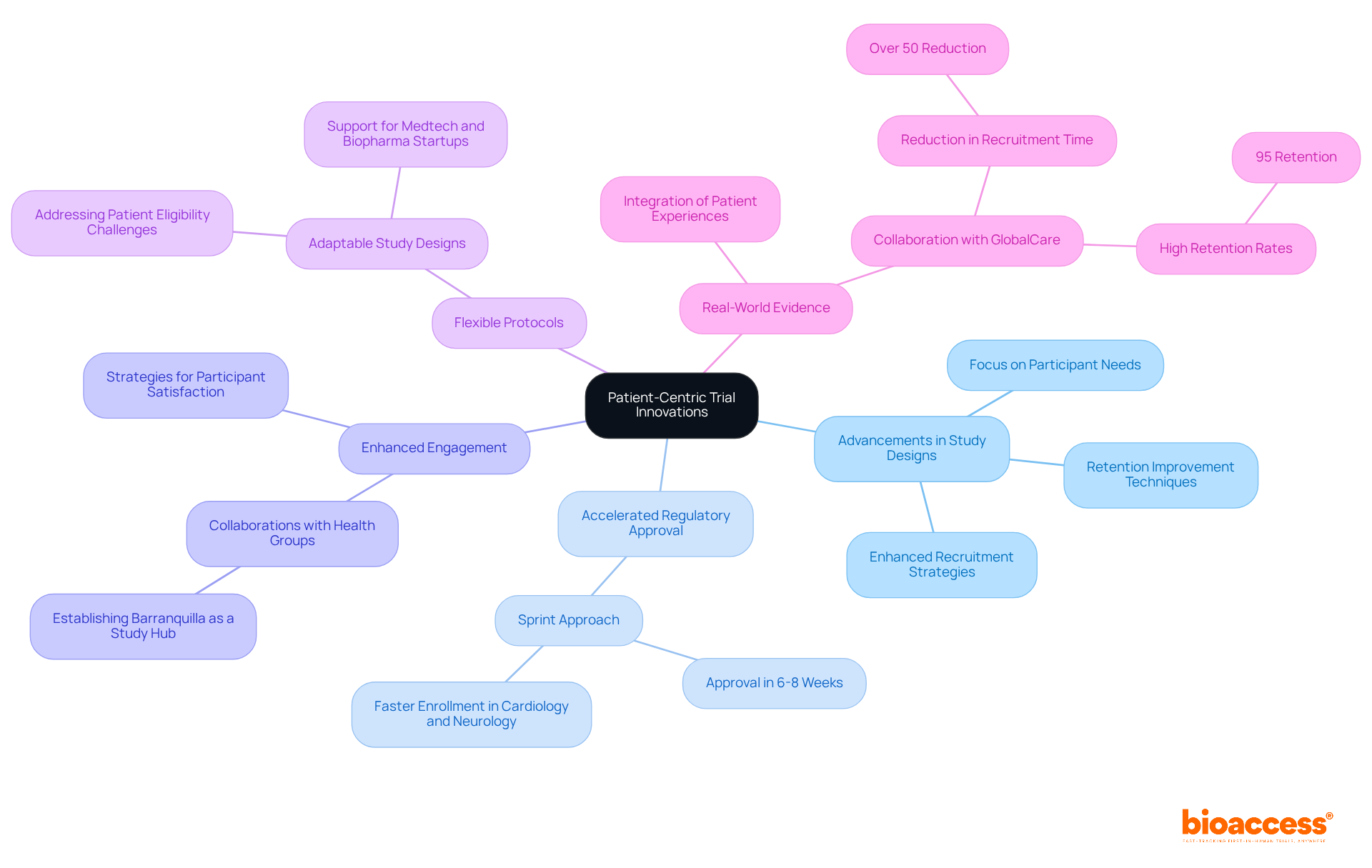
In the field of medical studies, access to extensive resources is essential for research directors. At bioaccess®, we leverage over 20 years of experience in Medtech to deliver a wealth of expertise in managing research studies, particularly in Latin America. Our focus includes:
Our dedicated team ensures that each test is organized efficiently, adheres to local regulations, and is tailored to meet the specific needs of our clients.
Our Clinical Trial Services encompass all aspects of clinical research, from feasibility studies and site selection to compliance reviews and trial setup.
Regulatory Expertise: With a profound understanding of INVIMA and its role as a Level 4 health authority, we adeptly navigate the complexities of medical device oversight and classification in Colombia.
Accelerated Study Services: Our commitment to expedited research study services facilitates faster routes to market, ensuring that advancements in medical technology reach those in need more swiftly.
Join bioaccess® in advancing medical technologies through innovation and quality in healthcare. Reach out to us today to discover how our expertise can enhance your medical study strategies.
Applied Clinical Trial Services Online serves as an indispensable resource for study directors intent on remaining abreast of the latest trends and advancements in medical studies. It offers professional perspectives and regulatory news critical for navigating the ever-evolving landscape of clinical trial services. Recent regulatory changes carry significant implications for testing operations, compelling research teams to adjust their strategies to uphold compliance and efficiency. For instance, the introduction of new guidelines mandates timely updates to research registration processes, directly impacting study timelines and data integrity.
Staying informed about these trends is not merely beneficial; it is crucial for sustaining a competitive edge within the industry. Experts emphasize that proactive engagement with regulatory updates can enhance the quality of Clinical Trial Services and patient safety. By leveraging practical strategies and insights from platforms like Applied Clinical Trials Online, project directors can adeptly manage these changes, ensuring their studies remain compliant and ultimately successful.
Versiti offers specialized Clinical Trial Services tailored to meet the unique requirements of hematological research. Their extensive expertise in blood disorders enables them to provide thorough assistance customized to the intricacies of these studies. Research directors can leverage Versiti's focused approach to ensure that their hematological investigations are executed with the utmost precision and care.
Current trends suggest an increasing focus on patient involvement in research studies, especially in hematology, where tailored methods can greatly influence results. Successful experiments have shown that customized protocols not only enhance patient compliance but also produce more dependable data. As the field of blood disorder research develops, Versiti remains at the leading edge, dedicated to promoting clinical trial services through expertise and a patient-centered approach.
The Food and Drug Administration (FDA) plays a critical role in offering regulatory guidance for clinical studies. Their comprehensive guidelines ensure that experiments are conducted ethically, prioritizing participant safety. Research leaders must remain knowledgeable about FDA regulations to ensure adherence and preserve the integrity of their studies. At bioaccess, we offer a wide range of Clinical Trial Services that include:
Our expertise, led by Katherine Ruiz—an authority in regulatory affairs for medical devices and in vitro diagnostics in Colombia—ensures that our clients navigate the complexities of regulatory requirements effectively.
Key regulatory aspects include:
Clinical study technology solutions are fundamentally transforming data management and analysis in clinical studies. By employing advanced technologies such as electronic data capture (EDC), cloud-based platforms, and artificial intelligence (AI), research directors can significantly enhance the efficiency and precision of their studies. These innovations simplify data management procedures and lead to improved overall results.
Recent advancements in EDC technology have further solidified its role in medical studies. For instance, the integration of AI capabilities within EDC systems has streamlined patient recruitment and improved trial design, resulting in faster and more reliable outcomes. A notable example is the EDCXtra platform, which enhances data collection processes while ensuring adherence to good practices in research. This system allows for real-time data entry and offers flexibility for sites that prefer traditional paper methods, ultimately improving the site experience and reducing administrative burdens.
Furthermore, bioaccess® provides expedited medical device research study services in Latin America, leveraging over 20 years of experience in overseeing various study types, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Follow-Up Studies. This specialized knowledge enables bioaccess® to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving significant cost savings of $25K per patient with FDA-ready data—no rework, no delays.
Experts in the field emphasize the importance of these technological advancements. As highlighted by industry leaders, the integration of EDC systems not only improves data accuracy but also empowers researchers to conduct studies with greater precision and efficiency. To maximize the benefits of these advancements, research directors should consider partnering with bioaccess® for their clinical trial services, ensuring access to cutting-edge technology and expert management that can drive successful outcomes.
The landscape of clinical trials is rapidly evolving, underscoring the critical role that effective clinical trial management plays in the success of research initiatives. Organizations like bioaccess® are setting new standards for conducting clinical trials, with a focus on speed, compliance, and patient-centric approaches, ensuring that innovations reach the market efficiently and safely.
Key insights reveal the importance of:
The collaboration between bioaccess® and various health organizations illustrates a commitment to enhancing the research environment, fostering local partnerships, and leveraging advanced technology to streamline processes. Moreover, the emphasis on regulatory expertise and adherence to Good Clinical Practice (GCP) guidelines positions these services as indispensable for research directors navigating the complexities of clinical trials.
As the demand for clinical trials continues to grow, it is essential for research directors to stay informed about the latest trends and advancements in the field. Engaging with innovative solutions and comprehensive services not only enhances the quality of research but also safeguards participant safety and data integrity. By embracing these essential clinical trial services, research leaders can significantly improve their study outcomes and contribute to the advancement of medical science.
What is bioaccess® and what services does it provide?
bioaccess® is a clinical trial management organization that offers global-first flexibility to accelerate studies for Medtech, Biopharma, and Radiopharma innovators. Their services include ethical approvals, patient enrollment, feasibility assessments, regulatory compliance, site management, and project oversight.
How quickly can bioaccess® achieve ethical approvals?
bioaccess® can achieve ethical approvals in an impressive 4-6 weeks.
How does bioaccess® improve patient enrollment processes?
Patient enrollment through bioaccess® is finalized 50% faster than in traditional markets, enhancing study efficiency.
What regions does bioaccess® operate in to facilitate clinical trials?
bioaccess® operates in Latin America, the Balkans, and Australia to leverage regulatory efficiency and diverse patient demographics.
What is the significance of bioaccess®'s partnership with Caribbean Health Group?
This partnership aims to transform Barranquilla into a premier destination for medical research in Latin America, enhancing the region's appeal and demonstrating bioaccess®'s commitment to local partnerships.
What are the key benefits of using bioaccess® for clinical trials?
The key benefits include speedy ethical approvals, faster patient enrollment, and access to varied patient demographics for thorough data gathering.
How does bioaccess® ensure regulatory compliance?
bioaccess® focuses on compliance with local and international regulations, facilitating the setup and approval processes necessary for medical device startups.
What is the role of project management in bioaccess®'s services?
Project management involves effective oversight of study status, monitoring adverse events, and managing recruitment issues, ensuring transparency and accountability throughout the study.
Why is a comprehensive approach important in clinical research?
A comprehensive approach enhances adaptability and increases the likelihood of success in studies, especially given the increasing complexity of medical research.
What advantages does Colombia offer for clinical trials?
Colombia provides cost efficiency, regulatory speed, high-quality healthcare, and robust patient recruitment strategies, making it an attractive location for first-in-human studies.