


In the complex realm of clinical research, the stakes are undeniably high, and the margin for error is razor-thin. Regulatory compliance stands as a cornerstone of ethical research practices, making meticulous audits absolutely essential. This article explores ten crucial clinical trial site audit checklists specifically designed for Bulgaria, providing insights that can significantly enhance the integrity and success of clinical trials.
What challenges do researchers encounter in ensuring compliance?
How can these checklists serve as a roadmap to navigate the intricate landscape of clinical trial audits?
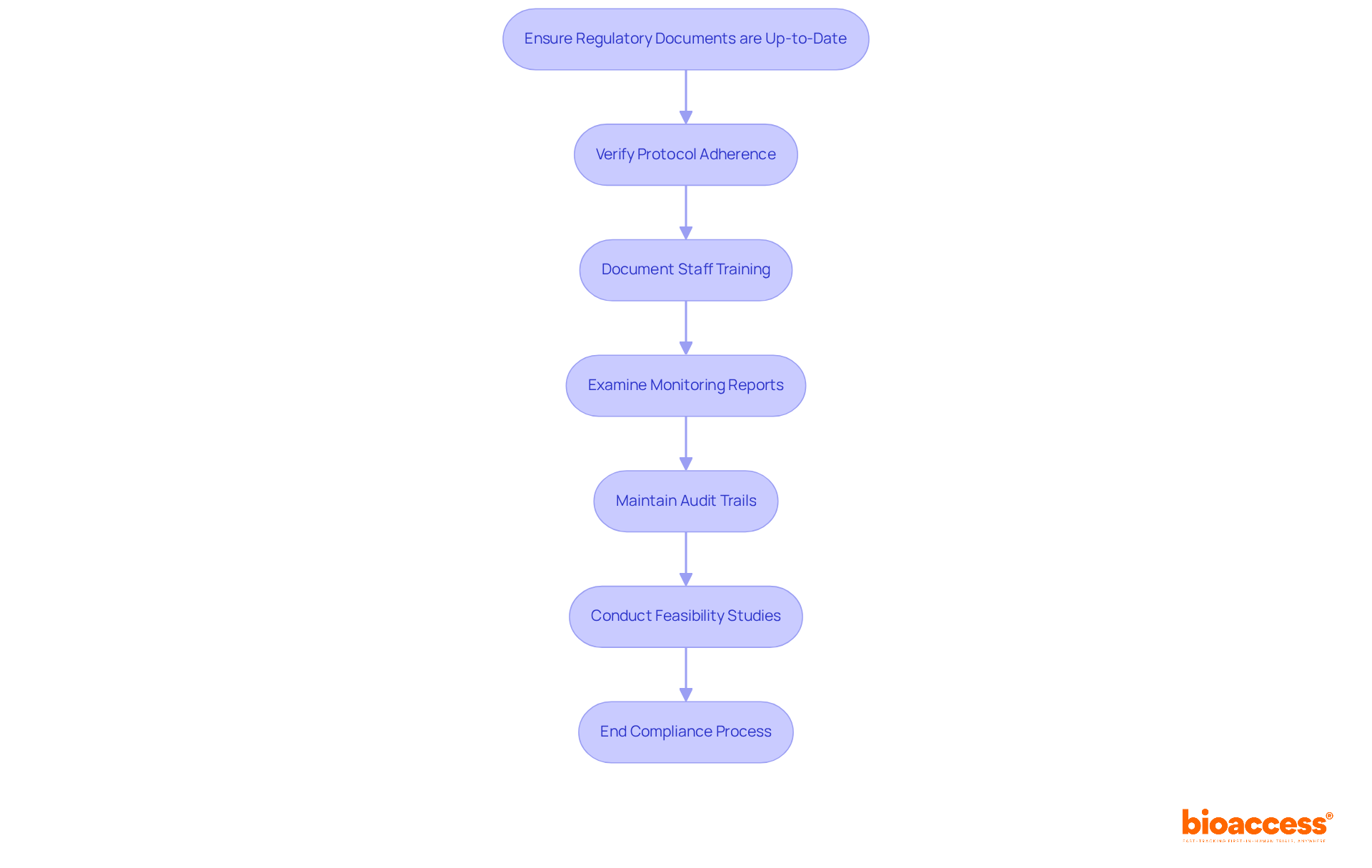
In clinical research, ensuring that all essential regulatory documents are present and up-to-date is paramount. This includes critical items such as the Investigator's Brochure and informed consent forms. Bioaccess provides comprehensive support in reviewing and offering feedback on study documents, ensuring compliance with country requirements and that all necessary regulatory documents are meticulously organized.
It is crucial to verify that experiments are conducted according to the approved protocol, including any amendments. Our services encompass the setup, start-up, and approval processes, which are vital for maintaining protocol adherence. By partnering with us, you can rest assured that your study will follow the established guidelines.
Documentation of training for all staff involved in the trial is essential to ensure their qualifications. Bioaccess emphasizes the significance of training and adherence, offering project management and monitoring services to guarantee that all personnel are adequately prepared for their roles in the study.
Monitoring reports must be examined to ensure adherence to Good Clinical Practice (GCP) guidelines. Our study project management services include thorough monitoring and reporting on study status, inventory, and adverse events, ensuring that GCP compliance is upheld throughout the research process.
It is also vital to ensure that tracking trails for data management systems are intact and accessible for review. Bioaccess's commitment to thorough reporting involves maintaining clear audit trails, which are crucial for compliance and transparency in clinical studies.
Furthermore, Bioaccess offers feasibility studies and site selection services, ensuring that the most appropriate research locations and principal investigators are chosen to enhance study results. By collaborating with us, you can navigate the complexities of clinical research with confidence.
Ensure all necessary documentation is in place, including the study protocol, informed consent forms, and regulatory approvals. These documents are foundational for compliance and must be readily accessible for review. Bioaccess provides extensive services that encompass feasibility studies, the selection of research locations and lead investigators, and support with import permits and nationalization of investigational devices. This guarantees that all required criteria are fulfilled for successful execution.
Assess the oversight of the testing location, concentrating on employee qualifications and training. Efficient site management is essential for upholding high standards and ensuring that all personnel are prepared to follow study protocols. Bioaccess's expertise in study setup and start-up processes, including obtaining ethics committee and health ministry approvals, supports this objective.
Implement robust processes to ensure information accuracy and integrity throughout the trial. Regular audits and checks, such as clinical trial site audit checklists in Bulgaria, are crucial to identify and correct any discrepancies, as absent information can significantly skew treatment comparisons. It is important to report confidence intervals alongside p-values to enhance the understanding of data interpretation and relevance.
Review the measures in place to protect participant safety and uphold ethical standards. This includes monitoring adverse events and ensuring that informed consent is obtained and documented properly. Bioaccess's project management and monitoring services are essential for ensuring participant safety during the study.
Following Good Clinical Practice (GCP) guidelines is essential for the credibility of clinical studies. Regular training and updates on GCP standards help ensure that all team members are aligned with current best practices, particularly with anticipated updates for 2024 that may refine existing protocols, as noted by Dr. Juan Cuya. Additionally, it is crucial to implement corrective actions following audits, particularly those outlined in clinical trial site audit checklists in Bulgaria, to maintain compliance and improve clinical study practices.
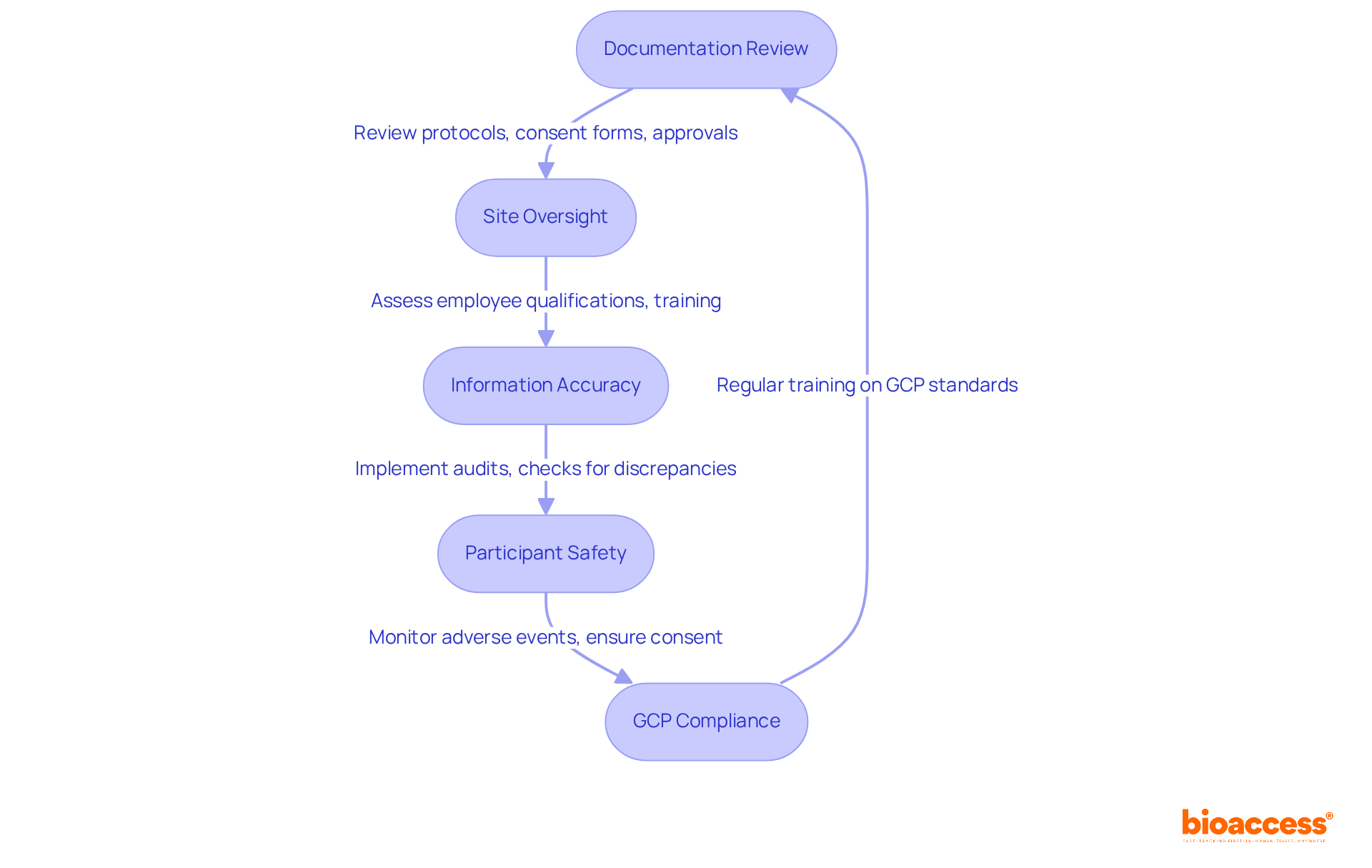
Adherence in clinical studies is not just important; it’s essential for protecting participant safety and maintaining the integrity of the research process. By following established regulations, trial sponsors safeguard the rights and welfare of participants, which is a cornerstone of ethical research practices. This commitment to adherence fosters trust among stakeholders and enhances the credibility of the research organization.
Moreover, maintaining information validity is crucial for compliance. Strict adherence to regulatory standards ensures that the data collected during trials is accurate and reliable, allowing researchers to draw significant conclusions about the safety and effectiveness of new treatments. For example, centralized monitoring has proven effective in identifying discrepancies and ensuring data integrity, as shown in various case studies.
Clinical research leaders emphasize the critical role of adherence in protecting participant safety. They warn that non-compliance can lead to serious legal repercussions, including hefty fines and potential litigation termination, jeopardizing the entire research effort. Additionally, adherence is vital for securing necessary approvals from regulatory bodies, facilitating the smooth progression of trials.
In conclusion, the impact of regulatory compliance on participant safety and data validity is profound. It serves as a foundational element that not only protects participants but also elevates the overall quality and credibility of clinical research.

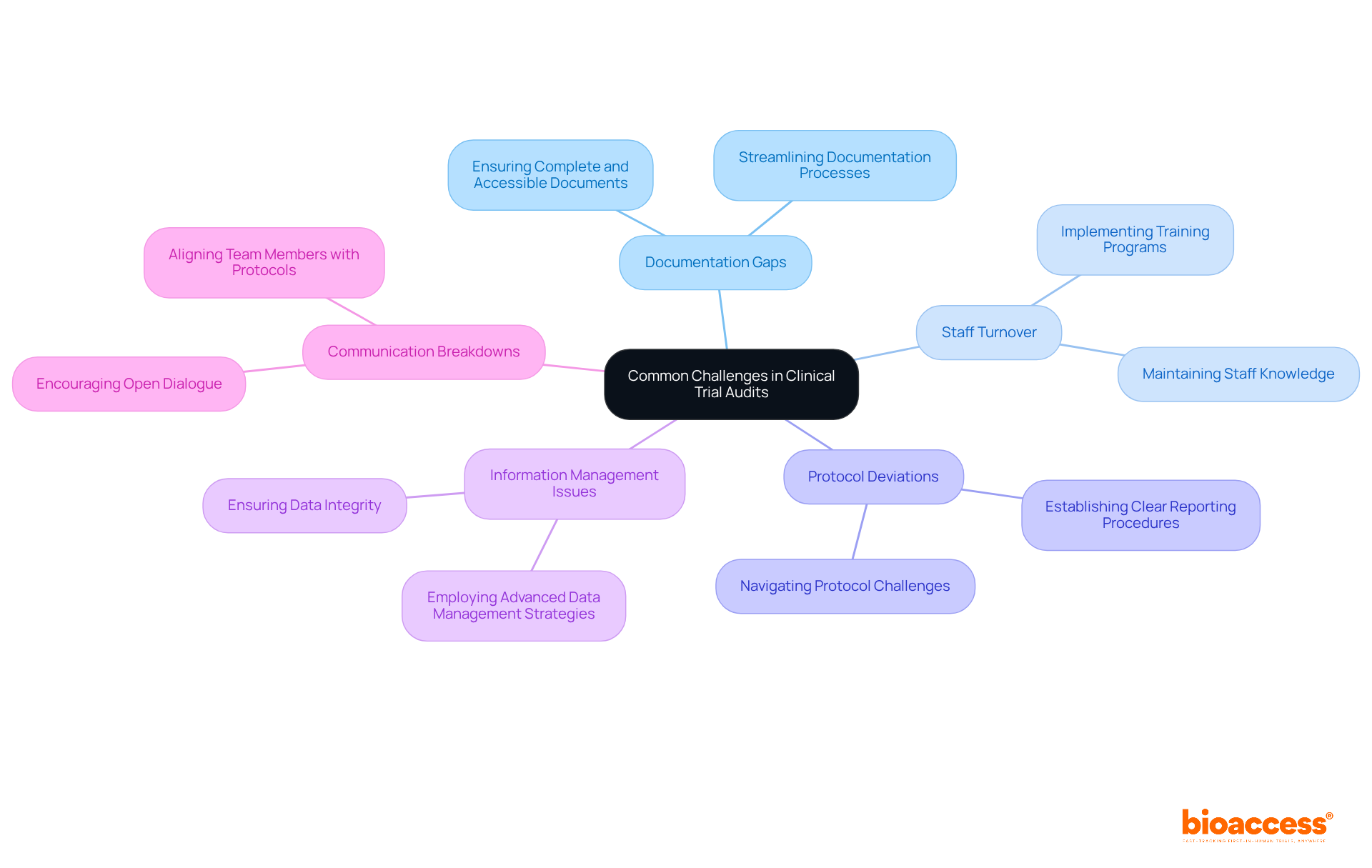
Documentation Gaps: To avoid delays during audits, it is crucial to ensure that all required documents are complete and easily accessible. Bioaccess's expertise in overseeing clinical studies, particularly in Early-Feasibility and First-In-Human research, plays a vital role in streamlining documentation processes.
Staff Turnover: Implementing training programs is essential for maintaining staff knowledge and ensuring continuity in the management of experiments. Bioaccess emphasizes the importance of skilled staff, ensuring that teams are well-versed in testing protocols and compliance requirements.
Protocol Deviations: Establishing clear procedures for reporting and managing deviations is key to minimizing their impact on the study. With Bioaccess's specialized knowledge, teams can adeptly navigate protocol challenges, ensuring adherence to regulatory standards.
Information Management Issues: Robust information management systems are necessary to ensure data integrity and facilitate easier evaluations. Bioaccess employs advanced data management strategies to uphold high standards of data quality throughout the trial process.
Communication Breakdowns: Encouraging open dialogue among team members is essential to keep everyone informed of assessment expectations and requirements. Bioaccess fosters a collaborative atmosphere, enhancing communication and ensuring that all team members are aligned with evaluation protocols.
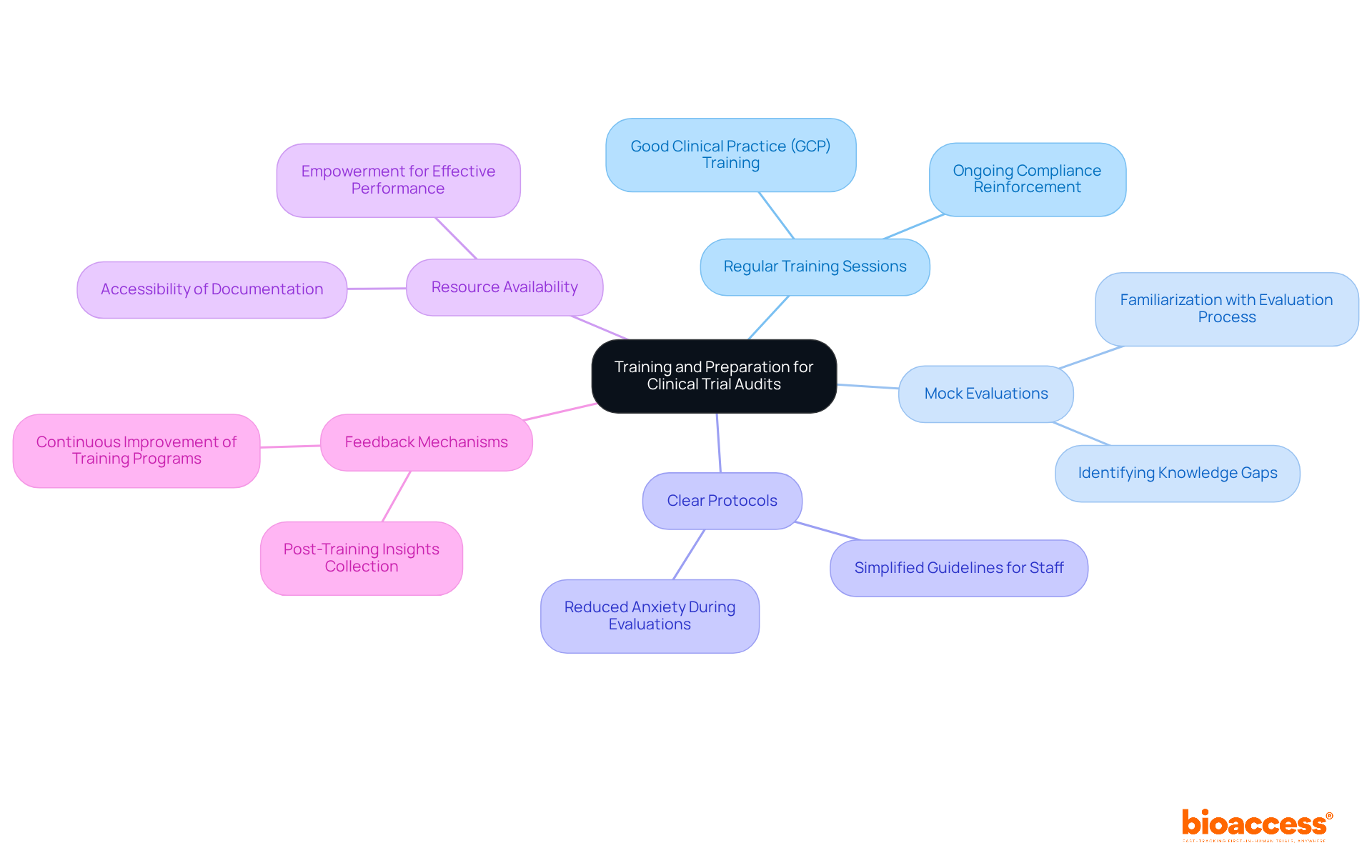
Regular Training Sessions: Ongoing training for all staff on Good Clinical Practice (GCP) is essential. This ensures that everyone is well-prepared and knowledgeable about the procedures that govern clinical research. By conducting these sessions regularly, we reinforce the importance of compliance and readiness in our team.
Mock Evaluations: Implementing mock evaluations serves a dual purpose: it familiarizes staff with the evaluation process and identifies areas that require enhancement. This proactive approach not only builds confidence among team members but also highlights potential gaps in knowledge or practice that can be addressed before actual evaluations take place.
Clear Protocols: Establishing straightforward guidelines for staff to follow during evaluations simplifies the process and alleviates anxiety. When staff members have a clear understanding of what is expected, they can perform their duties with greater assurance, leading to more accurate and effective evaluations.
Resource Availability: It is crucial to ensure that all necessary resources and documentation are readily accessible for examination. This accessibility empowers staff to perform their roles effectively, as they can quickly find the information they need to support their evaluations.
Feedback Mechanisms: Establishing robust feedback mechanisms post-training is vital for the continuous improvement of training effectiveness. By gathering insights from staff about their training experiences, we can refine our programs and ensure they meet the evolving needs of our team.
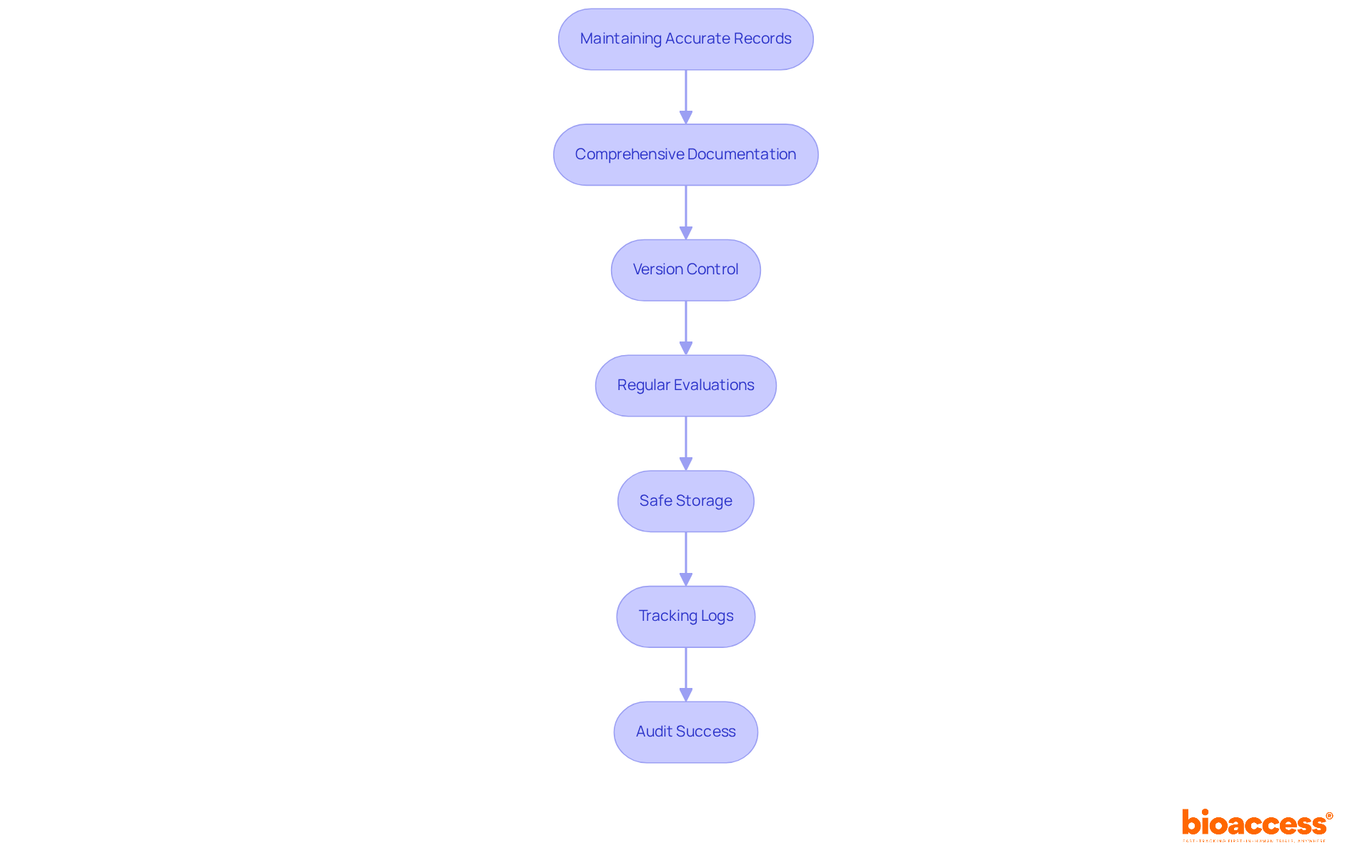
Keeping precise records is essential for success in clinical trials. Comprehensive documentation involves maintaining detailed records of all trial-related activities, including participant interactions and data collection. Implementing version control for all documents is crucial; it ensures that the most up-to-date versions are used during evaluations. This practice minimizes errors and enhances compliance with regulatory standards, allowing organizations to track modifications and revert to previous versions when necessary.
Regular evaluations of records should be performed to identify and correct any discrepancies before assessments take place. This proactive approach helps maintain the integrity of the data, ensuring that all documentation is accurate and complete. Safe storage of records is also vital; all documents must be kept securely and be easily accessible for review purposes.
Furthermore, preserving tracking logs for electronic records is essential. Audit trails track changes, capturing the identity of users making modifications, the time of changes, and the rationale behind them. This level of transparency protects information integrity and offers operational insights, aiding in the identification of inefficiencies and areas for enhancement. As specialists in the field highlight, efficient version control and thorough documentation practices are essential for attaining audit readiness and ensuring the success of clinical trial site audit checklists in Bulgaria.
Employing Electronic Data Capture (EDC) systems is essential for optimizing information collection and ensuring real-time access to research findings. This is particularly relevant within the framework of bioaccess's extensive clinical study management services. Their expertise in managing Early-Feasibility Studies, First-In-Human Studies, and other critical research highlights the importance of effective data management in today's Medtech landscape.
Utilizing audit management software allows for efficient monitoring of audit results and corrective measures, which is vital for maintaining regulatory standards in the demanding context of clinical studies. Furthermore, remote monitoring tools provide oversight and ensure adherence without the need for on-site visits, aligning with bioaccess's commitment to flexibility and specialized expertise in clinical research.
Data analysis plays a crucial role in identifying trends and potential issues before they escalate, thereby enhancing the quality of assessments overseen by bioaccess. Ultimately, leveraging online training platforms ensures that staff receive up-to-date training on compliance and readiness, reinforcing the high standards that bioaccess upholds in the clinical trial environment. This collaborative approach not only addresses key challenges but also positions bioaccess as a leader in clinical research.

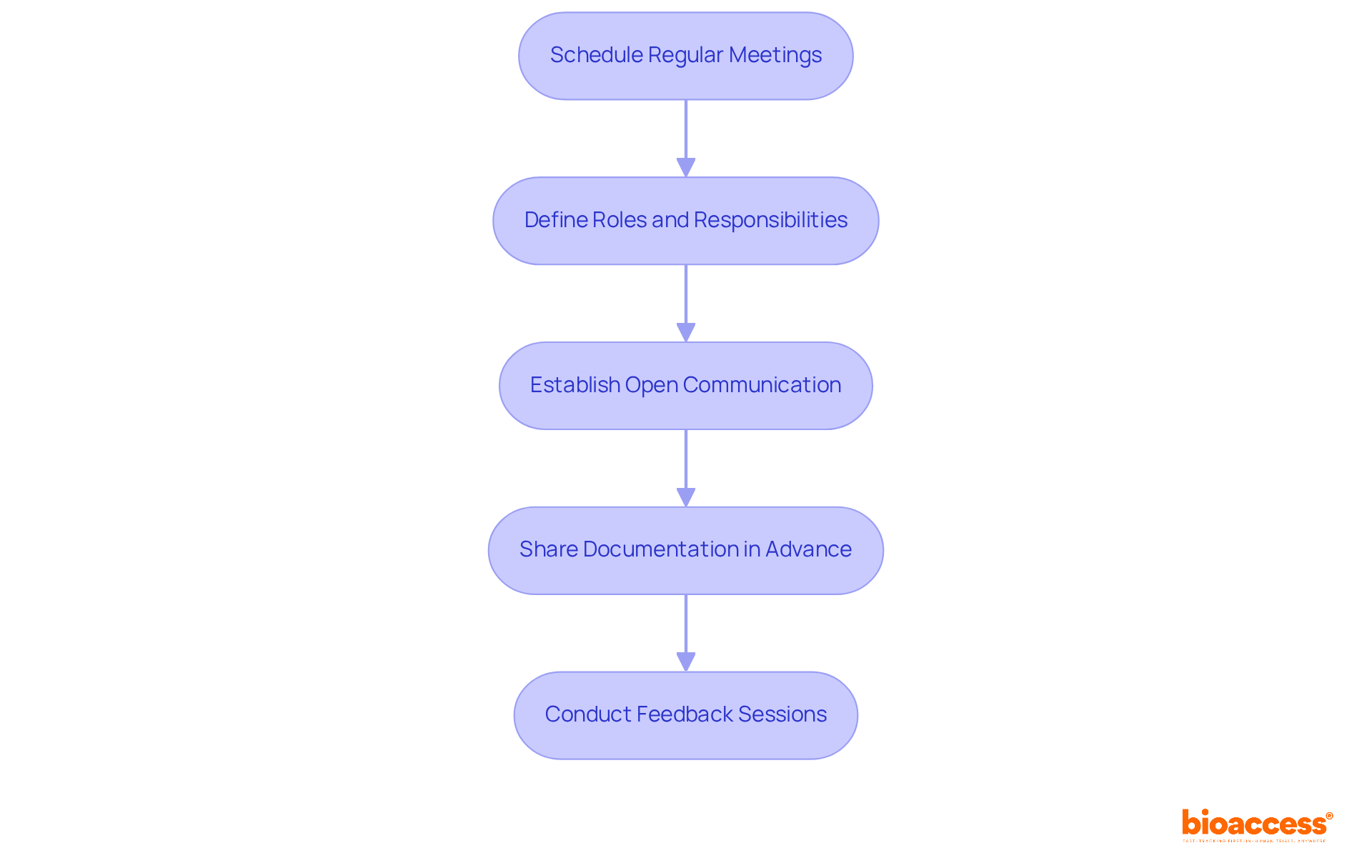
Regular meetings are essential; schedule them to discuss preparation and address any concerns among team members. Clearly defining roles and responsibilities for each team member during the evaluation process is crucial for ensuring accountability and efficiency. Establishing open channels of communication allows team members to ask questions and share vital information, fostering a collaborative environment. Ensure that all relevant documentation is shared with the team well in advance of the evaluation to facilitate thorough preparation. Finally, conduct feedback sessions after the review to discuss what went well and identify areas for enhancement in future evaluations.
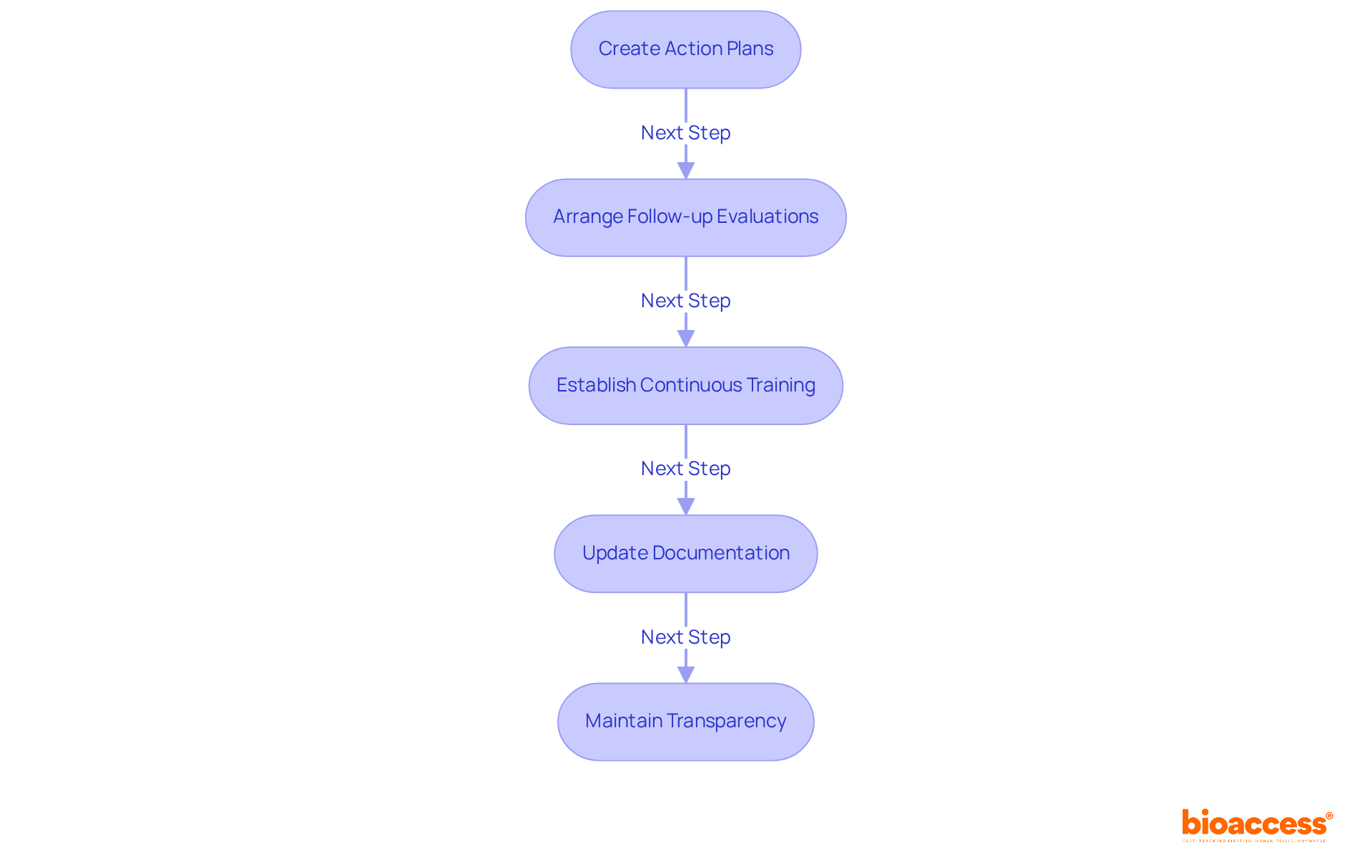
To effectively address evaluation results, it's crucial to create comprehensive action plans that detail specific measures for correcting identified problems. This proactive approach is essential for maintaining compliance and enhancing study integrity, particularly given that 5% of screen failures stem from inadequate follow-up actions. By leveraging bioaccess's pre-qualified networks, which include over 50 activated sites in less than eight weeks, you can significantly expedite this process.
Follow-up evaluations should be arranged to confirm the successful execution of corrective measures. Regular follow-ups are vital to ensure that improvements are sustained and that any new issues are promptly addressed. Delays in trial activation can severely impact accrual rates, making efficient follow-ups even more critical. With bioaccess's FDA/EMA/MDR-ready datasets and centralized monitoring, the efficiency of these follow-ups can be greatly enhanced.
Establishing continuous training initiatives for personnel based on evaluation results is another key step. Ongoing education helps prevent the reappearance of problems and fosters a culture of adherence and quality within the organization. Notably, participants in high-risk studies have 39 times the odds of being screen failures compared to those in low-risk studies, underscoring the need for targeted training. Bioaccess's expertise in patient recruitment can inform these training programs, ensuring that staff are well-equipped to handle diverse patient populations.
Updating documentation and protocols as necessary to reflect modifications made in response to review findings is essential. Keeping documentation current is critical for regulatory adherence and operational effectiveness, especially considering the median activation time of 167 days, which can influence study outcomes. Bioaccess's streamlined processes can assist in maintaining up-to-date documentation.
Finally, maintaining transparency by conveying assessment results and the measures taken to all pertinent stakeholders is vital. Effective communication fosters trust and collaboration, ensuring alignment with the corrective measures implemented. In an environment where low recruitment and retention rates can delay study completion, leveraging bioaccess's strong patient recruitment strategies can instill confidence in a more efficient and effective study process.
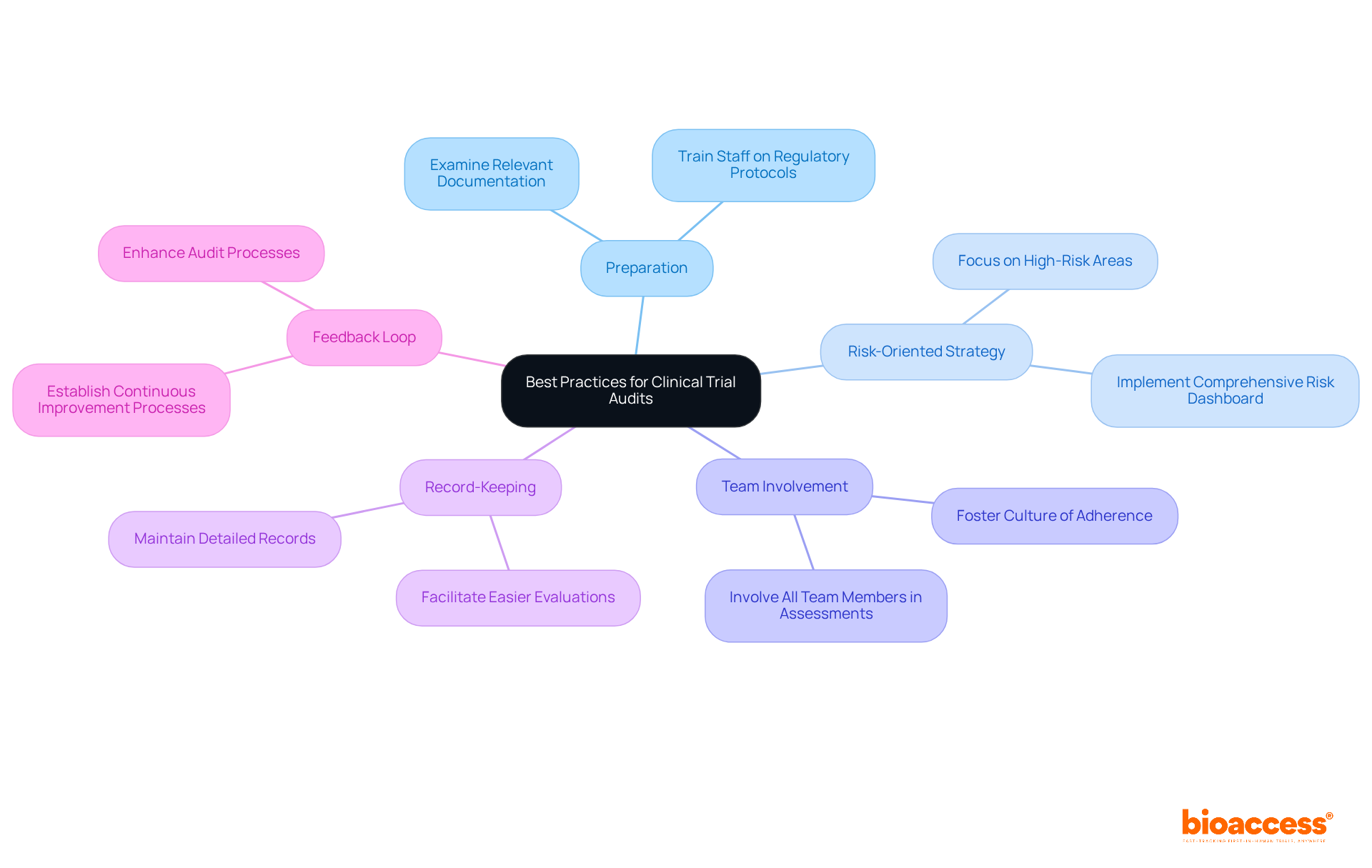
To ensure successful clinical evaluations, meticulous preparation is essential. This involves examining all relevant documentation and confirming that staff are well-trained in regulatory protocols. Implementing a risk-oriented strategy is crucial, focusing on areas with the highest likelihood of non-compliance. This approach aligns with bioaccess's commitment to comprehensive clinical study management services, including feasibility assessments and site selection.
Involving all team members in the assessment process fosters a culture of adherence and accountability, which is vital for maintaining high standards in clinical research. Furthermore, keeping detailed records throughout the study not only facilitates easier evaluations but also strengthens bioaccess's rigorous project management and reporting methods. Establishing a feedback loop is imperative for the continuous improvement of audit processes, ensuring that bioaccess remains a leader in clinical trial excellence.
Ensuring compliance during clinical trials is not just a regulatory requirement; it’s a critical element that protects participant safety and bolsters the integrity of research outcomes. The comprehensive audit checklists presented here are vital tools for clinical trial sites in Bulgaria, steering them through the intricate landscape of regulatory standards and best practices.
Key insights from this article underscore the necessity of:
By adhering to Good Clinical Practice (GCP) guidelines and fostering effective communication among team members, research organizations can significantly reduce common challenges faced during audits. Furthermore, utilizing technology, such as Electronic Data Capture systems, can streamline processes and enhance data integrity, thereby reinforcing compliance efforts.
In light of these discussions, it is crucial for clinical trial sites to prioritize adherence to regulatory standards and invest in training and resources that encourage ongoing improvement. This commitment not only safeguards the rights and welfare of participants but also enhances the credibility and success of clinical research overall. By embracing these best practices, organizations will be well-equipped to navigate the evolving landscape of clinical trials with confidence and integrity, ultimately leading to more reliable and impactful research outcomes.
What is the purpose of the bioaccess® Clinical Trial Audit Checklist?
The checklist ensures that all essential regulatory documents are present and up-to-date, helping to maintain compliance with country requirements and ensuring that experiments are conducted according to the approved protocol.
What types of documents are essential for compliance in clinical trials?
Essential documents include the study protocol, Investigator's Brochure, informed consent forms, and regulatory approvals.
How does Bioaccess support compliance in clinical trials?
Bioaccess provides comprehensive support in reviewing study documents, managing project oversight, ensuring staff training, and maintaining adherence to Good Clinical Practice (GCP) guidelines.
Why is training documentation important in clinical trials?
Training documentation is essential to verify that all staff involved in the trial are qualified and prepared for their roles, which is crucial for maintaining high standards during the study.
What is the significance of monitoring reports in clinical trials?
Monitoring reports help ensure adherence to GCP guidelines and provide thorough oversight of study status, inventory, and adverse events, which is vital for maintaining compliance throughout the research process.
What role do audit trails play in clinical trials?
Audit trails are crucial for compliance and transparency, ensuring that tracking trails for data management systems are intact and accessible for review.
What services does Bioaccess offer to enhance clinical trial execution?
Bioaccess offers feasibility studies, site selection services, support with import permits, and assistance with nationalization of investigational devices to ensure successful execution of clinical trials.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is essential for protecting participant safety, maintaining the integrity of the research process, and ensuring the credibility of the research organization.
What are the consequences of non-compliance in clinical trials?
Non-compliance can lead to serious legal repercussions, including fines and potential litigation, jeopardizing the entire research effort and affecting the ability to secure necessary approvals from regulatory bodies.
How can adherence to regulations affect data validity in clinical trials?
Strict adherence to regulatory standards ensures that data collected during trials is accurate and reliable, allowing researchers to draw significant conclusions about the safety and effectiveness of new treatments.