


The article primarily focuses on the critical importance of Case Report Forms (CRFs) in successful clinical research. It underscores their pivotal role in ensuring data quality, regulatory compliance, and efficient patient recruitment. By detailing how customized CRF designs, particularly in electronic formats, can significantly reduce errors, streamline data collection, and enhance participant engagement, the article makes a compelling case for their necessity. This ultimately contributes to more reliable and effective clinical trials, reinforcing the importance of adopting tailored approaches in the clinical research landscape.
The success of clinical research hinges on the effective collection and management of data, making Case Report Forms (CRFs) an indispensable tool in this process. As the landscape of clinical trials evolves rapidly, understanding the essential elements of CRFs can significantly enhance research outcomes. However, organizations striving for efficiency and compliance often encounter challenges that can undermine their efforts.
What are the critical CRF forms that can streamline clinical research success, and how can researchers avoid common pitfalls in their design and implementation?
bioaccess® creates customized CRF forms that are designed to meet the unique needs of each clinical study. This is crucial in the evolving landscape of clinical research, where tailored solutions are paramount for success. By utilizing extensive local regulatory expertise and understanding patient demographics, bioaccess® guarantees that CRF forms are compliant and optimized for swift information collection. This tactical method not only accelerates patient enrollment but also facilitates quicker trial commencement and data gathering.
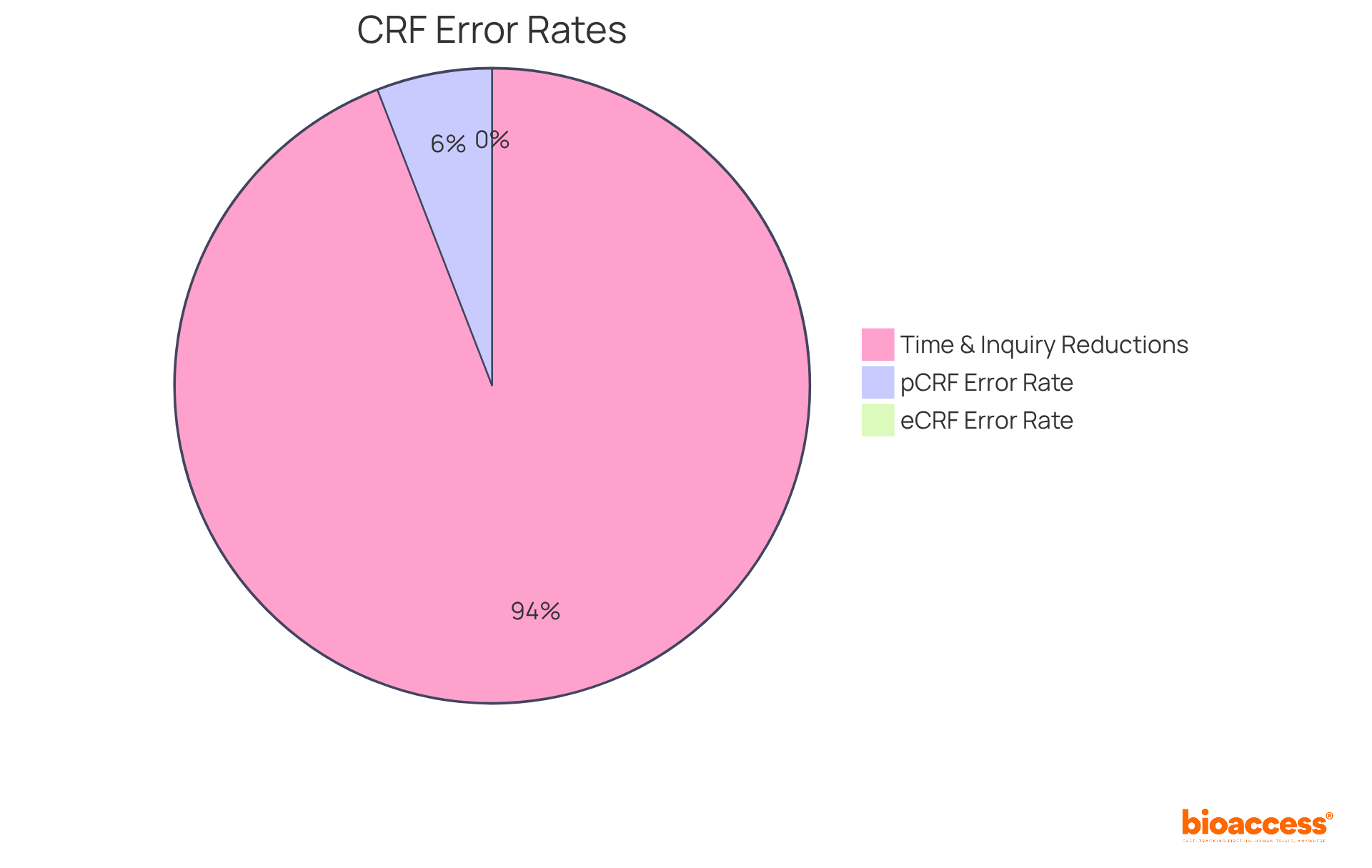
A well-organized CRF forms significantly enhance information quality and support faster decision-making in medical research. Recent trends reveal that organizations employing CRF forms see a notable reduction in entry mistakes, with electronic CRF forms achieving a 0% error rate compared to 5% for traditional paper formats. Case studies demonstrate that reorganizing CRF forms can result in a 30% reduction in entry time and a 50% decline in inquiries. These findings underscore the effectiveness of bioaccess®'s approach in optimizing research processes.
Moreover, the collaboration between bioaccess® and Caribbean Health Group, supported by Colombia's Minister of Health, positions Barranquilla as a key hub for medical studies in Latin America. This partnership enhances the overall environment for health research, showcasing the importance of strategic alliances in advancing clinical trials.
CRF forms are essential in clinical studies, ensuring consistent information gathering across various locations. This standardization is crucial for maintaining compliance with regulatory bodies, facilitating easier data comparison and analysis. The Clinical Research Management Specialist observes that employing standardized CRF forms significantly decreases the chances of mistakes and discrepancies, ultimately leading to more dependable study results. Furthermore, adherence to established guidelines is vital for meeting the stringent requirements set forth by regulatory agencies.
At Bioaccess, we offer extensive research study management services, including:
These services are designed to assist in the effective execution of CRF forms, ensuring that your trials are conducted efficiently and in complete adherence to regulatory standards. By collaborating with us, you can navigate the complexities of clinical research with confidence.

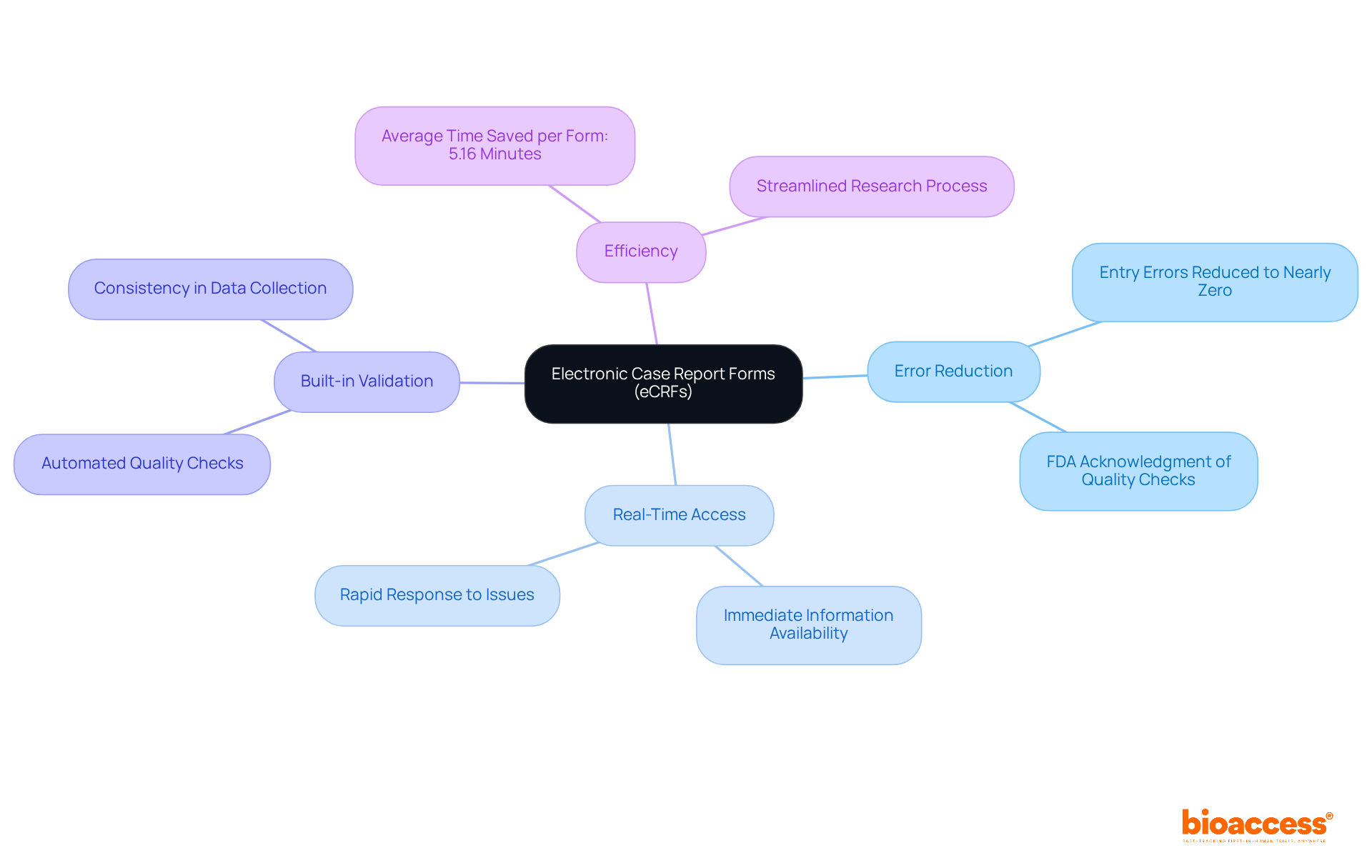
CRF forms, particularly Electronic Case Report Forms (eCRFs), represent a significant advancement in clinical information management. Unlike traditional paper forms, crf forms enable real-time information entry and validation, effectively minimizing the risk of transcription errors and enhancing data integrity. The FDA acknowledges in their guidance that the incorporation of prompts, flags, and quality checks in eCRFs substantially reduces mistakes and omissions during data entry. Research indicates that crf forms can reduce entry errors to nearly zero, which is a stark contrast to the typical 5% error rate associated with paper forms.
The advantages of eCRFs extend beyond merely reducing errors; they provide immediate access to information for oversight and evaluation, enabling rapid responses to any emerging issues during studies. Furthermore, eCRFs can incorporate built-in validation checks, ensuring that data is collected accurately and consistently throughout the study. This feature not only enhances the quality of the information but also streamlines the overall research process, positioning eCRFs as an indispensable asset for modern clinical studies. For example, estimates suggest that eCRFs can save an average of 5.16 minutes per crf forms, highlighting their efficiency in practical applications.
A successful study requires that CRF forms include several essential components to support thorough information gathering and improve study results. The inclusion of participant demographics is crucial for understanding the diversity of the population being studied. This information assists in guaranteeing that study outcomes are relevant across various demographic groups, addressing the FDA's recent focus on diversity in clinical studies. For instance, in 2020, only 8% of participants in new medication studies were Black, 6% were Asian, and 11% were Hispanic, highlighting the need for diverse representation.
Additionally, a comprehensive medical history offers crucial context for understanding study results. It allows researchers to identify potential confounding factors that could influence the efficacy and safety of the intervention. Historical distrust, exemplified by the Tuskegee Syphilis Study, emphasizes the significance of establishing confidence and ensuring varied involvement in medical studies.
Moreover, detailed documentation of trial procedure data is necessary to maintain protocol integrity and ensure the reproducibility of results. Capturing both primary outcomes and any adverse events is vital for assessing the treatment's safety and efficacy. This information is essential for regulatory submissions and for guiding future clinical practice.
Furthermore, ensuring adherence to protocol is crucial for preserving the integrity of the experiment. Gathering all necessary information points according to the study design not only enhances information quality but also aligns with regulatory expectations.
Authorities in Clinical Research Management, such as Ajay Singh, stress that integrating these components into CRF forms design is essential for obtaining high-quality information and successful study results. By prioritizing extensive information gathering, researchers can better comprehend the influence of their interventions and improve the overall efficiency of trials.
Actionable Tip: To effectively incorporate demographic information into CRF design, consider utilizing community engagement strategies to build trust and encourage participation from underrepresented groups.

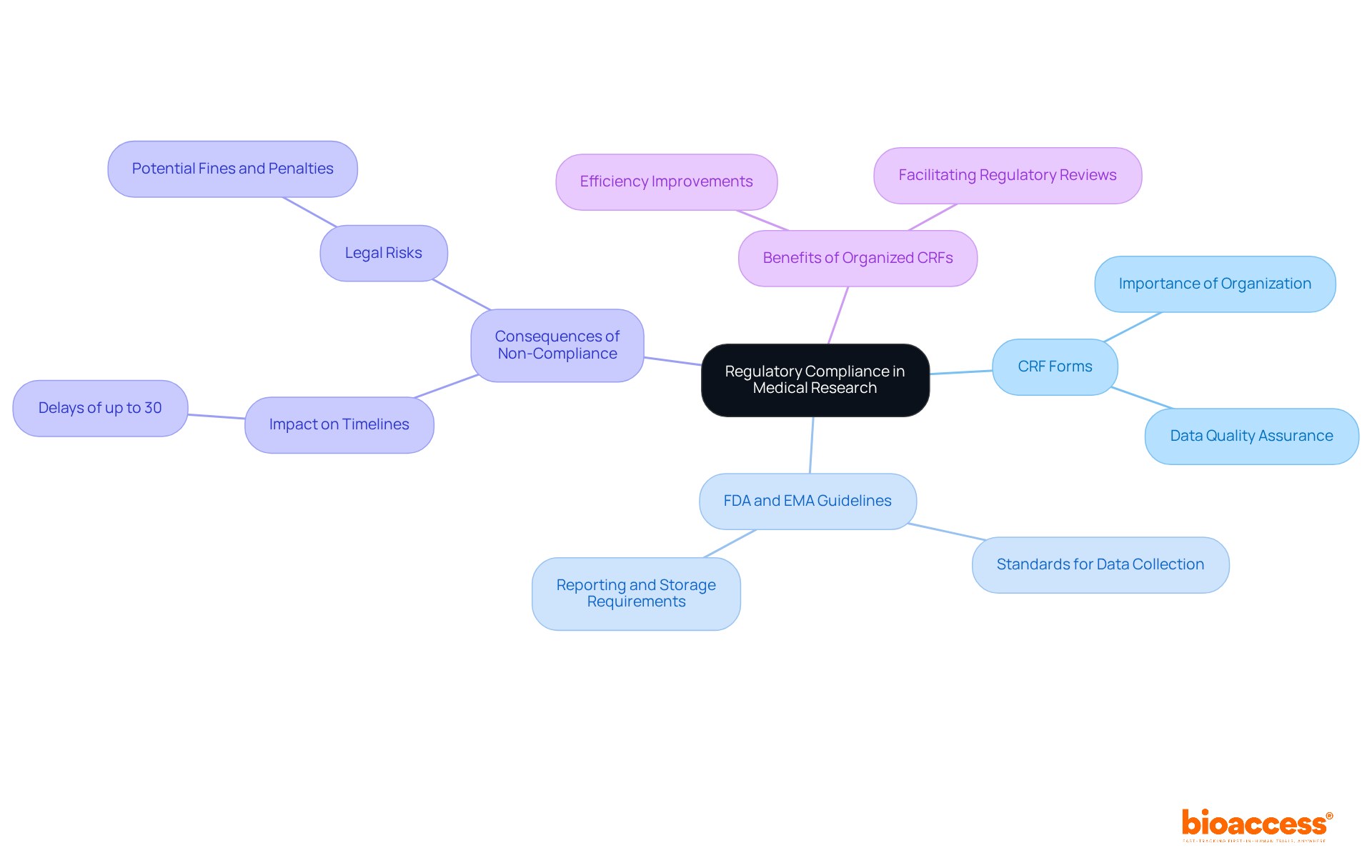
Regulatory compliance is paramount to the success of medical research, with crf forms playing a crucial role in this process. Adhering to guidelines established by the FDA and EMA is essential, as these organizations outline the standards for data collection, reporting, and storage. Non-compliance can significantly impact clinical study timelines, potentially delaying approvals by several months and exposing organizations to legal risks. Research indicates that non-compliance can lead to delays of up to 30%, underscoring the necessity for crf forms to be meticulously crafted in order to meet all regulatory standards. This diligence not only safeguards the integrity of the research but also enhances the likelihood of successful outcomes.
Specialists in the domain emphasize that well-organized crf forms are vital for maintaining compliance and ensuring accurate information capture and reporting, ultimately facilitating smoother regulatory reviews and approvals. Furthermore, bioaccess®'s extensive management services for research empower organizations to tackle these regulatory challenges with increased efficiency. The cost-to-speed ratio of bioaccess®'s FDA-ready information demonstrates significant savings, enabling earlier submissions and reduced patient recruitment expenses, thereby enhancing the overall efficiency of clinical trials.
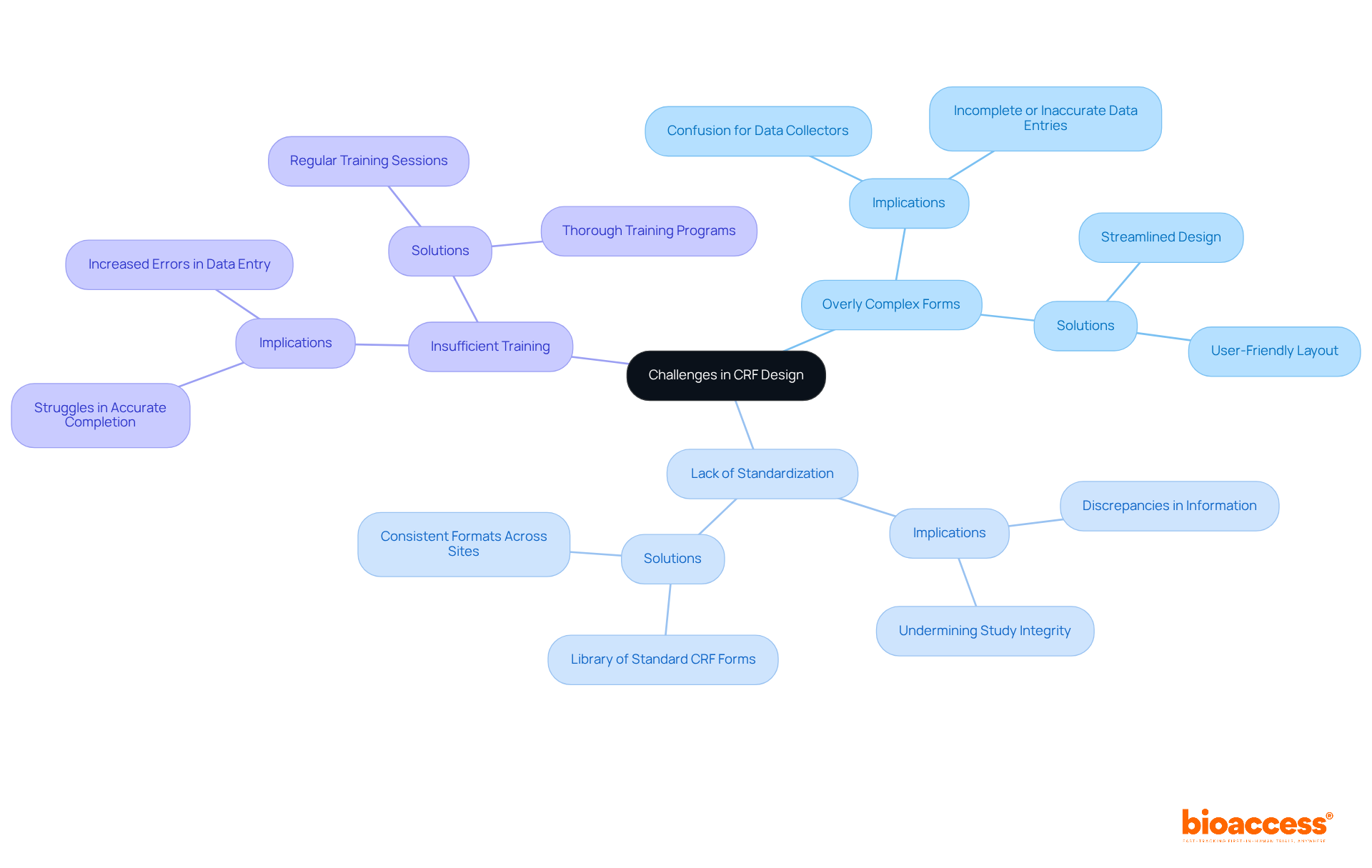
Creating CRF forms presents multiple challenges that, if ignored, can greatly undermine the quality of information. Key pitfalls include:
Tackling these challenges proactively can result in more dependable information gathering and ultimately improve the success of clinical research initiatives. Developing guidelines for CRF forms completion to assist investigators can also be a valuable step in mitigating these issues.
To ensure high-quality data collection through CRF forms, it is essential to adhere to several best practices.
Clear instructions: Providing detailed guidelines for completing each section of the CRF forms minimizes confusion and errors, establishing a strong foundation for accurate data collection.
Regular audits: Periodic reviews of completed CRF forms are crucial for identifying and rectifying errors. Research indicates that regular audits significantly enhance information quality; studies show that 95% of clinical trial sites implemented at least one procedure to ensure this quality.
Training Sessions: Continuous training for personnel engaged in information entry reinforces best practices and decreases entry mistakes. One study indicates that 71.1% of alterations in electronic case report forms (eCRFs) were due to entry errors. The Clinical Research Management Specialist emphasizes that following these practices, particularly the implementation of CRF forms, greatly improves the dependability and precision of the information gathered, ultimately leading to more substantial study results. Lauren Houston underscores that inadequate information quality can lead to erroneous conclusions and suggestions, highlighting the vital significance of information quality management in research trials.
By fostering a culture of ongoing enhancement through education and evaluations, research teams can ensure that their information gathering methods are both efficient and effective. Furthermore, it is essential to recognize that the average time spent on training is approximately 11.58 hours per person over 12 months, reflecting the commitment required for effective training sessions. Sherry Minor advises defining the 'W's (What, Who, Where, When) for TMF planning, which can enhance the structured approach needed in training and audits.
Data validation is a critical component of the clinical research process, ensuring that the information gathered through CRF forms is both accurate and reliable. Key validation techniques include:
The importance of information integrity in CRF forms cannot be overstated. Comprehensive validation procedures not only enhance the quality of the information collected in CRF forms but also facilitate more efficient regulatory reviews and approvals. Furthermore, the integration of automated checks has proven effective in safeguarding information integrity. Research indicates that studies employing these techniques experience fewer discrepancies, thereby enhancing the reliability of their results. As emphasized by industry specialists, preserving information integrity is essential for the success of research studies, as it directly impacts patient safety and the trustworthiness of research outcomes.
Moreover, the incorporation of machine learning models at various stages of the pipeline can identify anomalies in real-time, further refining the validation process. As Gregory L Ginn notes, the analysis of medical research information is crucial for discerning the true effects of therapies and differentiating these effects from random variations. Additionally, standardization during the design phase of CRF forms is key to ensuring uniformity and minimizing data entry errors, thus strengthening overall validation practices.
The design of CRF forms can significantly influence patient recruitment efforts in clinical trials. By utilizing strategies to enhance recruitment through CRFs, we can optimize the experience for participants:
Experts in Clinical Research Management emphasize that prioritizing patient-centric design in CRF forms not only fosters better engagement but can also lead to improved enrollment rates by up to 25%. Eli Chachak observes that effective CRF design emphasizes not just the information being collected but also the experience of the individual supplying that information. Considering that roughly 80% of trials are postponed or terminated because of recruitment challenges, the design of efficient CRF forms is essential for the success of research initiatives.
As clinical research continues to evolve, several key trends are shaping the design of Case Report Forms (CRFs):
Increased Use of Technology: The integration of artificial intelligence (AI) and machine learning (ML) is revolutionizing CRF design, significantly enhancing data collection and analysis efficiency. Studies indicate that the use of electronic case report forms (eCRFs) can reduce data entry time by up to 23% compared to traditional paper forms (pCRFs), showcasing the impact of technology on operational efficiency.
Focus on Patient-Centric Design: There is a growing emphasis on creating user-friendly interfaces that prioritize the participant experience. This shift is crucial, as recruitment challenges account for approximately 30% of clinical trial failures. By designing CRF forms that are intuitive and accessible, researchers can enhance participant engagement and retention.
Ongoing initiatives aim to standardize CRF forms across studies, which facilitates better data sharing and collaboration among researchers. The Clinical Research Management Specialist emphasizes that adjusting to these trends is crucial for enhancing trials and fulfilling regulatory requirements.
The evolution of CRF forms reflects a broader commitment to enhancing the efficiency and effectiveness of clinical research, ultimately leading to improved patient outcomes.
The significance of Case Report Forms (CRFs) in clinical research is paramount, serving as the backbone for data collection, compliance, and overall study success. By implementing tailored and standardized CRF designs, researchers enhance data quality, streamline information gathering, and ultimately improve clinical trial outcomes. The evolution towards electronic CRFs amplifies these benefits, allowing for real-time data entry, reduced error rates, and increased accessibility.
Key insights highlighted throughout the article include:
Effective CRF design strategies, such as user-friendly interfaces and regular training for data collectors, are essential to mitigate common pitfalls and enhance the reliability of the information gathered. The collaboration between organizations like bioaccess® and their commitment to optimizing CRF forms exemplifies the proactive measures needed to navigate the complexities of clinical trials.
Looking ahead, the integration of technology and a focus on patient-centric design will continue to shape the landscape of CRF development. Researchers are encouraged to adopt these innovations and best practices, as they not only facilitate compliance and data integrity but also foster greater participant engagement. By prioritizing these elements, clinical research can progress towards more efficient trials that ultimately lead to improved patient outcomes and advancements in medical science.
What are bioaccess® Case Report Forms (CRFs) designed for?
bioaccess® Case Report Forms are customized to meet the unique needs of each clinical study, ensuring compliance and optimized information collection.
How do bioaccess® CRFs impact clinical research efficiency?
They accelerate patient enrollment, facilitate quicker trial commencement, and enhance data gathering, leading to improved decision-making in medical research.
What advantages do electronic CRFs (eCRFs) offer over traditional paper forms?
eCRFs minimize transcription errors, enhance data integrity, and provide real-time information access, which reduces entry errors to nearly zero compared to a 5% error rate for paper forms.
How do standardized CRF forms contribute to clinical studies?
Standardized CRF forms ensure consistent information gathering across various locations, which helps maintain compliance with regulatory bodies and facilitates easier data comparison and analysis.
What are some services offered by bioaccess® related to CRF forms?
Bioaccess® offers services including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting to assist in the effective execution of CRF forms.
What is the significance of the collaboration between bioaccess® and Caribbean Health Group?
This collaboration, supported by Colombia's Minister of Health, positions Barranquilla as a key hub for medical studies in Latin America, enhancing the environment for health research.
What impact can reorganizing CRF forms have on data entry?
Reorganizing CRF forms can result in a 30% reduction in entry time and a 50% decline in inquiries, demonstrating the effectiveness of bioaccess®'s approach in optimizing research processes.
What are some features of eCRFs that enhance data quality?
eCRFs incorporate built-in validation checks, prompts, and flags that help ensure accurate and consistent data collection throughout the study.