


In the complex realm of clinical research, the importance of ethics board submission templates in Serbia stands out more than ever. These templates are essential tools for researchers navigating the intricate landscape of compliance, significantly enhancing the integrity and credibility of their studies. With anticipated changes in the approval process set for 2025, a pressing question arises: how can researchers effectively leverage these templates to secure timely approvals while upholding the highest ethical standards? This article explores ten crucial ethics board submission templates designed to streamline the approval process and reinforce the moral responsibilities inherent in medical research.
The bioaccess® ethics board submission templates in Serbia serve as an essential resource, meticulously crafted to meet the specific requirements of Serbian ethics committees. It includes vital elements such as:
By leveraging this template, researchers can prepare all necessary documentation ahead of time, significantly reducing the risk of delays due to incomplete submissions. This proactive approach not only streamlines the approval process but also enhances the overall quality of the submission, fostering a more efficient and effective review by ethics committees.
As Serbia's ethics committee approval process evolves, particularly with anticipated updates in 2025, the importance of utilizing ethics board submission templates in Serbia cannot be overstated. They are crucial for ensuring compliance and expediting approvals in clinical studies. In a landscape where timely and thorough submissions are paramount, the bioaccess® template emerges as a key player in navigating the complexities of clinical research.

The template provided by the Serbian Journal of Medical Society outlines essential instructions for authors to effectively address moral issues in their studies, utilizing ethics board submission templates in Serbia. It highlights critical aspects such as:
Recognizing the complexities surrounding informed consent is vital, particularly in emergency research scenarios where obtaining consent may not be feasible. Fundamental moral guidelines, including the Nuremberg Code and the Declaration of Helsinki, serve as crucial references for researchers, ensuring their investigations align with established ethical frameworks. By adhering to these guidelines, researchers can affirm that their studies are not only scientifically rigorous but also ethically sound, thereby enhancing the credibility and integrity of their work.
Moreover, it is imperative to underscore the moral responsibility to protect vulnerable groups, which may necessitate surrogate consent in instances where individuals are unable to provide informed consent. This approach is consistent with current ethical standards in Serbian medical research, which prioritize participant welfare and the responsible management of sensitive information, as specified in the ethics board submission templates in Serbia.

The Cromos Pharma submission template serves as a vital tool for researchers, urging them to spotlight Serbia's remarkable strengths, especially its swift patient recruitment capabilities and favorable regulatory environment. In 2025, investigators achieved a significant milestone by screening 18 patients in just four weeks, showcasing the efficiency of the patient enrollment process. Furthermore, Serbia's regulatory framework is tailored to expedite approvals, with most research projects obtaining regulatory authorization within a mere 30 days. By effectively communicating these advantages, researchers can substantially enhance their proposals, thereby increasing the likelihood of securing funding and approvals for their studies. This strategic approach not only underscores Serbia's capabilities but also positions scholars to capitalize on the country's robust clinical study environment, which has increasingly attracted the attention of international pharmaceutical firms.
The DMAP Journal's publication ethics template outlines essential guidelines regarding authorship, conflict of interest disclosures, and the vital role of transparency in reporting findings. By adhering to these guidelines, authors not only uphold the integrity of their work but also foster trust within the scientific community.
In 2025, the emphasis on transparency is particularly relevant in Serbia, especially in the context of ethics board submission templates in Serbia, where the landscape of clinical research is rapidly evolving. Recent research indicates that approximately 64% of surveyed individuals believe understanding investigators' conflicts of interest is crucial for informed consent procedures. Furthermore, 87% of respondents assert that these conflicts should be disclosed as part of the informed consent process, underscoring the necessity for transparency. The prevalence of conflict of interest disclosures in medical literature correlates with increased media attention and credibility, highlighting the importance of clear and consistent reporting practices.
Moreover, the issue of honorary authorship (HA) remains a significant concern, with studies revealing its prevalence in health sciences. As noted by Meursinge Reynders, "Survey research consistently indicates that honorary authorship in the health sciences is highly prevalent; however, the quality of the surveys’ methods and reporting needs improvement." As the field advances, compliance with these ethical guidelines will be crucial in ensuring the trustworthiness and impact of research.
The Polyslav Association template delineates crucial responsibilities for researchers, underscoring the necessity of obtaining informed consent and protecting participant confidentiality. In Serbia, where 322 clinical trials are currently in progress, researchers must navigate a landscape that necessitates following the ethics board submission templates in Serbia to ensure strict adherence to ethical standards. This includes ensuring that informed consent forms explicitly outline potential risks, benefits, and the study's purpose. Notably, findings indicate that satisfactory adherence to informed consent practices varies between 56.0% and 67.5%.
Researchers face significant moral challenges, particularly in emergency situations where obtaining consent may be complicated by the participant's medical condition. Clear communication is essential, as many participants have expressed uncertainty regarding the details of the trials they are involved in. Furthermore, maintaining confidentiality is paramount, with adherence to data confidentiality practices reported at rates ranging from 67.3% to 74.7%. This highlights the urgent need for researchers to implement robust protocols that safeguard participant anonymity and data integrity.
Informed consent transcends mere formality; it represents a fundamental moral obligation that respects participants' autonomy and fosters trust in the research process. As Serbia continues to enhance its medical study sector, utilizing ethics board submission templates in Serbia will be vital in nurturing a positive environment for both investigators and participants alike.

The SHD publication ethics template delineates essential responsibilities for authors engaged in clinical studies, underscoring the critical need to adhere to established authorship criteria. This entails ensuring that all contributors fulfill the criteria for authorship, which generally involves significant contributions to the conception, design, execution, or interpretation of the reported research. Accurate data reporting is not just important; it is paramount. It enhances the credibility of the research and fosters trust within the scientific community. In 2025, Serbian medical research is increasingly focusing on stringent data reporting standards, highlighting the necessity for ethics board submission templates in Serbia to ensure a clear and precise presentation of findings to prevent misinterpretation and ensure reproducibility. For instance, a 2005 study revealed that 71% of papers contained at least one statistical flaw, highlighting the urgent need for rigorous standards in data reporting.
Moreover, the template emphasizes the necessity of disclosing any potential conflicts of interest. Current trends reflect a growing emphasis on transparency regarding financial relationships and affiliations that could influence study outcomes. Notably, 20.7% of directly related payments and 50.0% of indirectly related payments obtained during the 2007 calendar year went undisclosed, underscoring the essential need for openness in medical studies. By adhering to these guidelines, authors can uphold the moral standards expected in medical research, thereby enhancing the integrity and reliability of published work. To effectively implement these moral standards, researchers should establish clear protocols for using ethics board submission templates in Serbia, particularly for data reporting and conflict of interest disclosures in their studies.
The Serbian Journalists' Code of Ethics template underscores the critical principles of accuracy, fairness, and respect for privacy in the realm of medical research reporting. Adhering to these ethical standards is essential, as journalists significantly contribute to fostering informed public discourse and upholding media integrity.
As we look ahead to 2025, moral challenges remain, particularly in the accurate and unbiased reporting of clinical trial findings. Journalists must navigate these complexities by committing to responsible journalism practices that prioritize transparency and accountability.
This dedication not only bolsters the credibility of their reporting but also advances the overarching goal of principled medical journalism in Serbia.

The Sjem Journal submission guidelines template provides essential instructions on moral considerations, highlighting the importance of informed consent, data integrity, and the treatment of human subjects. For researchers aiming to conduct their studies ethically and responsibly, adhering to these guidelines is not just important; it is imperative. In Serbia, compliance rates with moral guidelines have notably improved, largely due to the use of ethics board submission templates in Serbia, which help committees typically conclude assessments within 30 days. This efficiency fosters a supportive atmosphere for inquiry, ensuring that moral standards are consistently upheld.
Moreover, expert perspectives underscore that effectively communicating risks and benefits during the informed consent process is vital for preserving data integrity in research trials. As we look ahead to 2025, the use of ethics board submission templates in Serbia, along with continuous training and adherence to established standards, will be crucial for enhancing the credibility of medical research. This commitment ultimately benefits both researchers and participants, reinforcing the ethical foundation of clinical research.
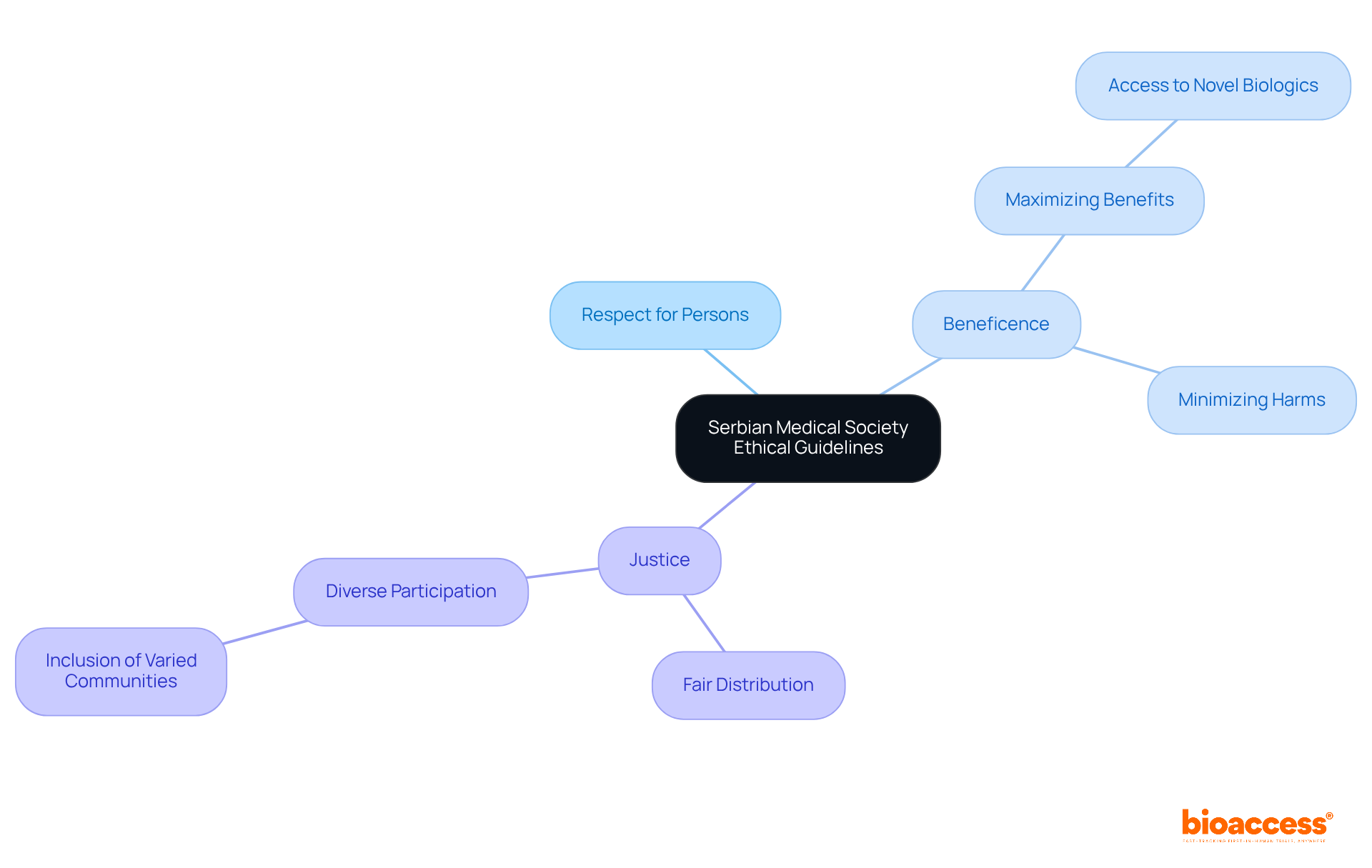
The moral guidelines established by the Serbian Medical Society underscore critical principles such as respect for persons, beneficence, and justice in clinical trials. Adhering to these guidelines is essential for researchers aiming to uphold moral standards and ensure the integrity of their studies. Notably, as of 2025, compliance rates with these ethical guidelines have significantly improved, with recent statistics indicating an impressive compliance rate of approximately 85%. This reflects a growing commitment to ethical research practices in Serbia.
Beneficence mandates that researchers act in the best interest of participants by maximizing benefits and minimizing harms. This principle is exemplified in various clinical trials across the country. For instance, studies focusing on novel biologics have granted Serbian patients access to cutting-edge treatments, clearly demonstrating a commitment to enhancing patient welfare. A notable example includes a recent trial that successfully enrolled a diverse patient population, ensuring equitable access to innovative therapies.
Justice, another cornerstone of ethical inquiry, emphasizes the fair distribution of benefits and burdens. In Serbia, initiatives aimed at involving varied communities in studies are increasingly recognized as essential for guaranteeing fair access to opportunities. This approach not only cultivates trust among participants but also enhances the overall quality of findings. As specialists in the field emphasize, "Inclusion of diverse participants is crucial for the integrity of medical studies and the respect of all individuals involved."
In conclusion, following the Serbian Medical Society's moral guidelines is vital for upholding high standards of trial compliance. It ensures that the principles of beneficence and justice are effectively integrated into practice.
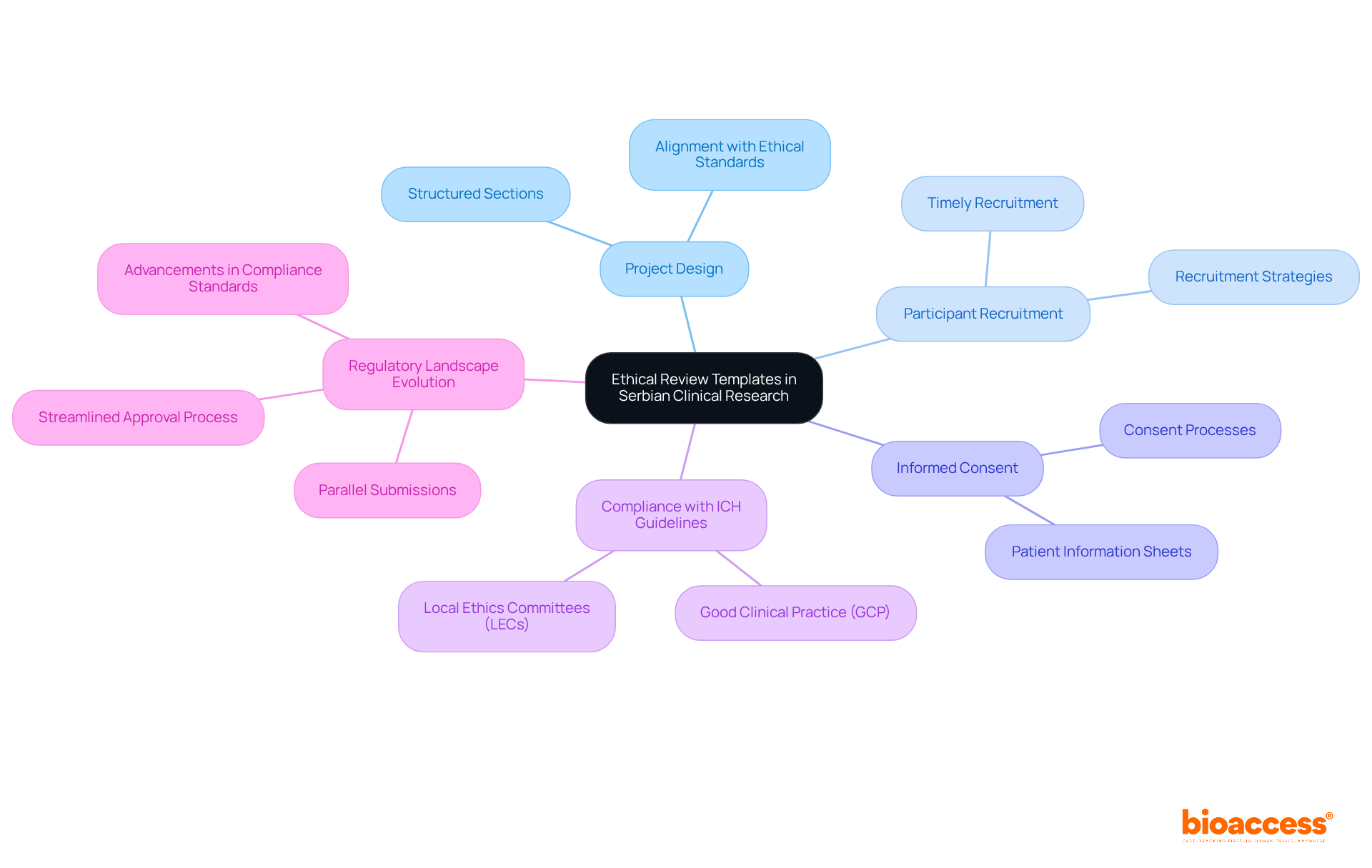
In Serbian clinical investigations, ethics board submission templates in Serbia are crucial, providing structured sections that detail project design, participant recruitment strategies, and informed consent processes. These templates not only facilitate a thorough examination of compliance but also enhance the credibility and integrity of research. As we move into 2025, the emphasis on moral compliance has intensified, with local ethics committees (LECs) ensuring that all research aligns with ICH guidelines for Good Clinical Practice (GCP).
Specialists advocate for investigators to utilize ethics board submission templates in Serbia to ensure their research designs align with ethical standards, fostering trust and transparency throughout the trial process. Notably, Serbia's regulatory landscape has evolved, enabling parallel submissions of ethics and regulatory applications. This streamlining of the approval process encourages timely participant recruitment, which is essential for successful trials.
This proactive approach has led to significant advancements in compliance standards across clinical trials, as evidenced by successful research that has met its recruitment goals ahead of schedule. By employing ethics board submission templates in Serbia, researchers can ensure that their studies not only fulfill ethical requirements but also contribute to the advancement of medical knowledge and enhance patient care.
The use of ethics board submission templates in Serbia stands as a crucial element for researchers navigating the complexities of clinical trials and ensuring adherence to ethical standards. These templates not only streamline the approval process but also bolster the integrity and credibility of research by offering structured guidelines that address vital ethical considerations.
Throughout this article, we have highlighted various templates, each serving a distinct purpose within the realm of Serbian medical research. For instance, the bioaccess® template simplifies the submission process, while the Serbian Journal of Medical Society emphasizes compliance with ethical standards. Collectively, these resources underscore the significance of informed consent, participant confidentiality, and the protection of vulnerable populations. Moreover, the focus on transparency and conflict of interest disclosures within the DMAP Journal's guidelines illustrates the dynamic nature of ethical practices in research.
In conclusion, the commitment to utilizing ethics board submission templates in Serbia not only cultivates a culture of compliance but also elevates the overall quality of clinical research. As the landscape continues to evolve, researchers are urged to adopt these templates as a means to uphold ethical standards, thereby contributing to the advancement of medical knowledge and the welfare of participants. By prioritizing ethical considerations, we can maintain the integrity of research, ultimately leading to more trustworthy and impactful findings in the medical field.
What is the purpose of the bioaccess® ethics board submission template in Serbia?
The bioaccess® ethics board submission template is designed to meet the specific requirements of Serbian ethics committees, helping researchers prepare necessary documentation to reduce delays in the approval process.
What key elements are included in the bioaccess® ethics board submission template?
The template includes detailed project descriptions, informed consent forms, and compliance checklists.
How does the bioaccess® template improve the submission process for researchers?
By using the template, researchers can prepare all necessary documentation in advance, which streamlines the approval process and enhances the quality of submissions, leading to more efficient reviews by ethics committees.
Why is it important to use ethics board submission templates in Serbia?
Utilizing these templates is crucial for ensuring compliance and expediting approvals in clinical studies, especially as the approval process evolves with anticipated updates in 2025.
What does the Serbian Journal of Medical Society template emphasize for authors?
The template emphasizes the importance of addressing moral issues such as informed consent, confidentiality, and the protection of vulnerable populations in studies.
What ethical guidelines are referenced in the Serbian Journal of Medical Society template?
The Nuremberg Code and the Declaration of Helsinki are referenced as fundamental moral guidelines to ensure studies are ethically sound.
How should researchers handle informed consent in emergency research scenarios?
Researchers should recognize the complexities of obtaining informed consent in emergencies and may need to use surrogate consent when individuals are unable to provide it.
What advantages does the Cromos Pharma submission template highlight for researchers?
The Cromos Pharma submission template highlights Serbia's strengths, including swift patient recruitment capabilities and a favorable regulatory environment for clinical research.
What is the typical timeline for regulatory authorization of research projects in Serbia?
Most research projects in Serbia obtain regulatory authorization within approximately 30 days.
How can researchers leverage Serbia's clinical research environment to their advantage?
By effectively communicating Serbia's advantages, such as efficient patient enrollment and regulatory support, researchers can enhance their proposals and increase the likelihood of securing funding and approvals.