


Understanding the clinical trial process is essential for volunteers. This includes recognizing the importance of informed consent and the potential health benefits of participation. Being well-informed about the steps involved, as well as the rights and responsibilities of volunteers, not only enhances their experience but also significantly contributes to the integrity and success of medical research. This knowledge empowers volunteers to engage meaningfully in the clinical trial process, ultimately fostering a more robust research environment.
Clinical trials stand at the forefront of medical innovation, yet a significant number of potential volunteers remain unaware of the intricacies involved in participation. Understanding the clinical trial process empowers individuals to make informed decisions, enhancing their overall experience and safety.
What essential insights should every clinical trial volunteer grasp to navigate this complex landscape effectively? This article delves into ten crucial aspects that can transform the volunteering journey, from comprehending informed consent to recognizing the profound impact of participation on personal health and community.
bioaccess® is dedicated to providing comprehensive support for research participants, ensuring they receive expert guidance throughout the process. By emphasizing regulatory efficiency and accommodating diverse patient groups, bioaccess® significantly enhances the research experience, empowering participants to partake in studies that could lead to groundbreaking medical advancements.
Our pre-qualified networks enable rapid site activation, with over 50 locations launched in less than 8 weeks, ensuring that participants can effectively engage in studies. The team at bioaccess® is resolute in ensuring that every volunteer feels informed and supported, making the research journey as seamless as possible while adhering to FDA, EMA, and MDR compliance standards.

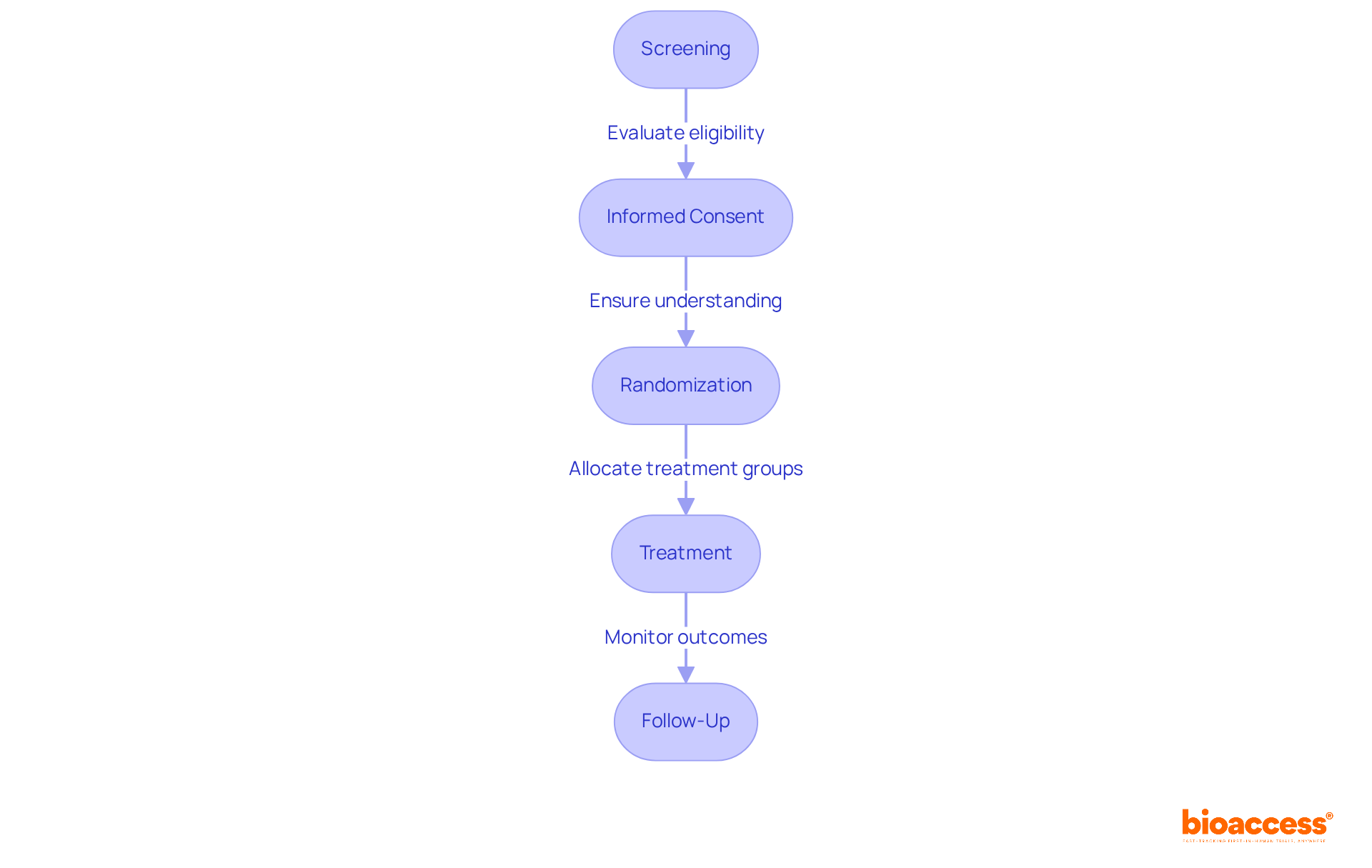
Volunteers must familiarize themselves with the clinical trial process, which typically encompasses several key steps:
Each step is vital for maintaining the integrity of the study and the safety of individuals. Informed consent not only safeguards the rights of clinical trial volunteers but also upholds ethical standards in medical research.
Engaging in research studies offers numerous health advantages, including access to innovative therapies that may not yet be available to the general population. Clinical trial volunteers typically undergo thorough medical evaluations and monitoring, which can lead to the early detection of health issues. Studies indicate that a significant portion of participants report improved health outcomes, underscoring the potential benefits of involvement.
Furthermore, participation in a research study empowers individuals, allowing them to contribute to the advancement of medical knowledge that can benefit future patients. This dual advantage of personal health improvement and contribution to scientific progress makes being clinical trial volunteers an appealing option for those seeking innovative treatment solutions.
Clinical studies often unite individuals facing similar health challenges, fostering a supportive community among participants. This connection not only enhances the experience but also provides essential emotional support and encouragement.
Numerous research locations bolster this concept by organizing group activities and discussions, where participants can share their experiences and insights. Such interactions cultivate a sense of belonging and empower participants to navigate their journeys collectively. The presence of support networks diminishes feelings of isolation, rendering the research process more enjoyable and enriching for all involved.
By establishing community engagement initiatives, research locations can create environments that resonate with bioaccess's mission to drive global health improvement through international collaboration and innovation in Medtech.

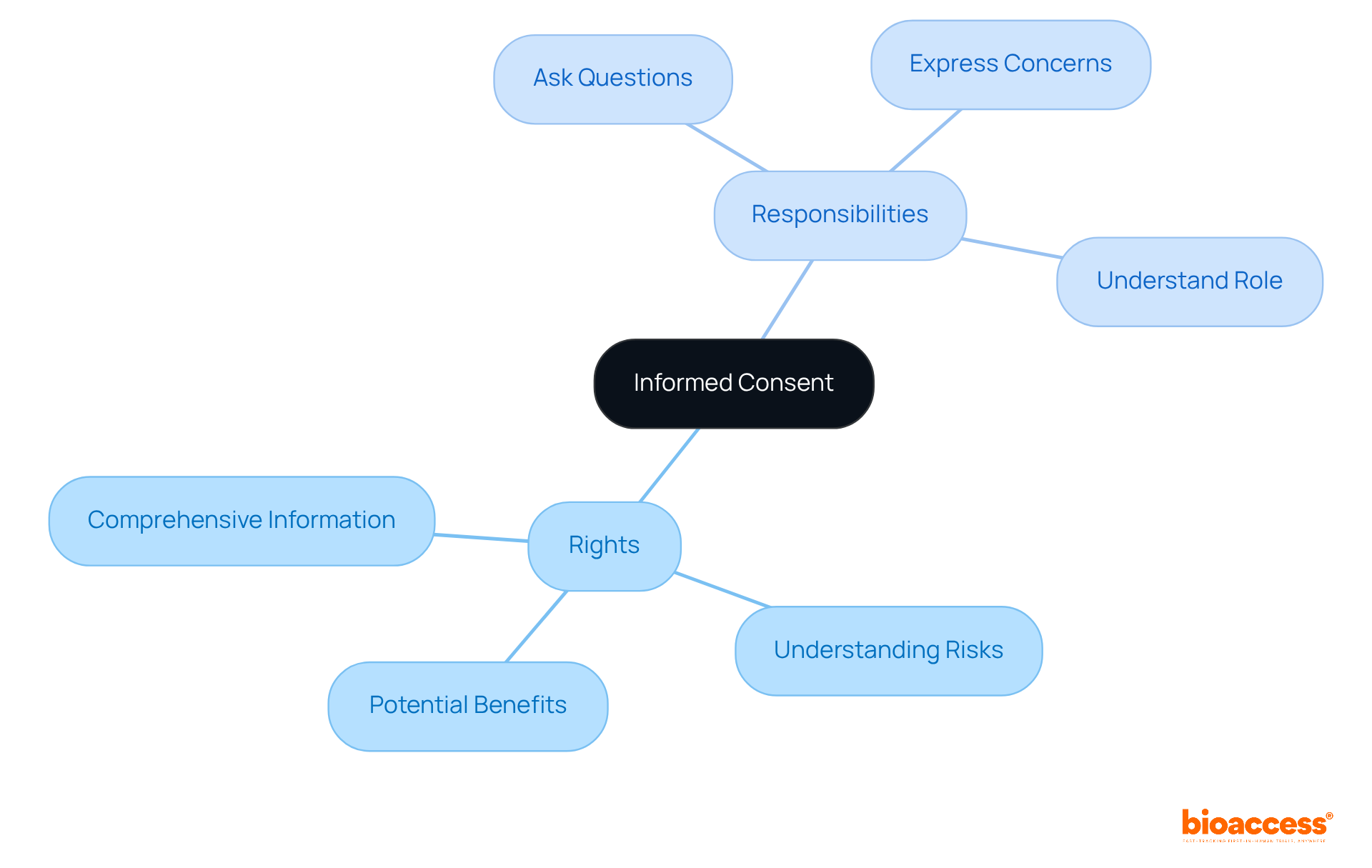
Informed consent stands as a cornerstone of research studies, ensuring that participants are fully aware of their involvement. Clinical trial volunteers have the right to receive comprehensive information regarding the study's purpose, procedures, risks, and potential benefits. They also carry the responsibility to pose questions and express any concerns prior to agreeing to participate. This understanding of rights and responsibilities is vital for making informed decisions and fostering trust in the research process.
Legal professionals emphasize that each individual must comprehend their role and the consequences of their engagement, reinforcing the ethical duty to prioritize awareness among those who assist. For instance, the Belmont Report underscores the principles of information, comprehension, and voluntariness as essential to informed consent.
By cultivating an environment where volunteers feel encouraged to engage in discussion, researchers can enhance the informed consent process, ultimately leading to more ethical and efficient studies.
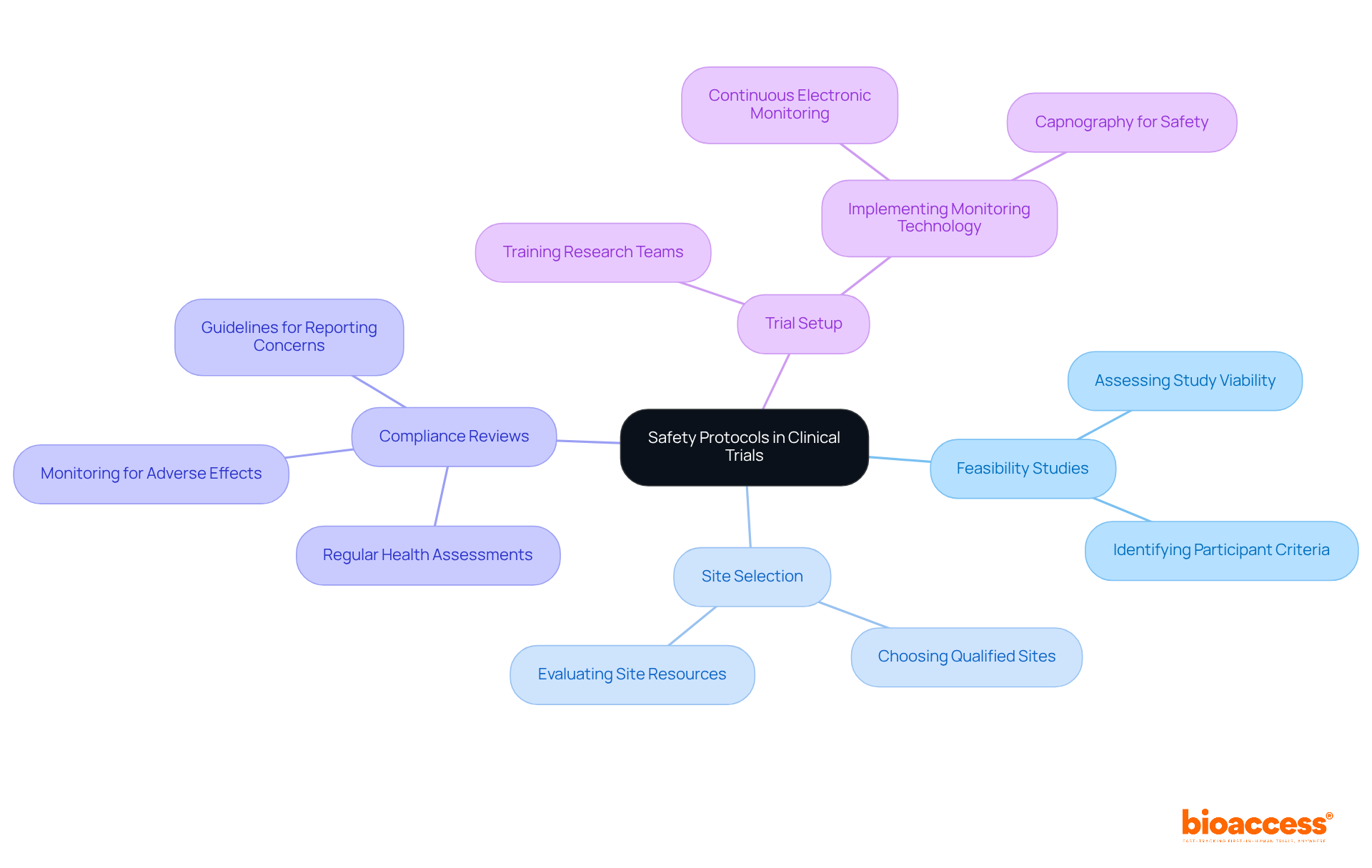
Safety remains paramount in research studies, underpinned by stringent protocols designed to protect participants. At bioaccess®, we provide comprehensive clinical trial management services, which encompass:
This meticulous approach guarantees that all safety measures are adhered to rigorously. Our protocols include:
To ensure that our research teams can promptly address any safety issues that may arise, they undergo extensive training to deliver comprehensive care for clinical trial volunteers throughout the study.
Recent advancements in monitoring technology, such as continuous electronic monitoring with capnography, significantly enhance participant safety. This technology enables real-time feedback, thereby minimizing the risk of adverse events. By prioritizing these safety measures, research studies not only foster public trust but also encourage greater involvement. This ultimately aids the progress of medical research and positively impacts local economies through job creation and healthcare enhancement.
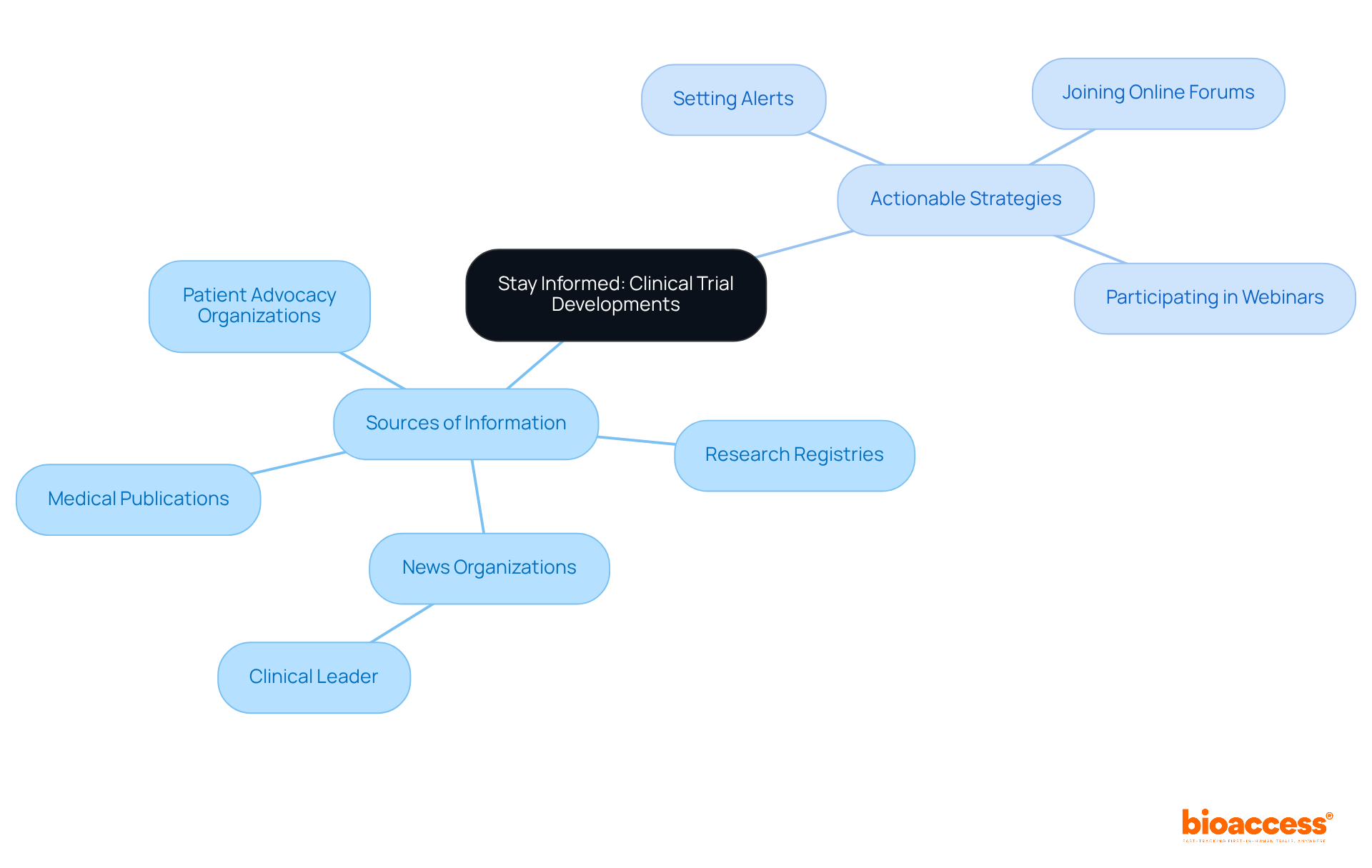
By engaging with a variety of trustworthy sources, clinical trial volunteers can efficiently stay informed about research developments. Medical publications and research registries are essential for accessing the latest study results and information. Moreover, news organizations that specialize in health research, such as Clinical Leader, provide timely updates on ongoing studies and advancements in Latin America and Colombia. Active participation in patient advocacy organizations and attendance at informative events can significantly enhance the understanding of clinical trial volunteers regarding the research landscape.
Studies indicate that a thorough understanding of medical study advancements is crucial for the involvement and support of clinical trial volunteers. For example, the BackInAction study illustrated how effective communication strategies can greatly improve participant outcomes, underscoring the necessity of clear and accessible information.
Additionally, health reporters emphasize the value of reliable sources for information regarding clinical trial volunteers. As noted by a prominent health reporter, "Regular updates empower participants to advocate for themselves and others within the clinical research community." By utilizing these resources, including media coverage from Clinical Leader, clinical trial volunteers can deepen their knowledge and make meaningful contributions to the progression of medical research.
Furthermore, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is vital for participants. INVIMA oversees the regulatory aspects of research studies and medical devices, recognized as a Level 4 health authority by PAHO/WHO. This insight can aid clinical trial volunteers in navigating the research environment more effectively.
Moreover, the recent release of the I CAN DO Surgical ACP study design document on October 2, 2025, highlights the continuous advancements in research, emphasizing the importance of remaining informed. Understanding diverse patient demographics, as illustrated by the iPATH research findings, can also provide valuable perspectives for supporters. To effectively stay updated on research developments, clinical trial volunteers should consider:
Some clinical trials necessitate overnight stays for monitoring purposes, and it is crucial for volunteers to be prepared for this possibility. Understanding what to expect is vital. Typically, arrangements will be made, ensuring that attendees have access to medical personnel for any inquiries or concerns.
It is advisable to bring personal items such as:
Being informed about what to expect can significantly enhance volunteers' comfort during their stay.
Numerous research studies highlight referral schemes that effectively motivate individuals to persuade others to enroll as clinical trial volunteers in clinical research. Volunteers can leverage these programs by sharing their positive experiences and the potential benefits of participation, including access to cutting-edge treatments and the opportunity to contribute to medical advancements. This approach not only raises awareness about research studies but also cultivates a sense of community and collective purpose among participants.
Research indicates that efficient referrals can significantly influence enrollment rates; studies reveal that up to 90% of potential candidates evaluated for research studies never complete the enrollment process. By actively engaging in referral initiatives, volunteers can play a pivotal role in advancing medical research and enhancing patient outcomes.
To encourage others to participate, contributors can emphasize both the personal and community advantages of research studies, share success narratives, and provide details on how to get involved.
Comprehensive research study management services, including feasibility assessments, site selection, and project management, bolster these referral programs, ultimately benefiting local economies through job creation and healthcare improvements.
Clinical trial volunteers play a crucial role in advancing medical research through their involvement in clinical studies. Their contributions provide researchers with critical data that can lead to groundbreaking treatments and therapies. For instance, the RECOVERY study quickly identified dexamethasone as a life-saving treatment for severely ill COVID-19 patients, exemplifying how volunteer efforts can yield significant medical breakthroughs. At the height of the pandemic, the communications team effectively engaged around 700-800 participants in online meetings, ensuring timely updates and fostering a collaborative environment.
The influence of research involvement extends beyond prompt study results; it shapes the future of healthcare. Volunteers not only contribute to the development of innovative medical solutions but also enhance the understanding of diseases and treatment efficacy. Their experiences and feedback are invaluable, as evidenced by the formation of public advisory panels that ensure acceptability and transparency.
Furthermore, bioaccess provides extensive clinical study management services, including:
This expertise ensures that trials are conducted efficiently and ethically, maximizing the potential for impactful outcomes. Biopharma leaders emphasize the significance of contributions from clinical trial volunteers in advancing medical science. Their insights highlight that without the commitment of unpaid helpers, many clinical advancements would be postponed or impossible. By recognizing the profound impact of their participation, volunteers can feel empowered to engage actively in the research process, knowing they are making a difference in the lives of countless individuals.
Engaging in clinical trials represents a significant opportunity for individuals to contribute to medical research while potentially benefiting their own health. Understanding the clinical trial process, from screening to informed consent, empowers volunteers to navigate their participation with confidence. By familiarizing themselves with the steps involved and the rights and responsibilities that come with volunteering, individuals can ensure a more enriching experience.
The article highlights essential insights for clinical trial volunteers, including:
From the rigorous safety measures in place to the personal and communal advantages of being involved, volunteers are not only enhancing their well-being but also playing a vital role in advancing medical knowledge and innovation.
Ultimately, participation in clinical trials is more than just a personal journey; it is a chance to be part of something larger. By staying informed and encouraging others to join, individuals can amplify their impact on the future of healthcare. Embracing the role of a clinical trial volunteer can lead to transformative experiences, both for themselves and for the countless lives that will benefit from the research they help to propel forward.
What is bioaccess® and what services do they provide?
bioaccess® is dedicated to supporting research participants by providing expert guidance throughout the clinical trial process. They emphasize regulatory efficiency and accommodate diverse patient groups, enhancing the research experience and empowering participants to engage in studies that could lead to medical advancements.
How does bioaccess® ensure rapid site activation for clinical trials?
bioaccess® utilizes pre-qualified networks to enable rapid site activation, having launched over 50 locations in less than 8 weeks, which allows participants to effectively engage in studies.
What are the key steps in the clinical trial process for volunteers?
The key steps in the clinical trial process include Screening (evaluating eligibility), Informed Consent (understanding the study's purpose and risks), Randomization (allocating individuals to treatment groups), Treatment (receiving the intervention), and Follow-Up (monitoring health outcomes and reporting side effects).
Why is informed consent important in clinical trials?
Informed consent is crucial as it safeguards the rights of volunteers and upholds ethical standards in medical research, ensuring that participants fully understand the study's purpose, procedures, risks, and benefits.
What health benefits can participants gain from engaging in clinical trials?
Participants in clinical trials can access innovative therapies not yet available to the public, receive thorough medical evaluations that may lead to early detection of health issues, and report improved health outcomes. Additionally, they contribute to advancing medical knowledge that can benefit future patients.
How does participation in clinical trials empower individuals?
Participation in clinical trials empowers individuals by allowing them to contribute to scientific progress and the advancement of medical knowledge while also potentially improving their own health outcomes.