

This article underscores the critical in vitro diagnostics (IVD) regulations that clinical research directors must grasp to achieve compliance and facilitate successful market entry. It asserts the necessity of expertise in areas such as:
By illustrating how meticulous preparation and strict adherence to these regulations can profoundly impact the success of IVD projects, the article aims to engage clinical research professionals in recognizing the importance of these guidelines.
Navigating the complex landscape of in vitro diagnostics (IVD) regulations is crucial for clinical research directors who aim to ensure compliance and accelerate their projects. The ever-evolving regulatory environment presents essential requirements and pathways that can significantly impact the success of IVD studies.
As directors strive to meet these stringent standards, what challenges lie ahead in fostering innovation and improving patient outcomes?
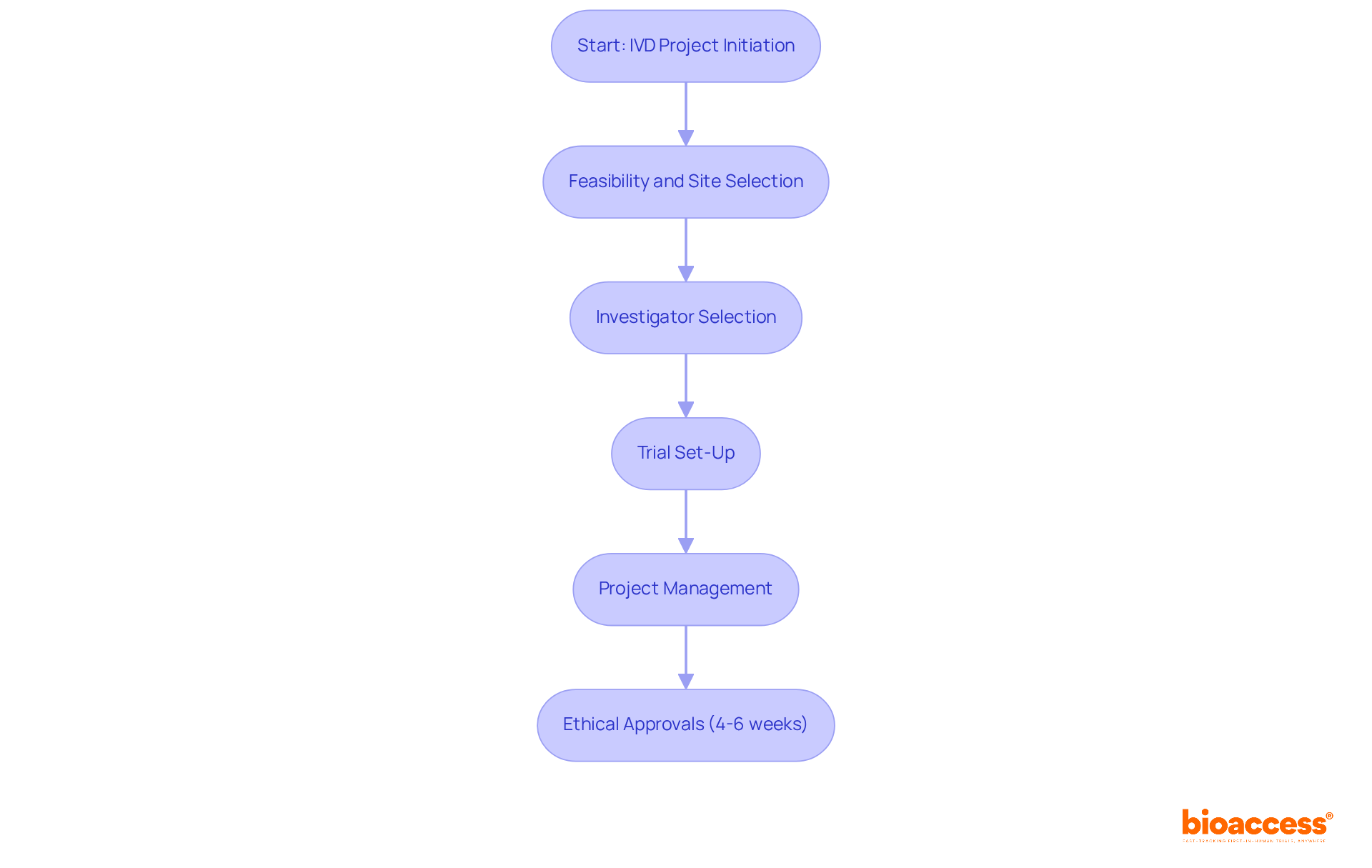
bioaccess® excels in facilitating swift approvals for in vitro diagnostics (IVD) studies by skillfully navigating the complexities of IVD regulations. By harnessing the regulatory agility of Latin America, the diverse patient populations in the Balkans, and the streamlined pathways available in Australia, bioaccess® achieves ethical approvals in a remarkable 4-6 weeks. This rapid process empowers trial directors to accelerate their IVD projects while adhering to IVD regulations, resulting in faster patient enrollment and quicker access to essential market insights.
Furthermore, bioaccess® provides comprehensive services, including:
This ensures compliance with local regulations while enhancing operational efficiency. The partnership with Caribbean Health Group positions Barranquilla as a prominent destination for medical trials in Latin America, backed by Colombia's Minister of Health. By implementing effective project management practices, bioaccess® guarantees that clinical trials are conducted smoothly, ultimately enhancing the development of innovative diagnostic solutions.
Clinical study directors should leverage bioaccess®'s expertise and resources to streamline their IVD projects in compliance with IVD regulations. This ensures timely access to market opportunities and improved patient outcomes. How can you benefit from this collaboration to address your clinical research challenges?
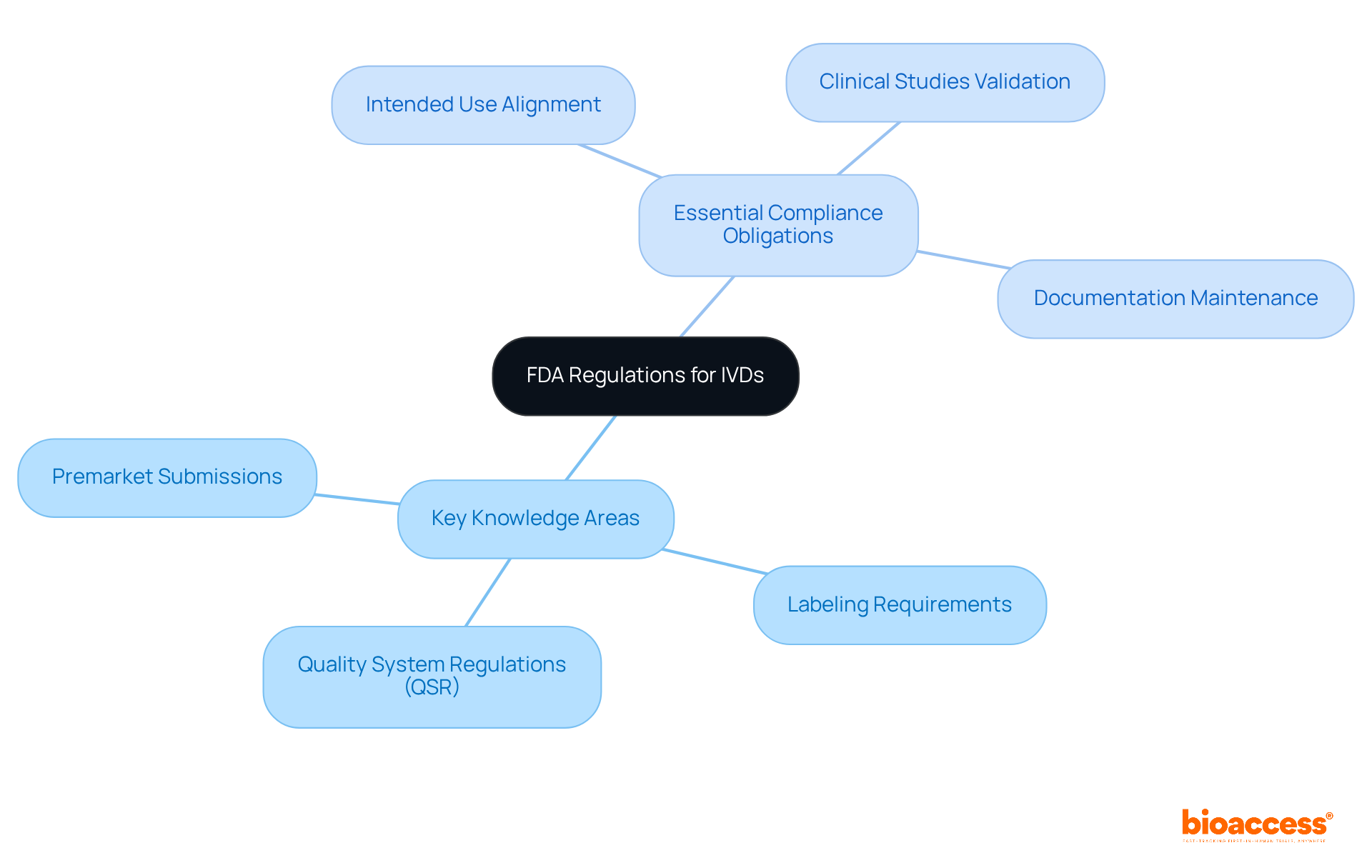
Clinical study directors must possess a thorough understanding of IVD regulations governing in vitro diagnostics (IVDs). This knowledge is critical, particularly regarding:
Essential compliance obligations involve:
In 2022, the FDA authorized 3,229 510(k) submissions, underscoring the importance of effective premarket strategies. Notably, nearly 32% of FDA 510(k) submissions failed the initial acceptance check, highlighting the necessity for comprehensive preparation and a deep understanding of regulatory expectations. Familiarity with IVD regulations is not merely beneficial; it is essential for successful product development and market approval.
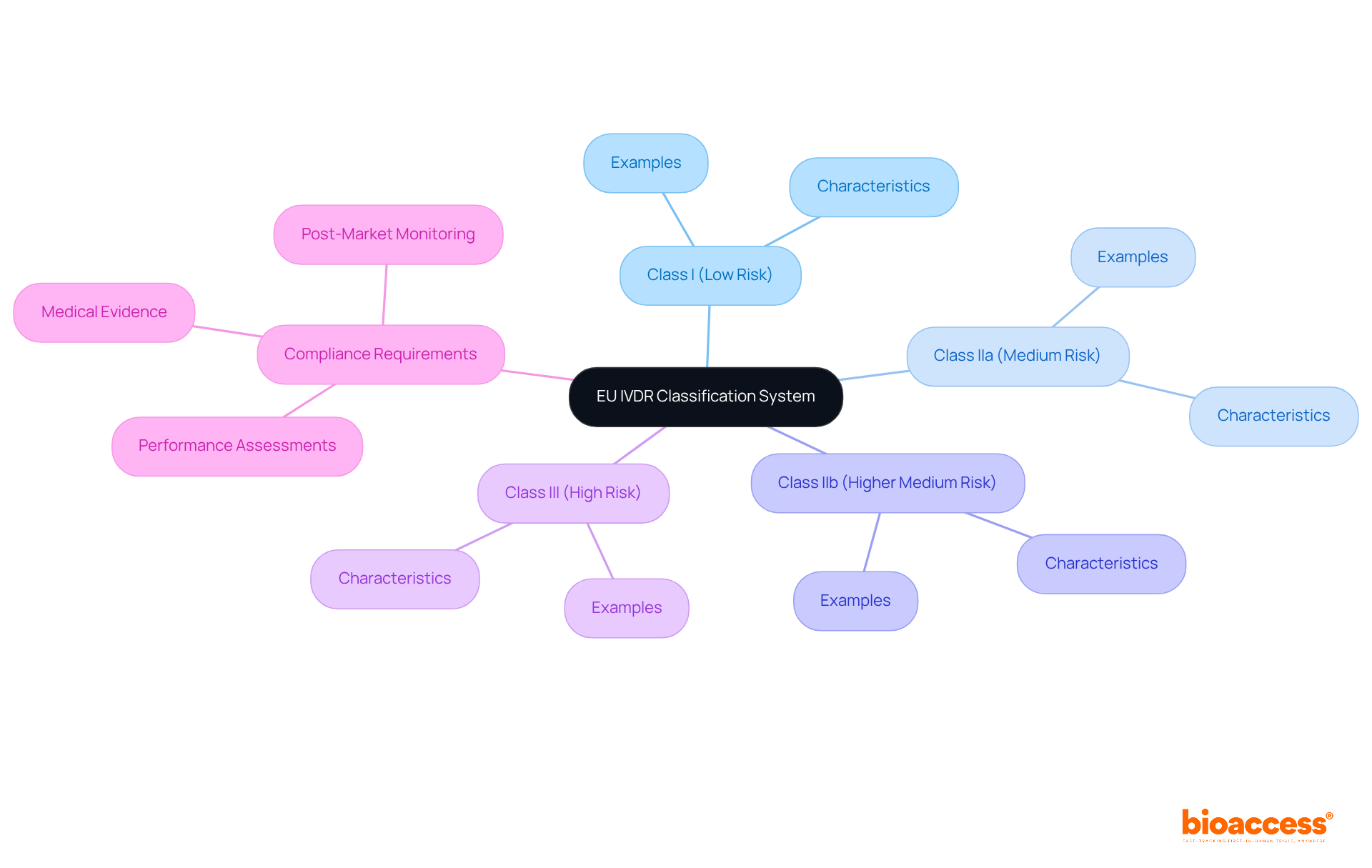
The EU IVDR introduces new classification rules and compliance standards that significantly impact IVD studies in accordance with IVD regulations. It is crucial for clinical study directors to grasp the new risk-based classification system, which categorizes IVDs into four classes based on their risk level. Adhering to the IVD regulations requires:
Staying informed about these changes is essential for ensuring that IVD products adhere to IVD regulations and meet EU market requirements.
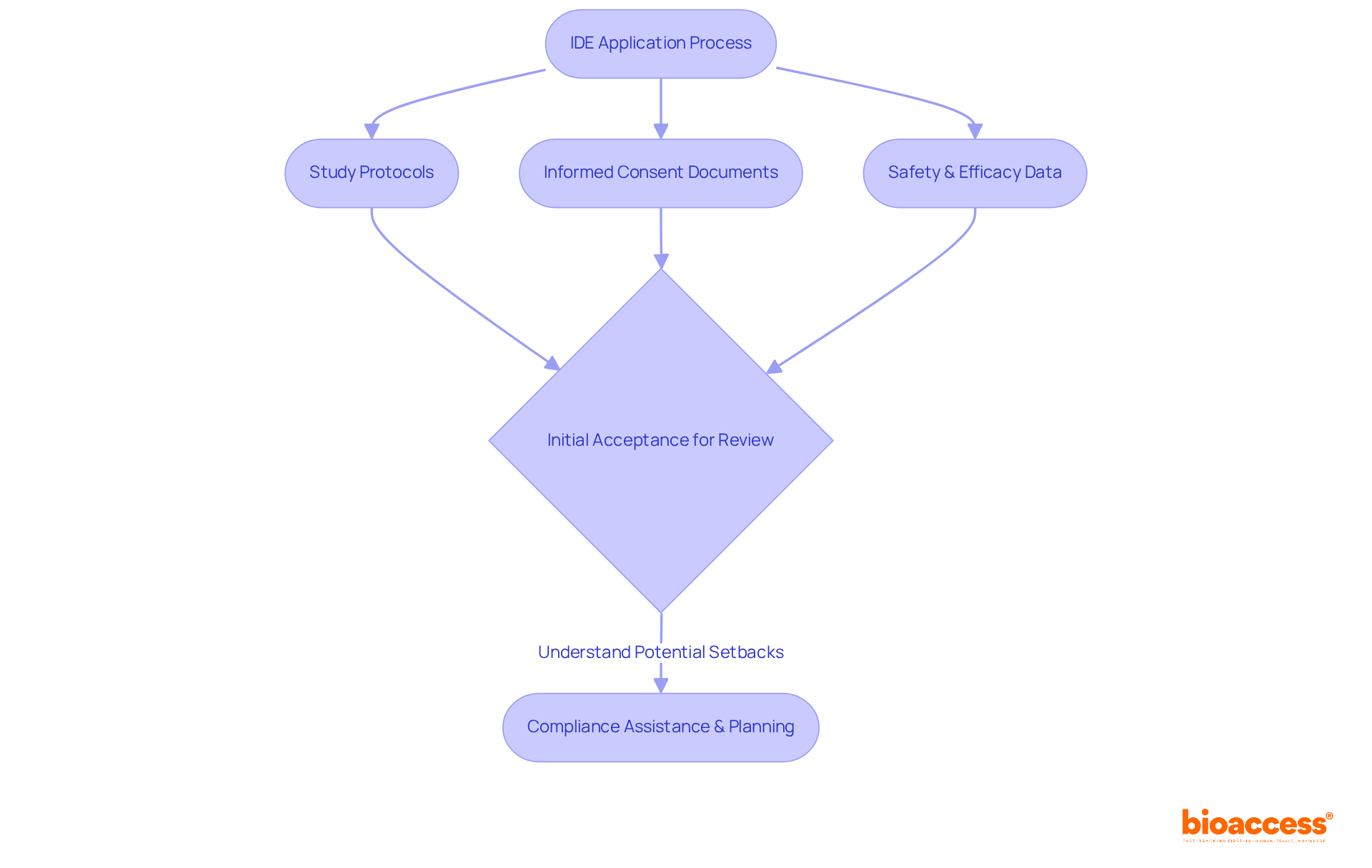
The Investigational Device Exemption (IDE) serves as a vital pathway, enabling study directors to conduct trials on in vitro diagnostics (IVDs) in accordance with IVD regulations without the necessity of premarket approval. To adeptly navigate the IDE process, directors are required to prepare a comprehensive application that includes essential components such as:
Notably, nearly 32% of FDA 510(k) submissions have failed the initial acceptance for review assessment in recent years, underscoring the importance of understanding the intricacies of the IDE process for securing timely approvals and ensuring the effective execution of research studies. Furthermore, the duration for completing the IDE process can vary significantly, making it crucial for directors to be well-informed about the requirements and updates to facilitate their submissions. Insights from medical study leaders highlight the importance of early compliance assistance with IVD regulations and thorough planning to mitigate potential setbacks and enhance the likelihood of favorable outcomes in IVD studies.
Clinical research directors face a pivotal decision when navigating the compliance landscape for IVD market entry: the choice between the 510(k) and Premarket Approval (PMA) pathways. The 510(k) pathway is typically more expedient, allowing for approval through the demonstration of substantial equivalence to an existing device. Notably, in 2021, 85% of 510(k) submissions received a Substantially Equivalent decision, highlighting its efficiency compared to the PMA process, which requires extensive clinical data and generally demands over a year for review.
As we approach 2025, updates to IVD regulations highlight the necessity for strategic compliance planning. This entails a comprehensive understanding of the specific requirements for each pathway, along with continuous education regarding evolving IVD regulations. Experts emphasize that early collaboration with compliance specialists can yield invaluable insights into the intricacies of these processes, ultimately facilitating successful navigation of the IVD regulations.
The decision between the 510(k) and PMA pathways transcends mere procedure; it represents a strategic choice that can profoundly influence the timeline and success of IVD product launches. By capitalizing on the advantages of the 510(k) pathway, innovators can accelerate their market entry while ensuring compliance with FDA regulations, thus enhancing their competitive edge in the rapidly evolving healthcare landscape.

Under the IVD regulations, performance evaluation is a critical component for IVD compliance. Research directors are tasked with conducting thorough evaluations to assess the analytical and therapeutic performance of their devices. This process encompasses:
Meeting these rigorous standards is essential for obtaining official approval and ensuring market success under the IVD regulations.
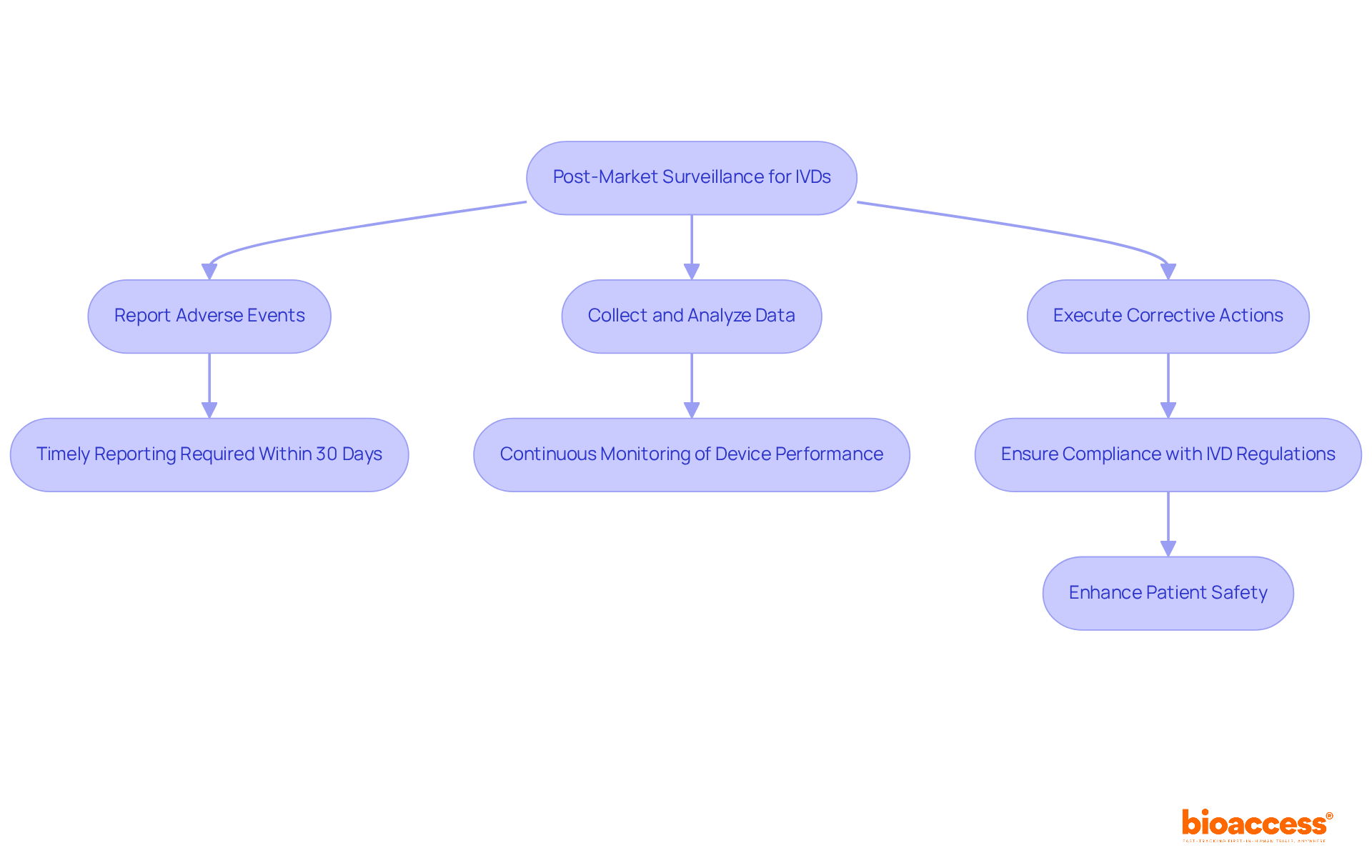
Post-market surveillance (PMS) is critical for ensuring the safety and compliance of in vitro diagnostics (IVDs) in accordance with IVD regulations once they enter the market. Clinical research directors must implement robust strategies for continuous monitoring of device performance. This entails:
Effective PMS not only guarantees compliance with IVD regulations but also plays a pivotal role in ensuring patient safety. Industry specialists assert that a proactive approach to overseeing IVD performance in accordance with IVD regulations significantly enhances compliance rates and fosters trust with oversight bodies. For example:
Furthermore, the urgency of timely adverse event reporting cannot be overstated; manufacturers are required to notify oversight organizations within thirty days of identifying any device-related fatalities or serious injuries. By prioritizing these practices, clinical study directors can ensure that IVDs remain safe and effective throughout their lifecycle, ultimately contributing to improved patient outcomes.
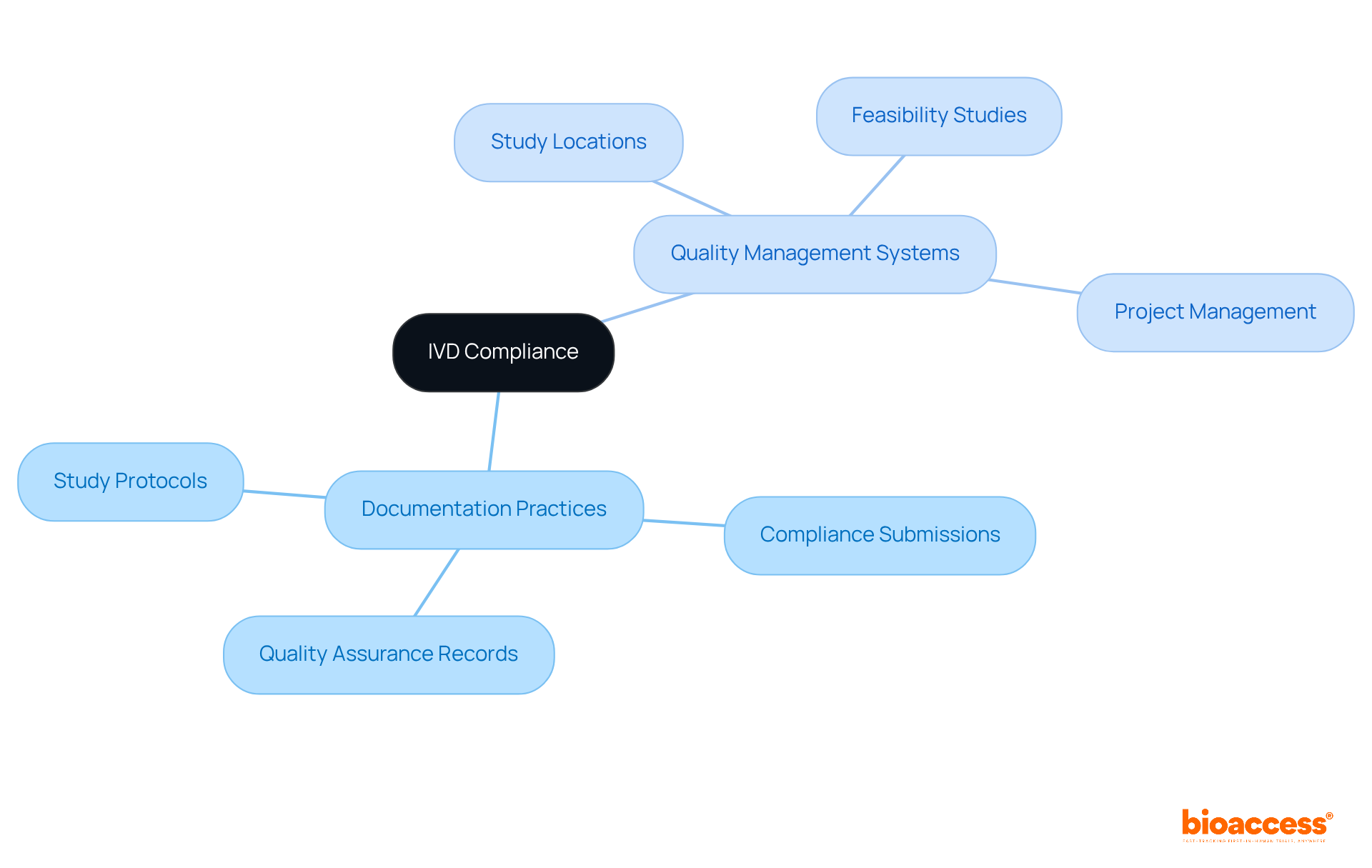
Effective documentation and quality management systems are foundational for meeting IVD regulations. Clinical study directors must establish comprehensive documentation practices that encompass study protocols, compliance submissions, and quality assurance records. At bioaccess, our service capabilities include feasibility studies and the selection of study locations and principal investigators, ensuring that all necessary compliance reviews are conducted. Implementing a robust quality management system standardizes and monitors processes, facilitating trial setup, import permits, and project management. This ongoing enhancement strategy not only improves overall study quality but also guarantees compliance with necessary standards.
Collaboration among stakeholders—investigators, sponsors, regulatory bodies, and ethics committees—is essential for enhancing oversight in IVD studies in accordance with IVD regulations. Clinical study directors must develop strong relationships with these entities to facilitate effective communication, streamline processes, and proactively address challenges. This collaboration not only enhances study outcomes and compliance with IVD regulations but also expedites the successful development of IVD products. By fostering a culture of collaboration, medical study directors can leverage the collective expertise of all participants, leading to more efficient and effective trials.

The terrain of IVD regulations is constantly evolving, requiring study directors to stay vigilant about updates and modifications. Engaging with compliance announcements, attending industry conferences, and participating in professional organizations are critical strategies for staying informed. A recent survey reveals that over 60% of clinical study directors face significant compliance challenges due to the rapid pace of regulatory changes. Industry leaders assert that proactive engagement with regulatory bodies and ongoing education can transform compliance from a hurdle into an opportunity for innovation. As IVD regulations evolve, adapting investigation strategies is essential for maintaining a competitive edge in the market. The commitment to staying informed not only ensures compliance with IVD regulations but also enhances the capability to effectively navigate the complexities of IVD research.

The landscape of in vitro diagnostics (IVD) regulations is intricate and ever-evolving, necessitating that clinical research directors remain acutely aware of the essential compliance requirements. Mastery of these regulations is not just advantageous; it is vital for ensuring the successful development and market entry of IVD products. By understanding the nuances of FDA regulations, EU IVDR standards, and critical pathways such as 510(k) and PMA, directors can significantly enhance their strategic approach to clinical trials and regulatory submissions.
Key insights underscore the importance of:
With the introduction of new classification rules and performance evaluation standards, as well as the necessity for robust post-market surveillance, directors must embrace a culture of continuous learning and adaptation. Engaging with resources like bioaccess® can streamline the regulatory process, offering invaluable support in navigating these challenges efficiently.
Ultimately, the commitment to staying informed about evolving IVD regulations not only ensures compliance but also fosters innovation within the field. By leveraging the knowledge and tools available, clinical research directors can meet regulatory demands while contributing to improved patient outcomes and the advancement of diagnostic solutions. Embracing this proactive approach is essential for thriving in the competitive landscape of IVD research and making a meaningful impact on healthcare.
What is bioaccess® and how does it assist in IVD research?
bioaccess® facilitates swift approvals for in vitro diagnostics (IVD) studies by navigating IVD regulations effectively, enabling ethical approvals in 4-6 weeks, which accelerates IVD projects and improves patient enrollment.
What services does bioaccess® provide for IVD projects?
bioaccess® offers comprehensive services including feasibility and selection of research sites, investigator selection, trial set-up, and project management, ensuring compliance with local regulations and enhancing operational efficiency.
Why is Barranquilla, Colombia, significant for medical trials?
Barranquilla is positioned as a prominent destination for medical trials in Latin America due to the partnership with Caribbean Health Group and support from Colombia's Minister of Health.
What are the key compliance requirements for IVDs according to FDA regulations?
Key compliance requirements include understanding premarket submissions, adhering to labeling requirements, and complying with quality system regulations (QSR).
What essential obligations must clinical study directors fulfill for IVDs?
Essential obligations include ensuring the IVD aligns with its intended use, conducting comprehensive clinical studies to validate performance, and maintaining meticulous documentation throughout the development process.
What does the FDA's 510(k) submission data indicate about IVD regulatory compliance?
In 2022, the FDA authorized 3,229 510(k) submissions, with nearly 32% failing the initial acceptance check, highlighting the need for thorough preparation and understanding of regulatory expectations.
What is the EU IVDR and how does it affect IVD studies?
The EU IVDR introduces new classification rules and compliance standards that impact IVD studies, requiring clinical study directors to understand a risk-based classification system and adhere to performance assessments, medical evidence, and post-market monitoring strategies.
Why is it important for clinical study directors to stay informed about IVD regulations?
Staying informed about IVD regulations is essential for ensuring that IVD products comply with market requirements and successfully navigate the regulatory landscape for product development and approval.