


This article highlights the essential types of medical equipment crucial for the success of clinical research. It underscores that the effectiveness of medical studies is heavily dependent on the quality and reliability of various equipment types—such as:
Each of these categories plays a critical role in ensuring patient safety, accurate data collection, and overall research integrity.
Understanding the Medtech landscape is vital as it addresses key challenges faced in clinical research. High-quality medical equipment not only facilitates effective treatment and monitoring but also enhances the accuracy of research findings. Consequently, the collaboration among researchers, medical professionals, and equipment manufacturers becomes imperative to overcome these challenges.
In conclusion, the importance of reliable medical equipment in clinical research cannot be overstated. By prioritizing quality and collaboration, stakeholders can ensure that their research efforts yield credible and impactful results. Next steps involve assessing current equipment capabilities and identifying areas for improvement.
In the fast-evolving landscape of clinical research, the right medical equipment can be the difference between success and failure. Each type of equipment, from diagnostic tools to life support systems, plays a crucial role in ensuring accurate data collection, patient safety, and effective treatment outcomes. As researchers navigate complex trials, the challenge lies not only in identifying essential equipment but also in understanding how each piece contributes to the overall integrity of the study.
What are the ten essential types of medical equipment that can empower clinical research and enhance healthcare delivery?
bioaccess® strategically positions itself in Latin America, the Balkans, and Australia to deliver exceptional research services. With the ability to secure ethical approvals in just 4-6 weeks and achieve enrollment rates that are 50% faster than traditional markets, bioaccess® empowers Medtech and Biopharma innovators to accelerate their products' journey to market. This agility is crucial in an environment where timely access to various medical equipment types can significantly impact outcomes and healthcare delivery.
With over 15 years of experience, bioaccess® possesses a deep understanding of the regulatory nuances and diverse participant demographics that greatly influence research success. Their steadfast commitment to ethical practices and high-quality research not only establishes them as a leader in the field but also makes them an invaluable partner for companies navigating the complexities of clinical trials. As the industry evolves, the focus on regulatory speed and ethical approvals remains critical, underscoring the essential role bioaccess® plays in advancing healthcare solutions.
Diagnostic equipment is pivotal in assessing individual health, incorporating a diverse array of tools such as stethoscopes, blood pressure monitors, and advanced imaging devices like MRI and CT scanners. These instruments are critical for gathering essential information on individual conditions, which is vital for evaluating the efficacy of new medical devices.
For instance, blood pressure monitors provide real-time insights into cardiovascular health, allowing researchers to monitor changes that may influence trial outcomes. Imaging technologies, particularly MRI and CT scanners, deliver detailed visualization of internal structures, facilitating the identification of underlying conditions that could impact evaluations.
Recent advancements in diagnostic tools, including high-resolution imaging technologies, have markedly enhanced the accuracy and efficiency of evaluations. By 2025, innovations such as AI-enhanced imaging systems are expected to further elevate diagnostic capabilities, leading to more precise assessments and improved outcomes for individuals.
Healthcare experts emphasize the importance of reliable diagnostic instruments, noting that their dependability directly affects the quality of medical studies. The integration of multimodal data—merging imaging, bio-signals, and patient history—has been demonstrated to improve diagnostic accuracy, diminishing the risk of misdiagnosis and enhancing treatment management for chronic diseases.
In summary, the effectiveness of medical research hinges on the quality of diagnostic equipment, underscoring the essential role these tools play in the research landscape.
Treatment equipment encompasses a diverse array of instruments essential for delivering care during research trials. Among these, infusion pumps emerge as critical devices for administering medications and fluids with precision, guaranteeing that patients receive the correct dosages at the appropriate intervals. A recent study underscores this significance, revealing that smart infusion pumps equipped with dose error reduction software can substantially enhance medication accuracy, potentially preventing 28% of infusion errors by alerting clinicians to dosing discrepancies. Furthermore, these smart infusion pumps maintain compliance rates exceeding 95% for safety standards, underscoring their reliability within healthcare environments.
Equally important are elastomeric pumps, which, being typically less expensive than more complex infusion systems, provide cost-effective solutions for medication delivery.
Surgical tools also hold paramount importance in medical studies, facilitating procedures that are integral to the investigative process. The effectiveness of these instruments is evidenced by hospitals that have adopted standardized procedures, reporting a remarkable 52% decrease in high-risk overrides. This statistic illustrates the impact of proper equipment usage on individual safety.
As we approach 2025, the role of infusion pumps in clinical studies is increasingly vital, particularly in managing complex treatment regimens associated with chronic and acute diseases. Their ability to sustain steady flow rates is crucial in critical situations, thereby enhancing the reliability of research outcomes. Additionally, the integration of infusion pumps with healthcare informatics systems improves tracking and monitoring, significantly reducing medication errors and enhancing safety for patients receiving care.
Training programs are indispensable for ensuring safe infusion practices, effectively minimizing programming errors and bolstering safety for individuals. In summary, the proper use and maintenance of medical equipment types, including infusion pumps and surgical instruments, are essential for ensuring safety and achieving reliable results in medical studies.
Monitoring devices are pivotal in tracking individual health during clinical studies, particularly in research conducted by bioaccess®, a leading contract research organization in Latin America. Devices such as:
deliver real-time data that reflect an individual's response to treatment. For example, heart rate monitors can alert researchers to any adverse reactions during a trial, facilitating immediate intervention. The ability to consistently track vital signs ensures that individual safety is prioritized and that potential issues are swiftly addressed, thereby enhancing the overall integrity of the research. With bioaccess®'s extensive expertise in managing:
the integration of medical equipment types is indispensable for achieving successful research outcomes.
Life support apparatus, which encompasses several medical equipment types including ventilators, defibrillators, and dialysis machines, is vital for individuals in critical condition or those participating in complex clinical trials. These devices are engineered to sustain life and support essential functions when individuals are unable to do so independently.
For example, ventilators deliver mechanical breathing assistance for individuals facing respiratory failure, significantly improving survival rates in critical care settings. Research indicates that effective ventilator management can reduce complications and enhance outcomes for individuals, underscoring their importance in medical studies. Moreover, healthcare professionals caring for individuals on ventilators must undergo annual competency evaluations, ensuring they are adequately trained to operate these essential devices.
Defibrillators are crucial in restoring normal heart rhythms during cardiac arrest, where every second is critical. Their prompt application can substantially elevate survival rates, rendering them indispensable in emergency situations. The integration of reliable medical equipment types for life support is essential not only for safety but also for the ethical conduct of clinical trials involving high-risk populations.
Additionally, advancements in dialysis machines have revolutionized healthcare since their inception in the early 1940s. Modern devices now feature automated control of dialysate intake and distribution, along with alert systems for unforeseen issues, ensuring that individuals with kidney failure remain healthy and toxin-free while awaiting replacement therapies. This evolution highlights the ongoing commitment to improving healthcare outcomes through innovative medical technology.
In summary, the availability of robust medical equipment types, particularly life support equipment, is crucial for ensuring patient safety and facilitating the ethical conduct of research studies, ultimately leading to enhanced health outcomes for patients in critical care.

Surgical tools are essential components of different medical equipment types used in a variety of medical processes, particularly in clinical studies. Key instruments such as scalpels, forceps, scissors, and retractors each serve distinct purposes that are critical for achieving successful outcomes. For instance, scalpels are primarily employed for making precise incisions, while forceps play a vital role in grasping and manipulating tissues during surgical procedures.
The impact of high-quality surgical tools on success rates is significant. A study involving 233 patients revealed a statistically significant reduction in surgical site infections (SSIs) through the implementation of a dedicated SSI prevention bundle. Additionally, the efficiency of surgical procedures is markedly improved when instruments are standardized, as demonstrated by Renown Health's initiative, which led to a 27% reduction in tray items and annual savings exceeding $50,000.
Innovations in surgical instruments continue to emerge, with advancements anticipated in 2025 that are expected to enhance research outcomes. The importance of utilizing quality surgical tools, which are among essential medical equipment types, is underscored by the fact that nearly 20% of all hospital-acquired infections in the United States are associated with SSIs, highlighting the necessity for meticulous instrument selection and management within medical environments. As the healthcare landscape evolves, the focus on reducing complications through advanced surgical tools remains essential for the success of medical studies.
In this context, partnering with bioaccess® ensures that research trials are supported by comprehensive management services, including:
These services are crucial for navigating the complexities of medical device trials, reinforcing the importance of collaboration in achieving favorable research outcomes.

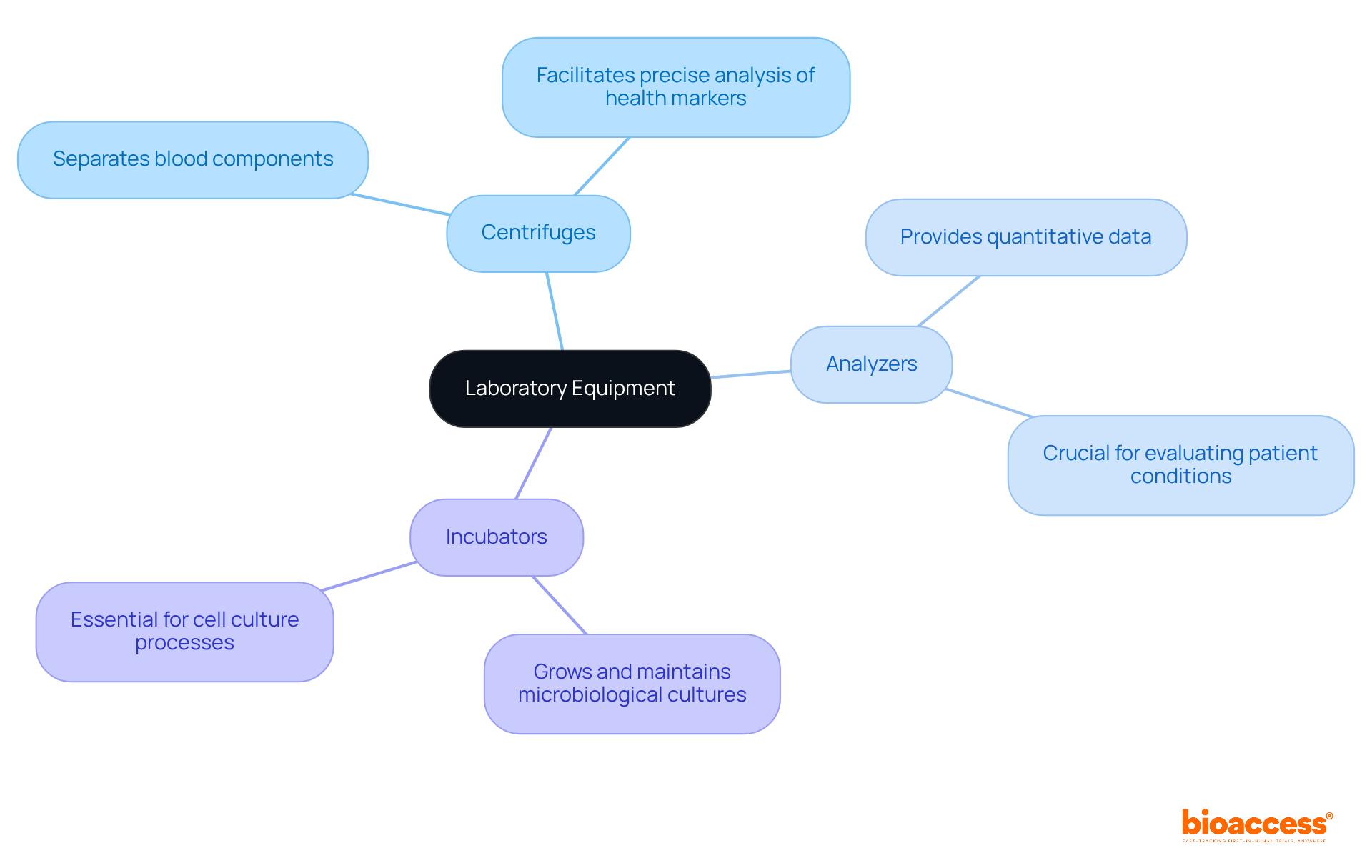
Various medical equipment types are essential for conducting diagnostic tests and analyses that are vital to medical research. Key medical equipment types, such as centrifuges, analyzers, and incubators, play an integral role in processing samples and generating reliable results. For instance, centrifuges effectively separate blood components, facilitating precise analysis of various health markers. Analyzers, conversely, provide quantitative data that is crucial for evaluating patient conditions.
The precision and dependability of these medical equipment types significantly impact the quality of data collected during clinical studies. Research shows that hospitals equipped with advanced molecular diagnostic tools have experienced a 30% increase in specialist referrals, highlighting the necessity of utilizing state-of-the-art equipment. Furthermore, automation in sample preparation has been proven to decrease test turnaround times, thereby enhancing overall efficiency in experimental environments.
As we approach 2025, the significance of centrifuges and analyzers in trials cannot be overstated. Their ability to deliver precise diagnostics is essential for advancing medical studies and improving outcomes for patients. Laboratory professionals assert that reliable medical equipment types are fundamental to achieving accurate testing and streamlined workflows, which ultimately drive progress in healthcare. Regular calibration and comprehensive training on the use of these tools are also critical for maintaining accuracy and ensuring safety in laboratory settings.
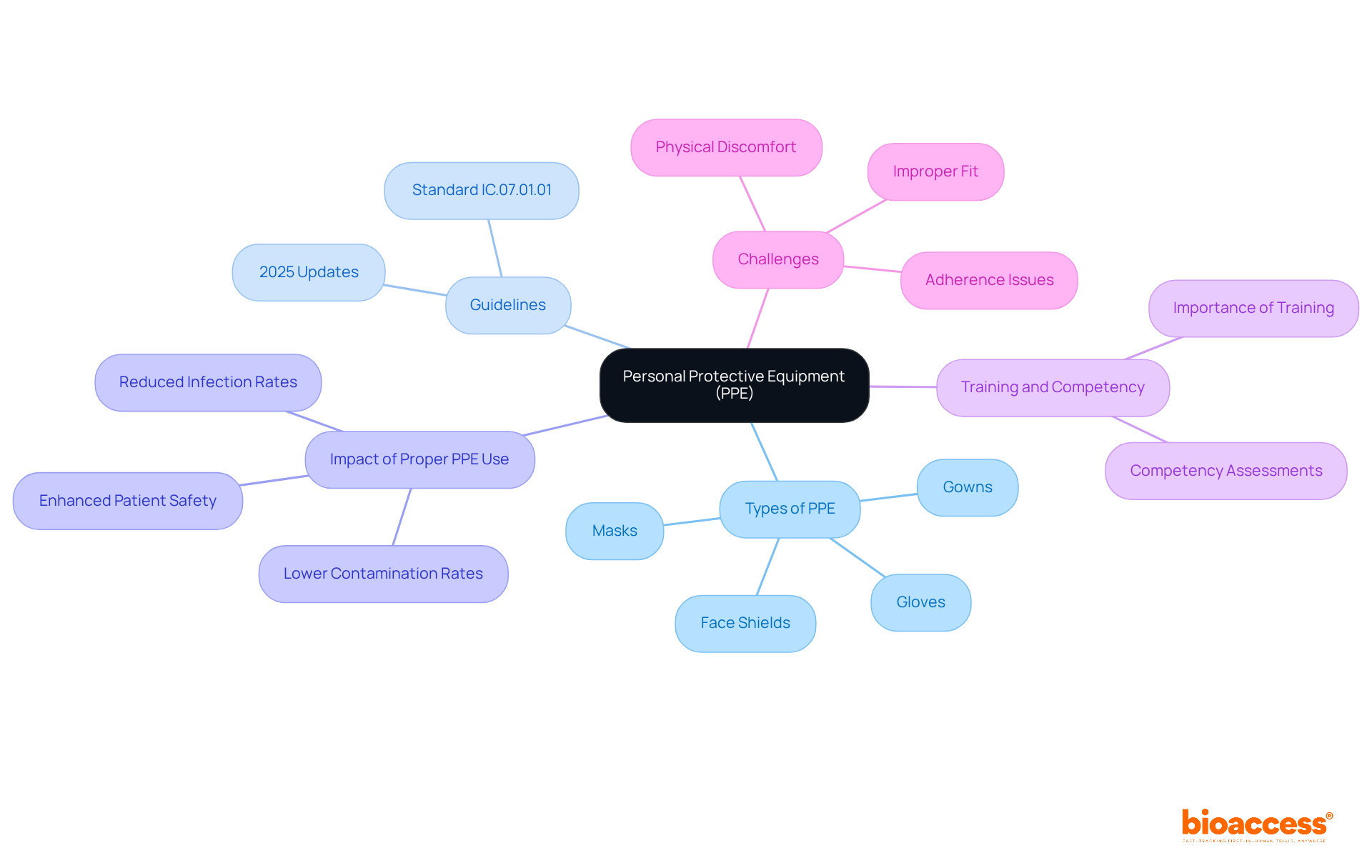
Personal protective equipment (PPE) is essential in research settings, as it protects healthcare workers and patients from infectious agents and hazardous substances. Key types of PPE include:
Each is designed to effectively mitigate exposure risks.
The latest guidelines for PPE in healthcare, set to take effect in 2025, underscore the necessity of properly fitting equipment to enhance protection. The updated standard IC.07.01.01 emphasizes High-Consequence Infectious Disease preparedness, detailing protocols for screening, isolation, and waste management. Experts in healthcare safety assert that adherence to PPE protocols significantly reduces infection rates in medical environments. For example, studies indicate that healthcare personnel (HCP) wearing short sleeves experienced less contamination than those in long sleeves, highlighting the critical role of appropriate attire in minimizing risks.
Real-world instances illustrate the impact of stringent infection control measures in healthcare studies. Facilities that implement comprehensive PPE training and competency assessments report lower contamination rates among HCP, showcasing the effectiveness of well-structured protocols. In these high-stakes environments, a commitment to PPE not only protects individuals but also upholds the ethical standards of medical research, ensuring that participant safety remains a top priority.
Assistive devices, including wheelchairs, walkers, and canes, are pivotal in enhancing mobility and independence, particularly within clinical studies centered on rehabilitation and recovery. These devices empower individuals to participate more actively in their care, facilitating their contributions to data collection during trials.
For example, walkers are essential for helping individuals regain balance and strength, while wheelchairs offer crucial mobility for those with limited physical abilities. The integration of assistive devices into clinical research not only leads to improved outcomes for individuals but also enriches the understanding of the effectiveness of various interventions.
Research demonstrates that the timely provision of mobility aids significantly boosts participant engagement in rehabilitation studies, ultimately promoting greater independence. Rehabilitation specialists underscore the necessity of mobility aids for recovery, as they allow individuals to navigate their environments more effectively, thereby encouraging involvement in therapeutic activities.
The importance of mobility aids in recovery studies is underscored by findings that show assistive devices can profoundly influence the trajectory of rehabilitation. By addressing mobility challenges, these aids not only facilitate physical recovery but also enhance the overall quality of life for individuals, enabling them to maintain greater autonomy in their daily activities. Furthermore, research indicates that factors related to device quality account for 20% of the variance in participation results, highlighting the critical role of reliable mobility devices in enhancing participant engagement and outcomes in rehabilitation studies. Additionally, the WHO's new wheelchair provision guidelines released in June 2023 reinforce existing standards and practices in assistive technology provision, emphasizing the significance of quality and accessibility in mobility aids.

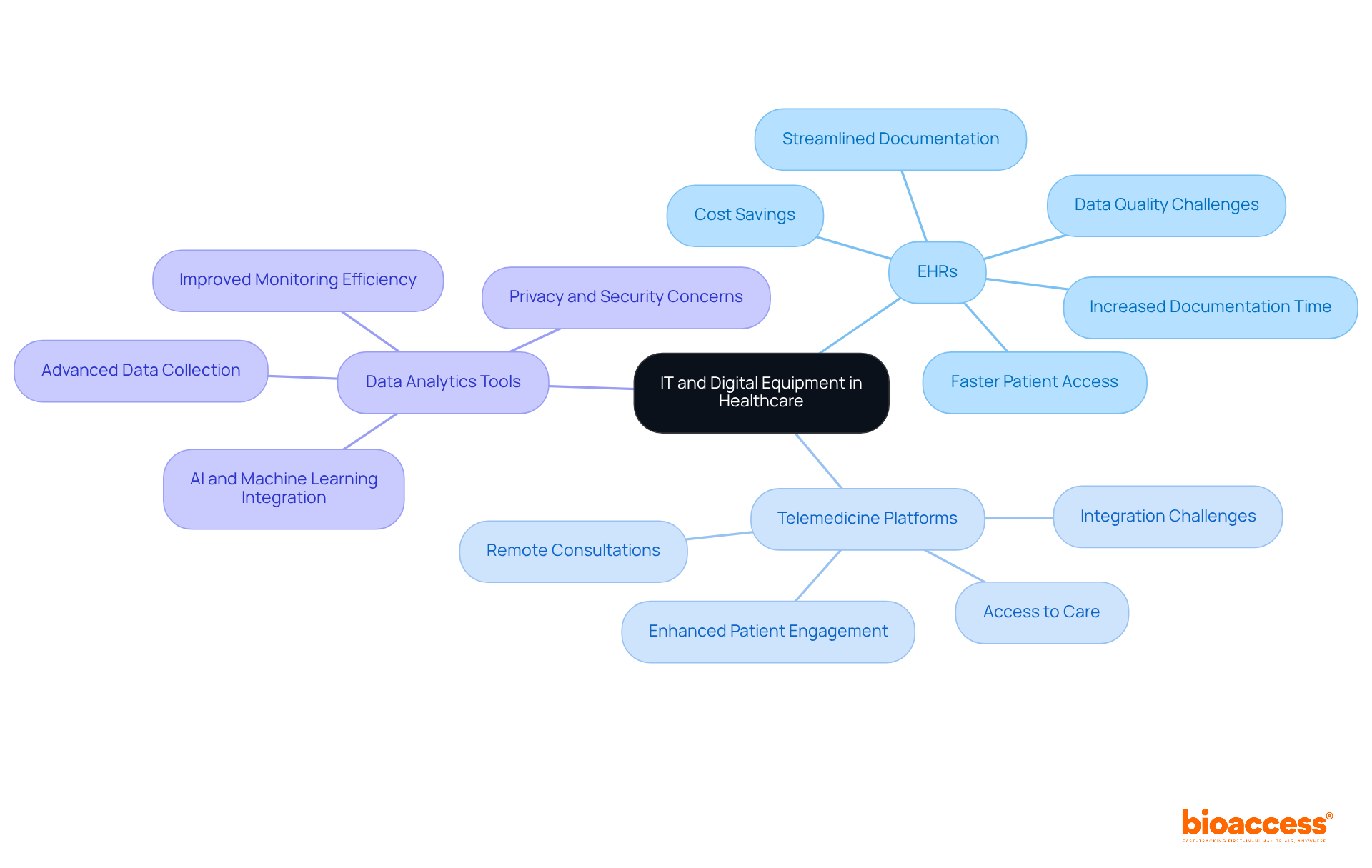
IT and digital medical equipment types are fundamentally transforming healthcare management, particularly within clinical research. Technologies such as electronic health records (EHRs), telemedicine platforms, and advanced data analytics tools are crucial for enhancing data collection and monitoring efficiency.
EHRs significantly streamline the documentation process, allowing researchers to access patient information swiftly and securely. This rapid access not only reduces the time spent on administrative duties but also minimizes documentation errors that could affect patient care. Research indicates that EHR integration can enhance the feasibility and efficiency of studies in recruitment, screening, and data collection. With bioaccess's FDA-ready information, studies can achieve participant enrollment 50% faster, resulting in substantial cost savings of $25K per individual, thus addressing common recruitment challenges faced by Medtech and biopharma startups.
Telemedicine platforms revolutionize patient engagement by enabling remote consultations, thereby expanding access to care. This capability is particularly vital in research studies, where participant involvement may be distributed across various locations. By integrating these digital solutions into medical studies, organizations can improve operational efficiency and elevate the overall quality of patient care, ensuring that innovative treatments reach patients more effectively and swiftly. Furthermore, comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—play a critical role in enhancing the success of medical initiatives.
Despite these advancements, operational challenges such as data quality and system diversity persist, complicating the integration of EMRs into healthcare studies. Addressing these challenges through policy reform and technological innovation is essential for maximizing the impact of EMRs on clinical research and healthcare delivery, ultimately contributing to job creation, economic growth, and improved healthcare outcomes.
The significance of medical equipment types in clinical research cannot be overstated; they serve as the backbone for successful trials and patient safety. Each category of equipment, from diagnostic tools to life support apparatus, plays a crucial role in ensuring that research outcomes are reliable and that patient care standards are upheld. The integration of advanced technologies and reliable instruments is essential for facilitating the ethical conduct of clinical studies, ultimately leading to improved healthcare solutions.
Throughout this article, various types of medical equipment have been highlighted, emphasizing their importance in different aspects of clinical research.
All contribute to a comprehensive approach to healthcare management and research efficacy.
As the landscape of clinical research continues to evolve, the call to action is clear: investing in high-quality medical equipment and embracing technological advancements is vital for enhancing patient outcomes and accelerating the development of innovative treatments. Stakeholders in the healthcare sector must prioritize the integration of reliable equipment and robust systems to navigate the complexities of clinical trials effectively, ensuring that the future of healthcare is not only innovative but also safe and effective for all patients involved.
What is bioaccess® and what services does it provide?
bioaccess® is a research service provider that operates in Latin America, the Balkans, and Australia, specializing in accelerating clinical research for medical devices and biopharma innovators.
How quickly can bioaccess® secure ethical approvals?
bioaccess® can secure ethical approvals in just 4-6 weeks.
How does bioaccess®'s enrollment rate compare to traditional markets?
bioaccess® achieves enrollment rates that are 50% faster than those in traditional markets.
Why is the agility of bioaccess® important for healthcare delivery?
The agility of bioaccess® is crucial because timely access to various medical equipment types can significantly impact patient outcomes and healthcare delivery.
What experience does bioaccess® have in the field?
bioaccess® has over 15 years of experience and a deep understanding of regulatory nuances and diverse participant demographics that influence research success.
What commitment does bioaccess® have regarding its research practices?
bioaccess® is committed to ethical practices and high-quality research, establishing itself as a leader in the field.
What role do diagnostic equipment play in patient assessment?
Diagnostic equipment, such as stethoscopes and imaging devices, is essential for assessing individual health and gathering information critical for evaluating new medical devices.
How do advancements in diagnostic tools affect medical research?
Recent advancements, including high-resolution imaging technologies, enhance the accuracy and efficiency of evaluations, leading to improved outcomes.
What are the key types of treatment equipment mentioned?
Key treatment equipment includes infusion pumps, elastomeric pumps, and surgical tools, all of which are essential for delivering care during research trials.
How do smart infusion pumps improve medication accuracy?
Smart infusion pumps equipped with dose error reduction software can prevent 28% of infusion errors by alerting clinicians to dosing discrepancies.
What is the significance of surgical tools in medical studies?
Surgical tools are crucial for facilitating procedures integral to the investigative process, and their proper usage can significantly enhance patient safety.
Why is training important for infusion practices?
Training programs are essential for ensuring safe infusion practices, minimizing programming errors, and bolstering safety for patients receiving care.
How does the integration of infusion pumps with healthcare informatics systems benefit clinical studies?
This integration improves tracking and monitoring, significantly reducing medication errors and enhancing patient safety.