


The article highlights the essential skills that every clinical trials statistician must master to uphold the integrity and reliability of clinical trial outcomes. It underscores the critical role of statistical methodologies, including regression analysis and ANOVA, while also stressing the necessity for ongoing education and the capacity to mitigate biases. These elements collectively enhance accurate data interpretation, which is vital for improving patient outcomes in medical research. By mastering these skills, statisticians can significantly contribute to the credibility of clinical trials, ensuring that the findings are both reliable and actionable.
The intricate world of clinical trials hinges on the expertise of statisticians who navigate complex data to ensure the validity of medical research outcomes. Mastering essential skills in statistical analysis not only enhances the reliability of findings but also significantly influences patient care and treatment protocols. However, with the rapid evolution of methodologies and the growing demands of healthcare, how can clinical trials statisticians stay ahead of the curve and effectively mitigate challenges such as bias and misinterpretation? This article explores ten critical skills every clinical trials statistician must master to thrive in this dynamic field and contribute meaningfully to medical advancements.
At bioaccess®, the role of a clinical trials statistician is paramount for mastering data analysis to uphold the integrity and reliability of clinical trial outcomes. The organization employs advanced analytical methodologies that are essential for a clinical trials statistician to assess the efficacy and safety of innovative medical technologies.
By adhering to best practices—such as ensuring sufficient sample sizes and conducting appropriate data distribution evaluations—bioaccess® aids the clinical trials statistician in mitigating common analytical errors that can compromise study validity. This expertise not only accelerates the research process but also enhances the credibility of findings, ultimately leading to improved patient outcomes and strengthened regulatory compliance.
The emphasis on robust statistical structures, including sensitivity evaluations and clearly defined estimands, further bolsters the dependability of medical research, ensuring that the results are both significant and actionable.

Statistical evaluation methods in clinical trials are integral to effective data assessment. Among these, regression evaluation stands out as pivotal for elucidating relationships between variables, allowing researchers to model and predict outcomes based on diverse factors.
Concurrently, Analysis of Variance (ANOVA) is essential for comparing means across multiple groups, thus facilitating the identification of significant differences in treatment effects.
Furthermore, survival analysis plays a crucial role in studies focused on time-to-event data, offering valuable insights into patient outcomes over time.
Mastery of these methodologies is indispensable for a clinical trials statistician, as they underpin robust study designs and ensure precise interpretations of results.
Current trends reveal an increasing emphasis on advanced regression techniques and ANOVA applications, reflecting the evolving landscape of medical research and underscoring the necessity for a clinical trials statistician to adapt to these advancements.

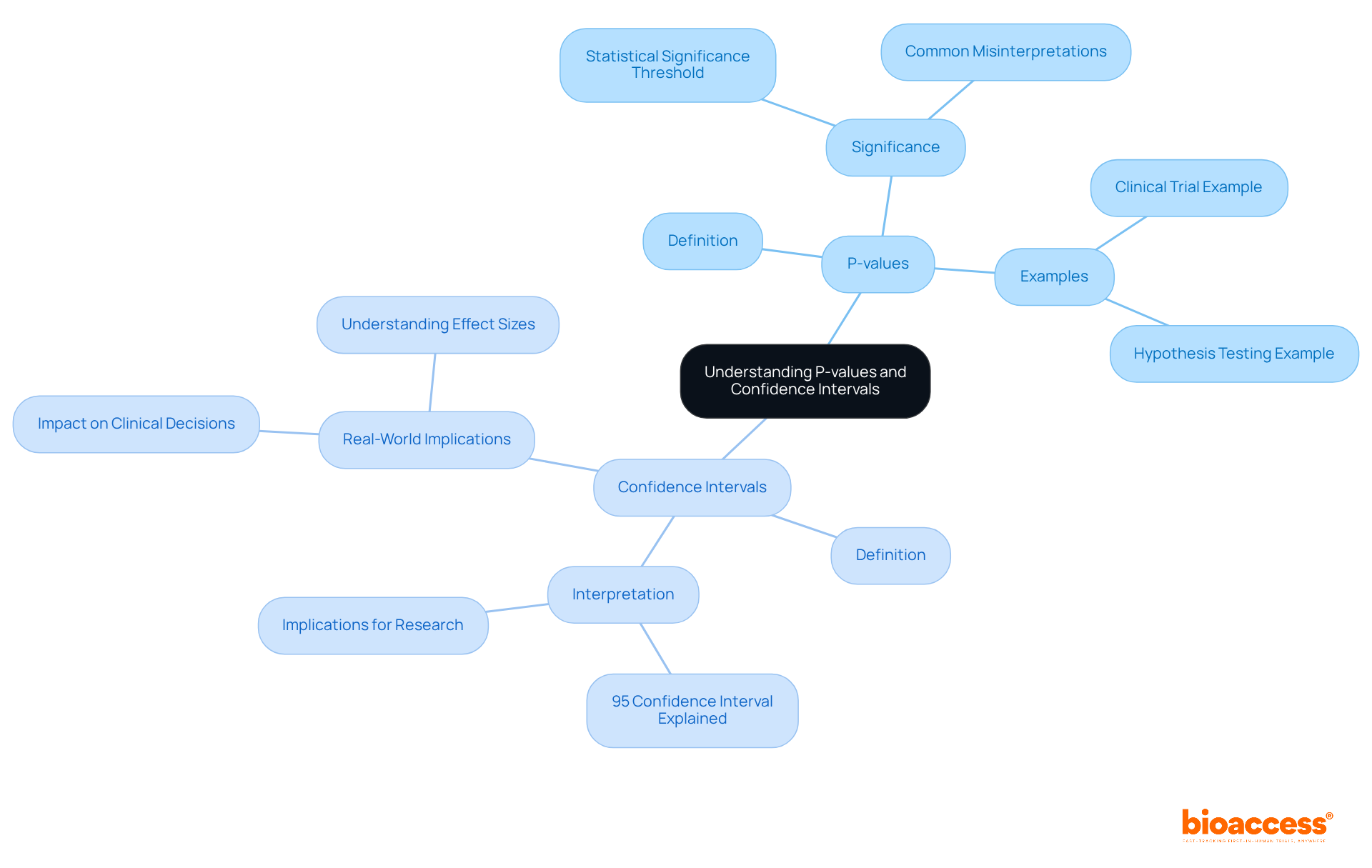
Understanding the importance of significance is essential in evaluating whether the outcomes of a medical study are likely a result of randomness. A common threshold for significance is a p-value of less than 0.05, which indicates that there is less than a 5% probability that the observed results occurred by random chance.
However, it is crucial to consider the practical importance of findings alongside numerical significance. A numerically significant result may not always translate into meaningful practical outcomes, underscoring the need for a comprehensive evaluation of research findings.

Preventing bias in statistical evaluation is essential for the reliability of medical studies. Bias can arise from various sources, including:
To mitigate these biases, implementing strategies such as:
is crucial. By ensuring that the study design minimizes bias, researchers can enhance the reliability of their findings, thereby supporting more accurate conclusions about the efficacy of treatments.

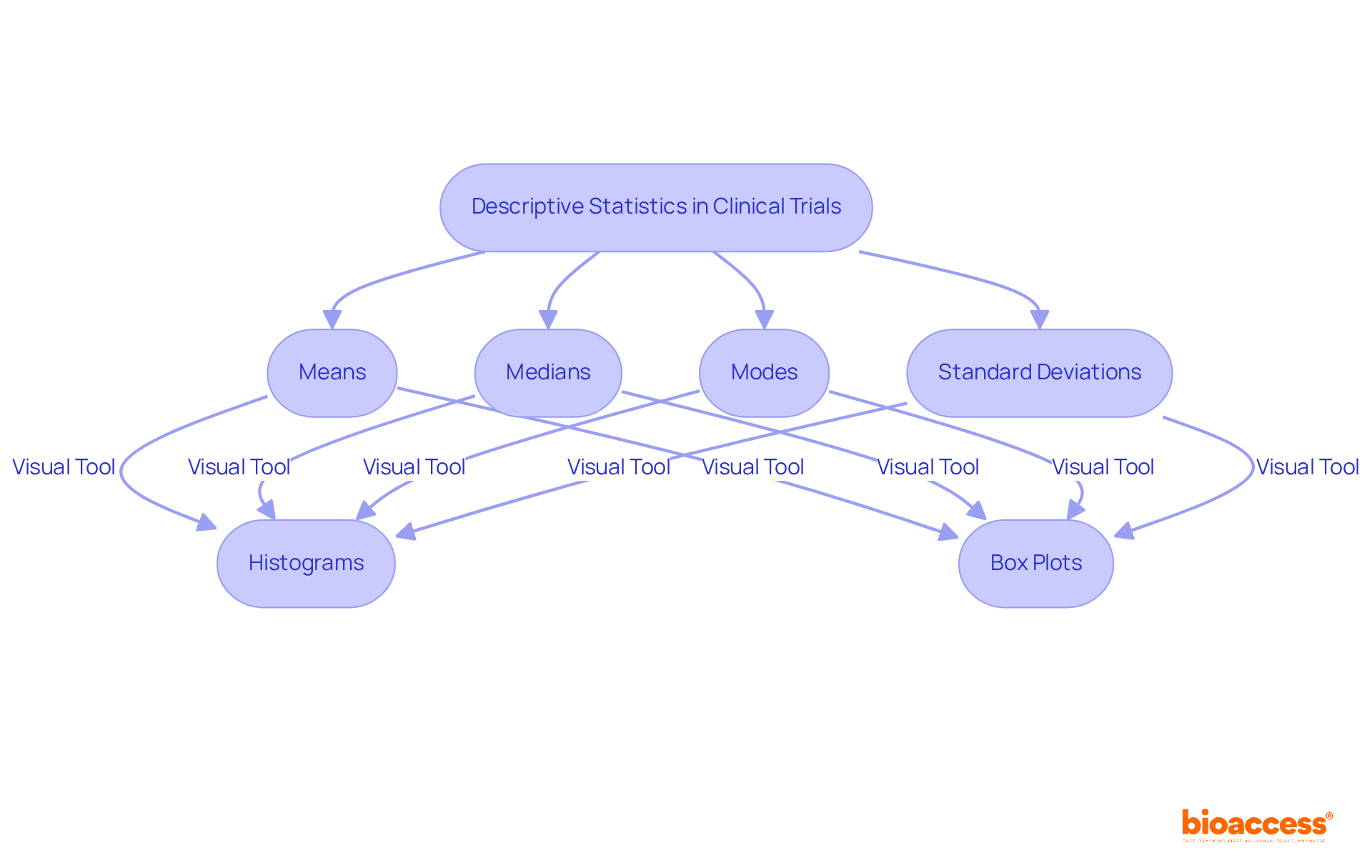
Descriptive statistics play a pivotal role in summarizing the characteristics of participants in clinical research studies conducted by a clinical trials statistician. Metrics such as means, medians, modes, and standard deviations provide a comprehensive overview of the data, which enables a clinical trials statistician to discern the distribution and central tendencies within their study populations. Furthermore, visual representations like histograms and box plots significantly enhance the communication of findings. These tools facilitate a clearer understanding for stakeholders, allowing them to appreciate the implications of the data more readily.
Inferential statistics empower researchers to make predictions and generalizations about broader populations based on sample data. This method is crucial in clinical research for a clinical trials statistician, where common techniques such as:
serve as foundational tools for drawing conclusions from study results. For instance, confidence intervals provide a range of values that likely include the true population parameter, offering insights into the precision of estimates derived from samples. Furthermore, the significance of results is vital in assessing the reliability of observed treatment effects, ensuring that findings are not merely coincidental. Continuous data evaluations are essential for identifying patterns or adverse occurrences during studies, which may necessitate adjustments to research protocols. This quantitative approach not only enhances the reliability of results but also aids a clinical trials statistician in making critical decisions in medical research, ensuring that conclusions are firmly grounded in rigorous data analysis.

P-values and confidence intervals serve as fundamental components of statistical evaluation in medical studies. A p-value quantifies the probability of observing the results under the assumption that the null hypothesis is true, while a confidence interval delineates a range of values likely encompassing the true effect size. For instance, a 95% confidence interval implies that if the study were conducted repeatedly, 95% of the intervals would capture the true population parameter. Grasping these concepts is crucial for a clinical trials statistician, as it enables them to assess the reliability and significance of their findings, ultimately enhancing the credibility of their research outcomes.
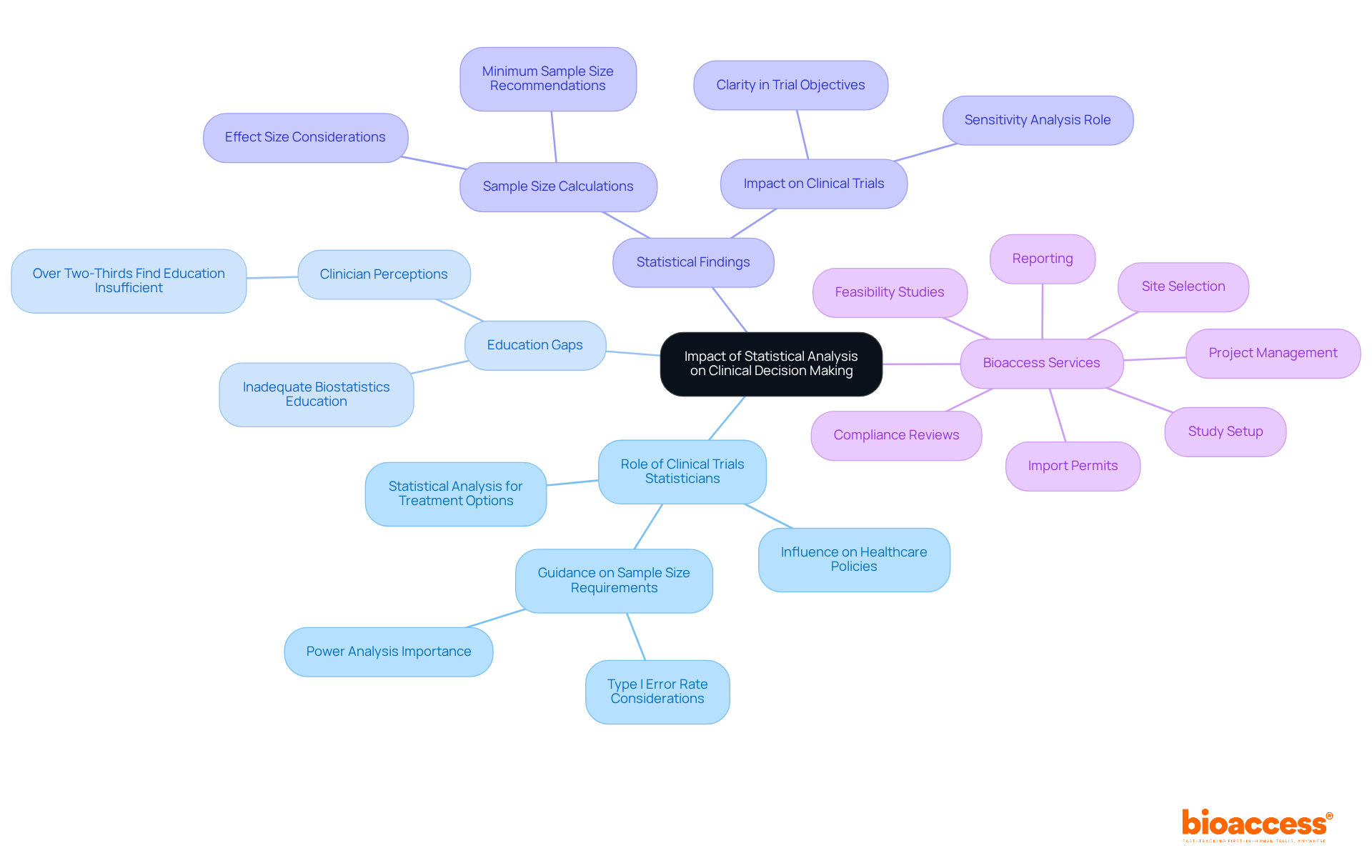
The role of a clinical trials statistician is indispensable in medical decision-making, as they provide statistical analysis and evidence-based insights that shape treatment options and patient care strategies. For instance, a clinical trials statistician can help ensure that clinical trial results directly inform healthcare providers regarding the efficacy and safety of new therapies, empowering them to make well-informed recommendations to patients. Notably, over two-thirds of clinicians perceive the level of biostatistics education offered to medical students as inadequate, underscoring a gap that could impact future healthcare decisions.
Furthermore, the impact of statistical findings extends to healthcare policy decisions and guidelines. A clinical trials statistician can use rigorous data analysis to pave the way for the establishment of new protocols that enhance patient outcomes. Recent data indicate that maintaining a Type I error rate below 0.05 often necessitates a sample size of at least 239, which a clinical trials statistician would advise, to achieve a power of 0.8, ensuring that studies yield reliable results. This underscores the critical importance of sufficient sample size considerations in medical research, which a clinical trials statistician must take into account, as smaller differences require larger sample sizes to achieve significant results.
Within the realm of comprehensive research management services, bioaccess offers capabilities such as:
This thorough management not only guarantees adherence to regulatory standards but also promotes international collaboration, advancing global health improvements through innovative Medtech solutions.
In conclusion, the integration of robust data evaluation by a clinical trials statistician in medical studies not only directs treatment choices but also profoundly influences healthcare policies and provider recommendations, ultimately enhancing patient care.
Proficiency in research terminology is essential for effective communication in medical studies. Fundamental terms such as 'mean,' 'median,' 'variance,' and 'standard deviation' serve as the backbone of data examination, enabling researchers to summarize and interpret information accurately.
Understanding 'type I error'—the probability of incorrectly rejecting the null hypothesis—and 'type II error'—the probability of failing to reject a false null hypothesis—is crucial for grasping the implications of hypothesis testing. For instance, a commonly accepted significance level is 0.05, indicating a 5% chance of a type I error in a study. This knowledge empowers researchers and clinicians to engage in informed discussions about study design, interpret results effectively, and understand their implications for patient care.
Furthermore, data evaluations can uncover negative occurrences or patterns during clinical studies, underscoring the importance of thorough mathematical techniques. Interim evaluations may lead to the early conclusion of a trial if the treatment is found to be ineffective or harmful, emphasizing the vital role of continuous numerical assessment.
In case studies involving inferential statistics, researchers employ hypothesis testing and regression analyses to draw conclusions about broader populations based on sample data. Descriptive statistics are also essential for summarizing participant characteristics and treatment outcomes, aiding in effective data interpretation.
This integration of research terminology not only enhances the reliability of medical studies but also fosters collaboration among researchers, ensuring that results are conveyed clearly and precisely.

Embracing ongoing education is essential for researchers in statistics to remain abreast of advancements in statistical methods and technologies. Regular training, attending workshops, and engaging with professional organizations significantly enhance skills and knowledge. Given the estimated physician deficit of up to 86,000 by 2036, along with a projected 34.1% increase in the population aged 65 and above, the need for a clinical trials statistician in medical studies is more urgent than ever. Staying informed about emerging trends in data analysis, including machine learning and big data analytics, can greatly improve the quality and efficiency of medical research.
As David J. Skorton, President and CEO of AAMC, emphasizes, "The medical education community and policymakers are making real progress in our efforts to meet the projected health care needs of our communities, but we must not be complacent."
This unwavering commitment to lifelong learning ultimately contributes to better patient outcomes and more effective clinical trials, highlighting the critical role of a clinical trials statistician in addressing the evolving challenges in healthcare through continuous professional development.

Mastering the essential skills of a clinical trials statistician is crucial for ensuring the integrity and reliability of medical research outcomes. This underscores the importance of various statistical methodologies, from descriptive and inferential statistics to understanding significance and avoiding bias. Such competencies not only enhance the validity of clinical trials but also significantly contribute to informed decision-making in healthcare.
Key arguments highlight the necessity of advanced statistical techniques, including:
These techniques facilitate accurate data interpretation and help mitigate biases that can skew results. Furthermore, the integration of continuous learning and adaptation to emerging trends in data analysis is vital for clinical trials statisticians to remain effective in their roles, especially in a rapidly evolving medical landscape.
Ultimately, the role of a clinical trials statistician extends beyond mere data analysis; it is integral to shaping treatment protocols and improving patient outcomes. As the demand for skilled statisticians continues to grow, a commitment to ongoing education and proficiency in statistical methods will be essential for addressing the challenges of modern healthcare and ensuring that clinical research continues to drive advancements in patient care.
What is the role of a clinical trials statistician at bioaccess®?
The clinical trials statistician at bioaccess® is responsible for mastering data analysis to ensure the integrity and reliability of clinical trial outcomes by employing advanced analytical methodologies.
How does bioaccess® support clinical trials statisticians?
bioaccess® supports clinical trials statisticians by ensuring sufficient sample sizes, conducting appropriate data distribution evaluations, and helping to mitigate common analytical errors that can compromise study validity.
What are the benefits of adhering to best practices in statistical analysis during clinical trials?
Adhering to best practices accelerates the research process, enhances the credibility of findings, improves patient outcomes, and strengthens regulatory compliance.
What statistical methods are commonly used in clinical trials?
Common statistical methods include regression evaluation for modeling relationships between variables, Analysis of Variance (ANOVA) for comparing means across multiple groups, and survival analysis for studying time-to-event data.
Why is it important for a clinical trials statistician to master statistical methodologies?
Mastery of statistical methodologies is essential for ensuring robust study designs and precise interpretations of results, which are critical for the validity of clinical research.
What is the significance of a p-value in clinical research?
A p-value of less than 0.05 is commonly used as a threshold for statistical significance, indicating that there is less than a 5% probability that the observed results occurred by random chance.
How should researchers evaluate the significance of study findings?
Researchers should consider both the numerical significance (such as p-values) and the practical importance of findings, as a numerically significant result may not always lead to meaningful practical outcomes.