


The article delineates ten essential skills for a Clinical Operations Manager, underscoring the critical nature of:
Each skill is indispensable for ensuring efficient clinical trials, maintaining compliance, and aligning research activities with organizational goals. This comprehensive skill set is not merely advantageous; it is vital for achieving success in this pivotal role.
In the intricate world of clinical research, the role of a Clinical Operations Manager is pivotal, demanding a unique blend of skills that can significantly influence the success of medical studies. These professionals are the backbone of clinical trials, navigating complex regulatory landscapes and fostering effective communication among diverse stakeholders.
This article delves into the ten essential skills that define an effective Clinical Operations Manager, exploring how these competencies enhance operational efficiency and drive impactful outcomes in patient care and medical innovation.
What challenges do these managers face in mastering such a multifaceted role? How can they leverage their skills to overcome these challenges?
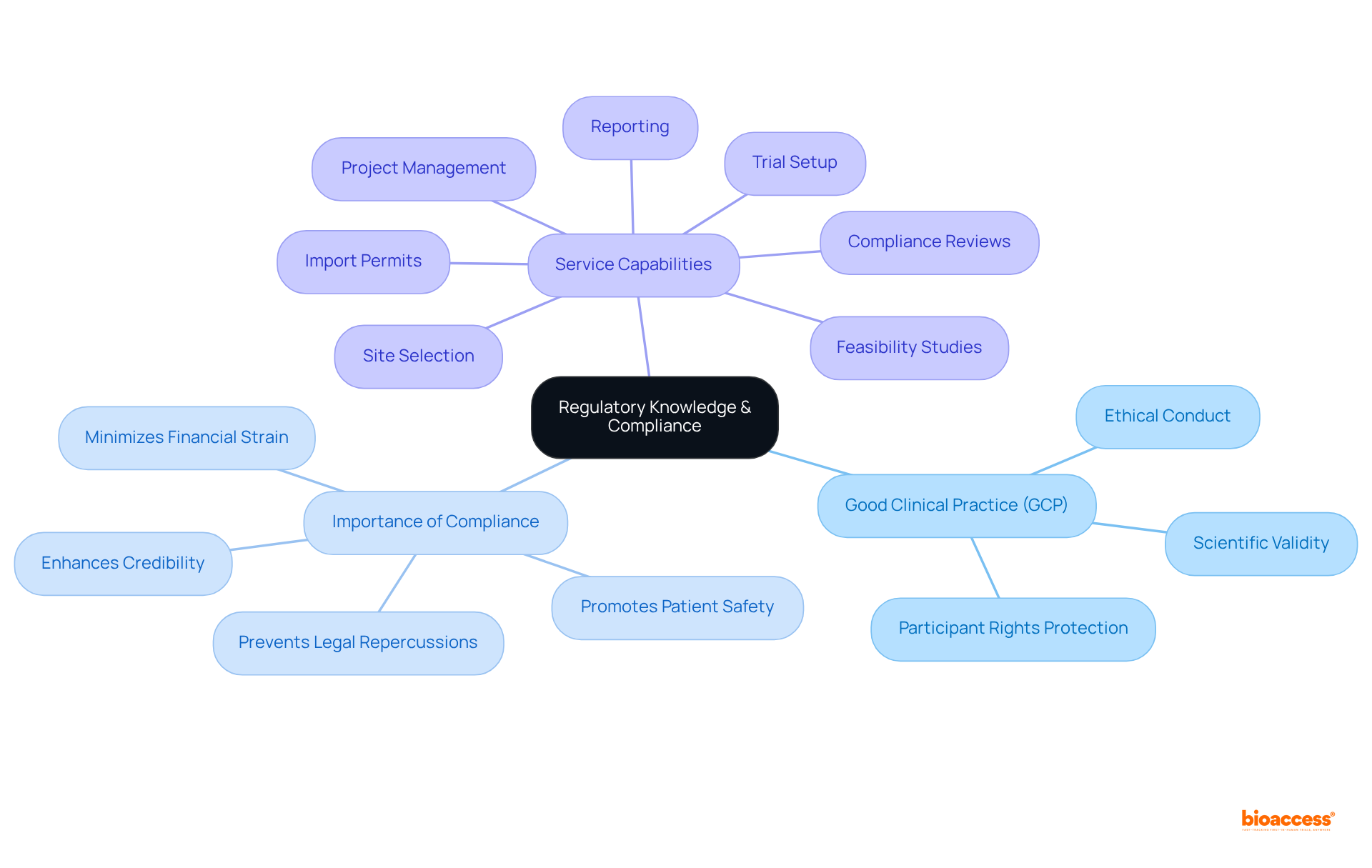
A clinical operations manager must possess a thorough understanding of the regulatory framework overseeing research studies, particularly the principles of Good Clinical Practice (GCP). GCP adherence is essential, as it directly influences study outcomes by ensuring that research is conducted ethically and scientifically, thereby enhancing the validity and reliability of results. Successful medical studies that adhere to GCP not only protect participant rights but also enhance the credibility of the investigation, fostering trust among stakeholders. Continuous education on regulatory updates is essential, as it protects the organization from legal repercussions and ensures that all studies meet the necessary compliance standards. As emphasized by industry experts, a clinical operations manager plays a fundamental role in maintaining rigorous compliance with GCP to advance medical knowledge while prioritizing patient safety.
Nossos recursos de serviço incluem:
These services are essential for maintaining GCP standards. The financial consequences of non-compliance can strain research budgets and impede future study capabilities. A strong pharmacovigilance system is essential for overseeing the safety of investigational products during research studies, further emphasizing the significance of thorough regulatory compliance.
A clinical operations manager in healthcare must excel in project management to effectively oversee the various stages of clinical studies. This role involves:
By employing project management tools, processes can be streamlined, and communication among team members can be enhanced. Ultimately, this leads to a more efficient execution of tests, which is crucial for the role of a clinical operations manager in the fast-paced Medtech landscape.
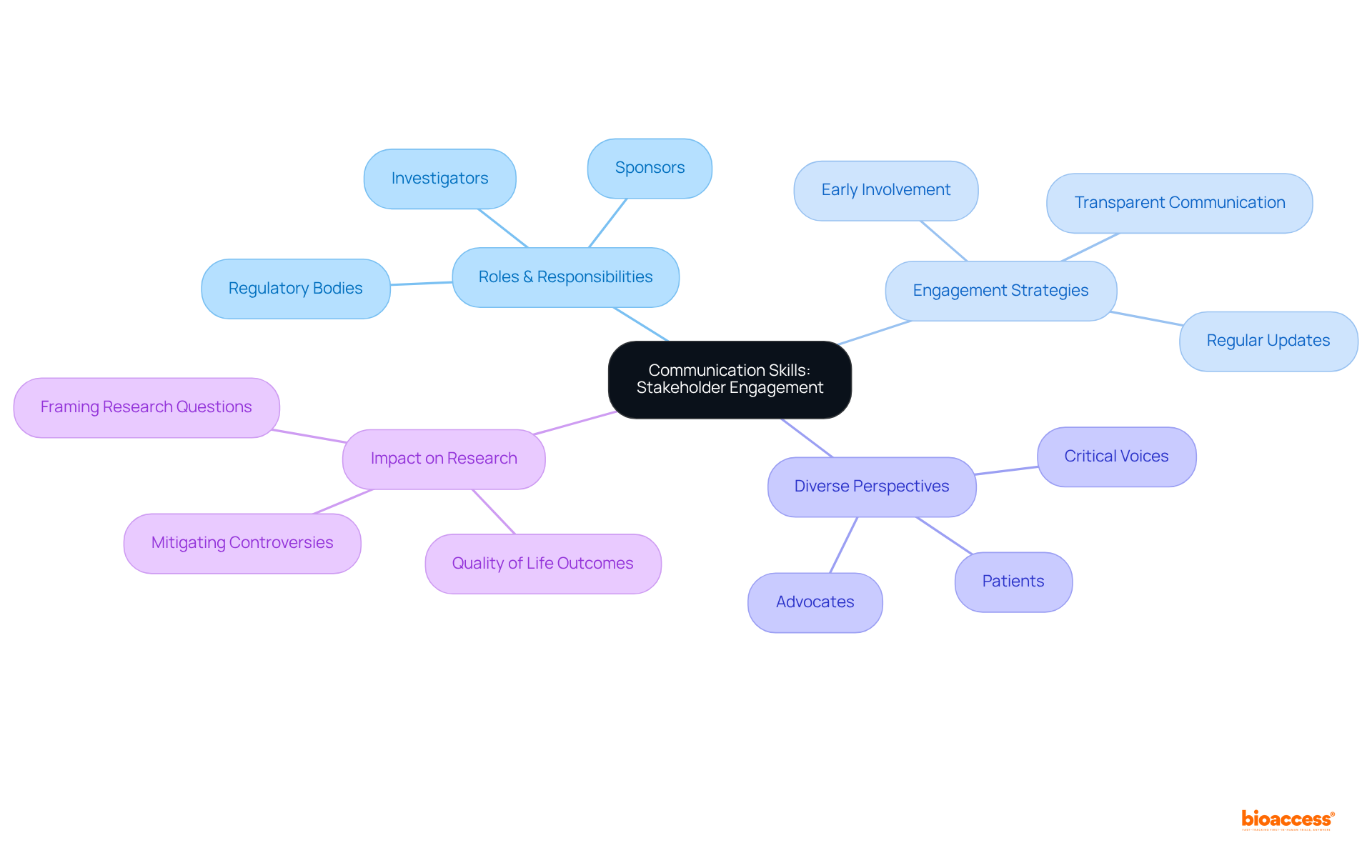
A clinical operations manager must excel in communication to effectively engage stakeholders, including investigators, sponsors, and regulatory bodies. Clear and concise communication is crucial for ensuring that all parties comprehend their roles and responsibilities, which is vital for the success of the proceedings. Regular updates and meetings foster transparency, allowing for prompt addressing of concerns. Engaging stakeholders early in the process not only enhances the relevance of research outcomes but also builds credibility and buy-in.
Studies have shown that stakeholder involvement can significantly influence the framing of research questions. Moreover, incorporating diverse perspectives, including those of patients and advocates, can lead to more nuanced questions and improve the overall quality of the review process. This proactive engagement strategy helps anticipate potential controversies, ensuring smoother reviews and better acceptance of findings. Ultimately, this enhances the likelihood that systematic reviews will be effectively utilized in practice.
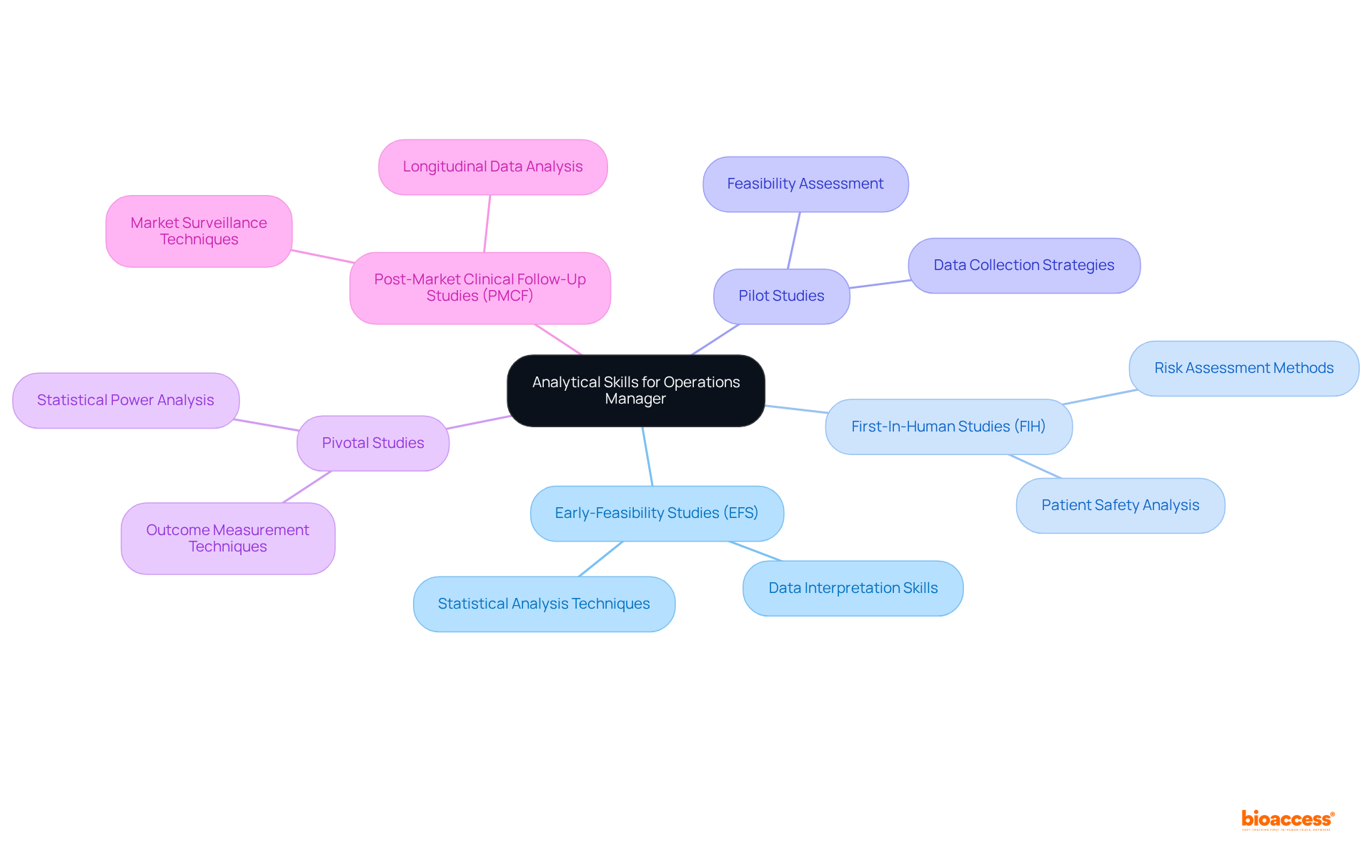
An Operations Manager must exhibit strong analytical capabilities to evaluate data collected during research studies, particularly within the diverse projects managed by bioaccess. This role necessitates a solid understanding of statistical methods, enabling the manager to extract significant insights from data gathered in:
The expertise and adaptability required to oversee these medical device clinical trials are essential, as they empower the manager to thoroughly assess trial results and make informed decisions about the study's continuation or modification. Such decisions ultimately influence patient outcomes and the overall success of the research.
A clinical operations manager must exhibit strong leadership abilities to inspire and manage their team effectively, particularly when confronted with challenges such as convoluted regulatory requirements and competition from established players in the medical device industry. This role necessitates:
By fostering a positive and collaborative work environment, the manager can enhance team performance and ensure that everyone is aligned with common goals. Furthermore, effective leadership by a clinical operations manager is crucial for navigating the intricacies of research management, including:
These elements are essential for the success of medical device startups.
To effectively navigate the myriad challenges that arise during research studies, a Clinical Operations Manager must exhibit exceptional problem-solving abilities. This responsibility encompasses not only the early identification of potential issues but also the formulation of robust strategies to mitigate them. By embracing a proactive approach to problem-solving, managers can ensure that projects remain on schedule and within budget, ultimately yielding successful outcomes.
As one seasoned clinical operations manager noted, the ability to anticipate and address obstacles can significantly impact project timelines, highlighting the importance of these skills in achieving medical study objectives.
Furthermore, employing organized problem-solving methodologies enables teams to respond swiftly to unforeseen challenges, thereby enhancing overall efficiency and effectiveness.
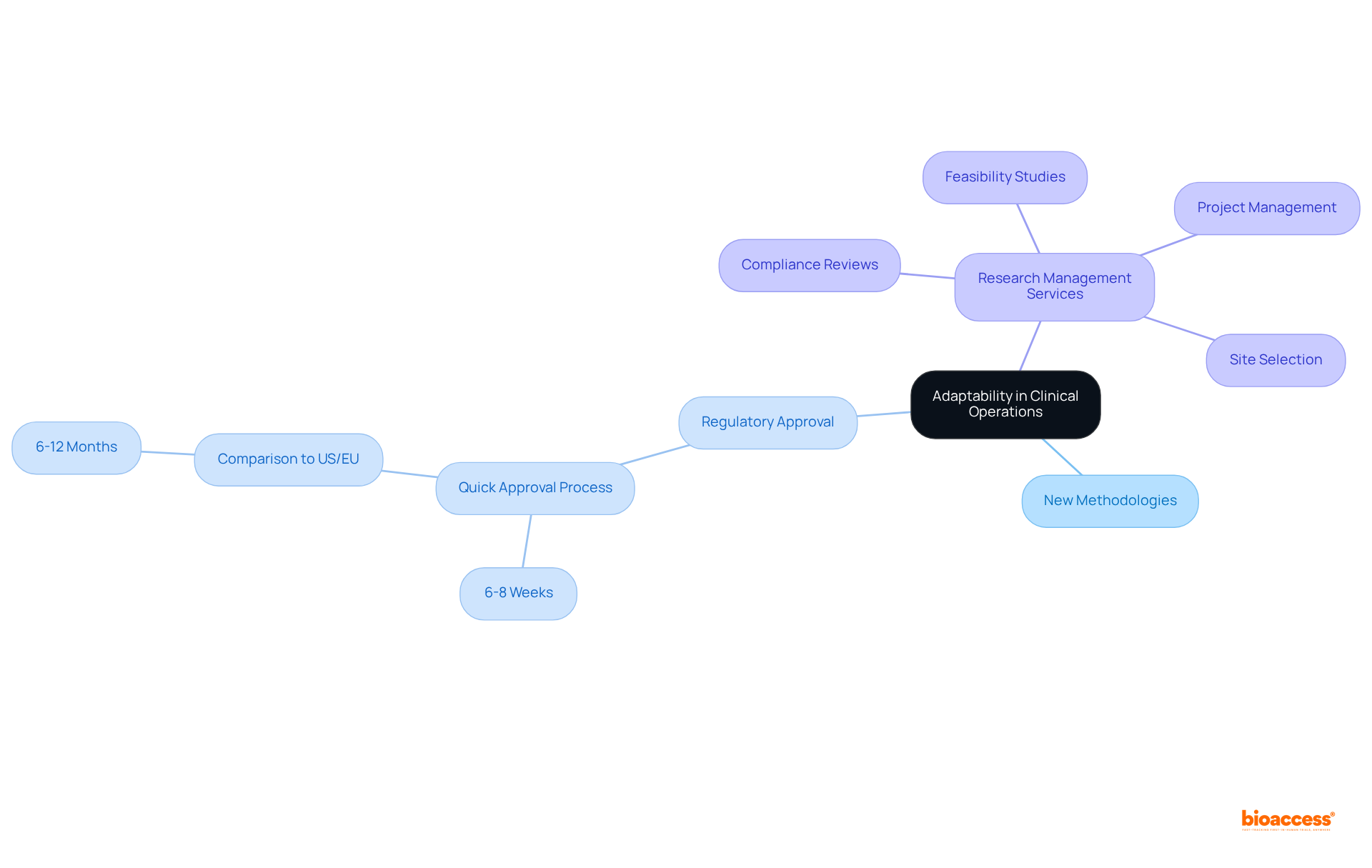
A clinical operations manager must exhibit flexibility to respond to the ever-evolving landscape of medical studies. This adaptability includes openness to new methodologies, technologies, and regulatory shifts, exemplified by bioaccess®'s remarkable capability to secure regulatory approval in just 6-8 weeks—significantly quicker than the typical 6-12 months in the US/EU. By embracing this flexibility, the clinical operations manager can adeptly modify experimental protocols and strategies as needed, ensuring that the research remains relevant and effective in achieving its objectives.
Furthermore, a deep understanding of extensive research management services—such as:
enables the clinical operations manager to efficiently navigate the complexities of patient recruitment and regulatory hurdles.
A Clinical Operations Manager is required to demonstrate exceptional financial insight to effectively manage the budgets and resources associated with research studies. This responsibility encompasses the development of precise budget forecasts and the ongoing monitoring of expenditures to ensure optimal resource allocation.
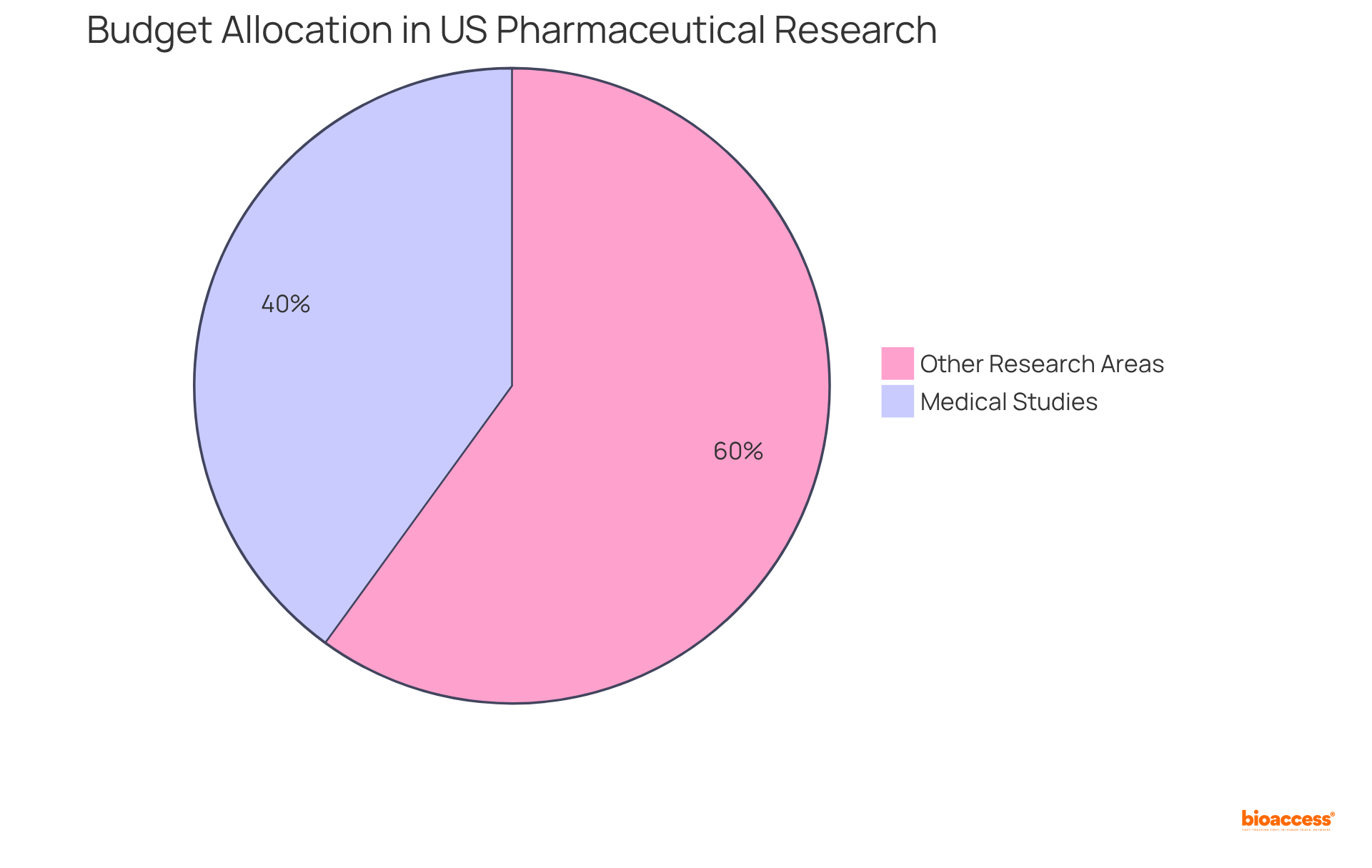
Effective financial supervision is paramount for a clinical operations manager; research indicates that budget excesses can lead to costs for sponsors ranging from $600,000 to $8 million for each day a study is postponed. Notably, medical studies account for nearly 40% of the US pharmaceutical research budget, totaling approximately $7 billion annually.
By employing robust budgeting techniques, such as Functional Service Provider (FSP) models, a clinical operations manager can improve cost estimation accuracy and establish realistic timelines, ultimately preventing cost overruns and ensuring that projects stay within financial constraints.
Furthermore, bioaccess offers comprehensive management services for studies, which include:
These services are vital for effective financial management. As industry specialist Patricio Ledesma aptly states, "Every dollar saved in medical studies can be reinvested into groundbreaking innovation," underscoring the critical nature of meticulous financial oversight in fostering medical advancements.
A robust technical skill set in research management systems (CTMS) and associated technologies is essential for a Clinical Operations Manager. Mastery of these tools is essential for optimizing data collection, monitoring, and reporting processes. The clinical operations manager ensures that the effective utilization of CTMS significantly enhances operational efficiency, leading to improved data accuracy and streamlined communication among team members.
As bioaccess®, a prominent Contract Research Organization with over 15 years of experience in research services, emphasizes, the clinical operations manager plays a crucial role in utilizing CTMS to ensure adherence to regulatory standards, such as those established by INVIMA, and facilitating the successful execution of trials in Latin America. This capability ultimately supports the rapid advancement of medical innovations.
Key challenges for medical data managers include handling incomplete data and ensuring compliance with data privacy regulations, underscoring the importance of skilled CTMS utilization. In addressing these challenges, the clinical operations manager can leverage collaboration and expertise in CTMS to drive significant improvements in the clinical research landscape.
A clinical operations manager is essential in integrating strategic thinking into operational processes, ensuring that these efforts align with the organization's overarching goals. This alignment requires a deep understanding of the organization's mission and objectives, enabling the clinical operations manager to prioritize research studies that directly support these aims. By embracing a strategic approach, the manager not only enhances the relevance of medical research but also maximizes its potential impact on patient outcomes and the broader healthcare landscape. As emphasized by industry leaders, aligning trials with organizational goals transcends mere operations; it is a strategic imperative that defines the success of clinical initiatives.
A clinical operations manager plays a pivotal role in the success of clinical trials, requiring a diverse skill set that encompasses regulatory knowledge, project management, communication, and adaptability. Mastering these essential skills not only ensures compliance with industry standards but also enhances the overall efficiency and effectiveness of clinical research, ultimately leading to improved patient outcomes and advancements in medical science.
The article highlights ten critical skills necessary for a clinical operations manager, including:
Each skill contributes to navigating the complexities of clinical trials, from maintaining rigorous compliance with regulations to fostering stakeholder engagement and utilizing technology for streamlined operations. Emphasizing these competencies can significantly impact the quality and relevance of research outcomes.
In a rapidly evolving medical landscape, the importance of these skills cannot be overstated. As clinical operations managers face new challenges and opportunities, embracing continuous learning and adaptation will be crucial. By honing these essential skills, managers can not only enhance their own effectiveness but also drive innovations that benefit the healthcare industry as a whole. The call to action is clear: invest in developing these competencies to ensure that clinical operations remain aligned with organizational goals and responsive to the needs of patients and stakeholders alike.
What is the importance of Good Clinical Practice (GCP) in clinical research?
Good Clinical Practice (GCP) is essential in clinical research as it ensures that studies are conducted ethically and scientifically, which enhances the validity and reliability of results. Adherence to GCP protects participant rights and fosters trust among stakeholders.
What are the responsibilities of a clinical operations manager regarding regulatory compliance?
A clinical operations manager is responsible for maintaining rigorous compliance with regulatory frameworks, particularly GCP. This includes understanding regulatory updates, conducting compliance reviews, and ensuring that all studies meet necessary standards to protect the organization from legal repercussions.
What services are offered to maintain GCP standards in clinical trials?
Services offered to maintain GCP standards include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What are the financial implications of non-compliance in clinical research?
Non-compliance can lead to financial consequences that strain research budgets and impede future study capabilities.
Why is project management important for a clinical operations manager?
Project management is crucial for a clinical operations manager as it involves developing timelines, allocating resources, and ensuring team alignment with project goals, leading to efficient execution of clinical trials.
How can project management tools benefit clinical studies?
Project management tools can streamline processes and enhance communication among team members, resulting in more efficient execution of clinical trials.
What communication skills are necessary for a clinical operations manager?
A clinical operations manager must excel in clear and concise communication to effectively engage stakeholders, ensuring that all parties understand their roles and responsibilities, which is vital for the success of clinical studies.
How does stakeholder engagement influence research outcomes?
Engaging stakeholders early in the research process enhances the relevance of outcomes and builds credibility. It can also lead to more nuanced research questions and improve the overall quality of the review process.
What is the significance of incorporating diverse perspectives in research?
Incorporating diverse perspectives, including those of patients and advocates, can lead to better framing of research questions and enhance the likelihood that systematic reviews will be effectively utilized in practice.
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
Forcura scales up with Growth Era and increases pipeline velocity by 200% (https://growthera.com/case_studies/forcura)
The TCM Group | Case studies (https://thetcmgroup.com/case_studies)
We are looking for an driven CRAII to join our clinical team, home-based in Perth or Brisbane, looking after a growing portfolio of Oncology trials with an opportunity to work across other… | Krisztina Auth (https://linkedin.com/posts/krisztina-auth_iconstrategicsolutions-australia-clinicalresearchassociate-activity-7285815144339664896-FCBw)