


This article delineates ten essential steps for effective medical device risk management, underscoring the critical nature of systematic processes such as risk analysis, control measures, and continuous improvement. These processes are vital to ensuring safety and compliance in the Medtech landscape. Adherence to ISO 14971:2019 standards is paramount, complemented by proactive strategies that incorporate comprehensive documentation and post-production activities. Ultimately, these measures lead to the introduction of safer medical devices into the market, reinforcing the importance of a robust risk management framework.
Navigating the intricate landscape of medical device risk management is vital for ensuring patient safety and regulatory compliance. With the stakes higher than ever, manufacturers must adopt a systematic approach that not only identifies and mitigates risks but also aligns with global standards like ISO 14971:2019. This article outlines ten essential steps that empower organizations to enhance their risk management strategies, fostering a culture of accountability and continuous improvement.
How can manufacturers effectively integrate these practices to not only meet regulatory demands but also drive innovation in medical technology?
bioaccess® offers specialized clinical research services that significantly enhance safety oversight for medical device innovators. By leveraging extensive expertise in early-phase clinical studies, bioaccess® ensures that all safety activities comply with stringent regulatory standards. This includes meticulous selection of research locations and principal investigators, as well as strict adherence to country-specific requirements. Such proficiency in navigating complex regulatory environments allows companies to concentrate on innovation while prioritizing security and efficacy throughout the development process.
The integration of robust hazard mitigation strategies within medical device risk management not only minimizes potential risks but also fosters a culture of compliance and accountability. This commitment ultimately leads to safer medical products reaching the market. Notably, successful collaborations, such as with Avantec Vascular for their first-in-human clinical study in Latin America, exemplify how bioaccess®'s services are effectively applied in real-world scenarios. By enhancing clinical research practices, bioaccess® plays a pivotal role in improving safety statistics for medical instruments, thereby reinforcing their dedication to medical device risk management and advancing healthcare innovation.

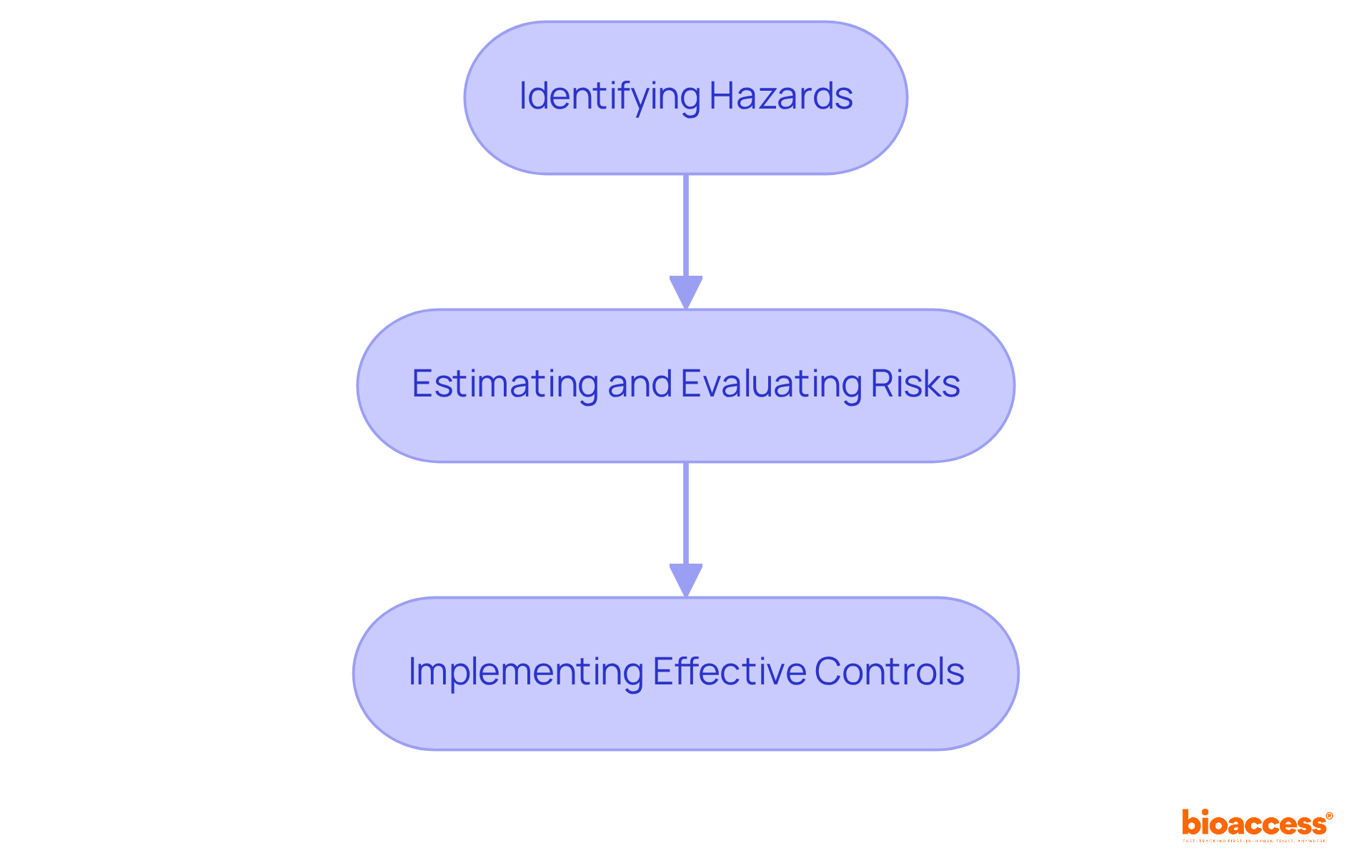
ISO 14971:2019 stands as the definitive global standard for medical device risk management concerning hazards associated with medical devices. This standard delineates a systematic approach for medical device risk management, which includes:
Understanding this standard is imperative for manufacturers, as it directly influences the reliability and efficacy of their products. By adhering to ISO 14971:2019, manufacturers not only enhance patient safety but also cultivate trust with regulatory bodies and stakeholders.
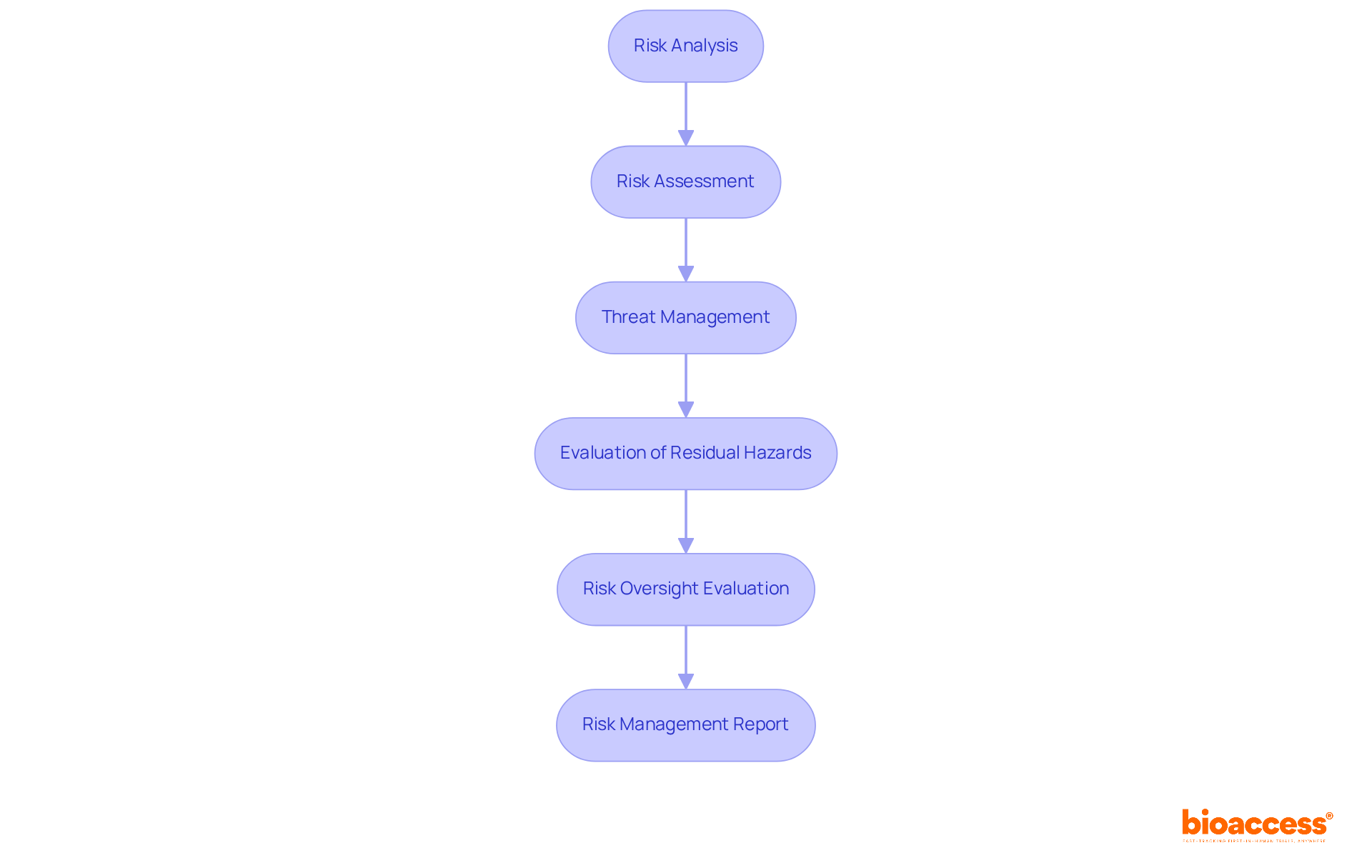
Implementing a structured six-step process for medical device risk management is essential for enhancing the safety and effectiveness of medical devices. The steps are as follows:
This systematic approach not only aligns with ISO 14971 standards but also promotes medical device risk management while fostering a culture of safety and continuous improvement throughout the product lifecycle. Furthermore, the Management Plan must outline the scope of activities, intended use of products, roles and responsibilities, and acceptability criteria, ensuring a comprehensive framework for the management process.
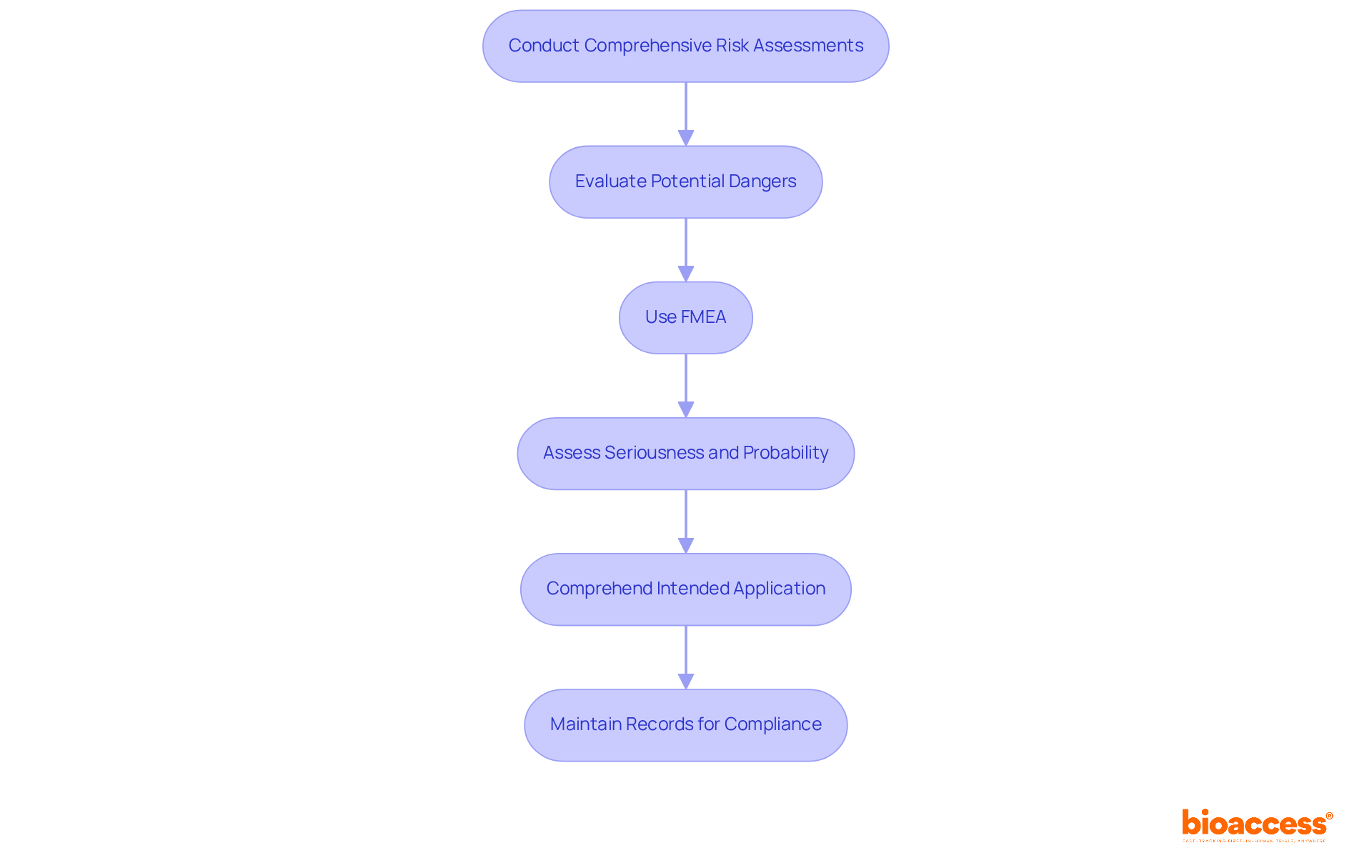
Thorough evaluations of potential dangers are essential for medical device risk management as they help in recognizing hazards related to medical instruments. This process entails a comprehensive examination of the device's design, materials, and intended use within the framework of medical device risk management to reveal factors that could affect patient safety.
According to ISO 14971, the potential for harm is defined as the combination of the probability of occurrence of harm and the severity of that harm. Instruments such as Failure Mode and Effects Analysis (FMEA) are crucial for medical device risk management, as they systematically identify and prioritize threats, ensuring that all potential dangers are addressed early in the development process.
FMEA enables teams to assess the seriousness and probability of failure modes, promoting proactive management of potential issues. As highlighted by industry specialist Peter Sebelius, comprehending the intended application of a medical instrument is vital, as it directly affects the safety profile.
Furthermore, keeping records of who participated in the analysis and when it was conducted is crucial for adherence to ISO 14971. The risk control file should compile all documents created during the risk process, serving as a comprehensive reference throughout the medical device's lifecycle.
By employing these methodologies, manufacturers can enhance safety and compliance through effective medical device risk management, ultimately leading to more successful market entry. Medical device risk management is not a one-time task but a continuous process that must adapt to new information and changes in the product lifecycle.
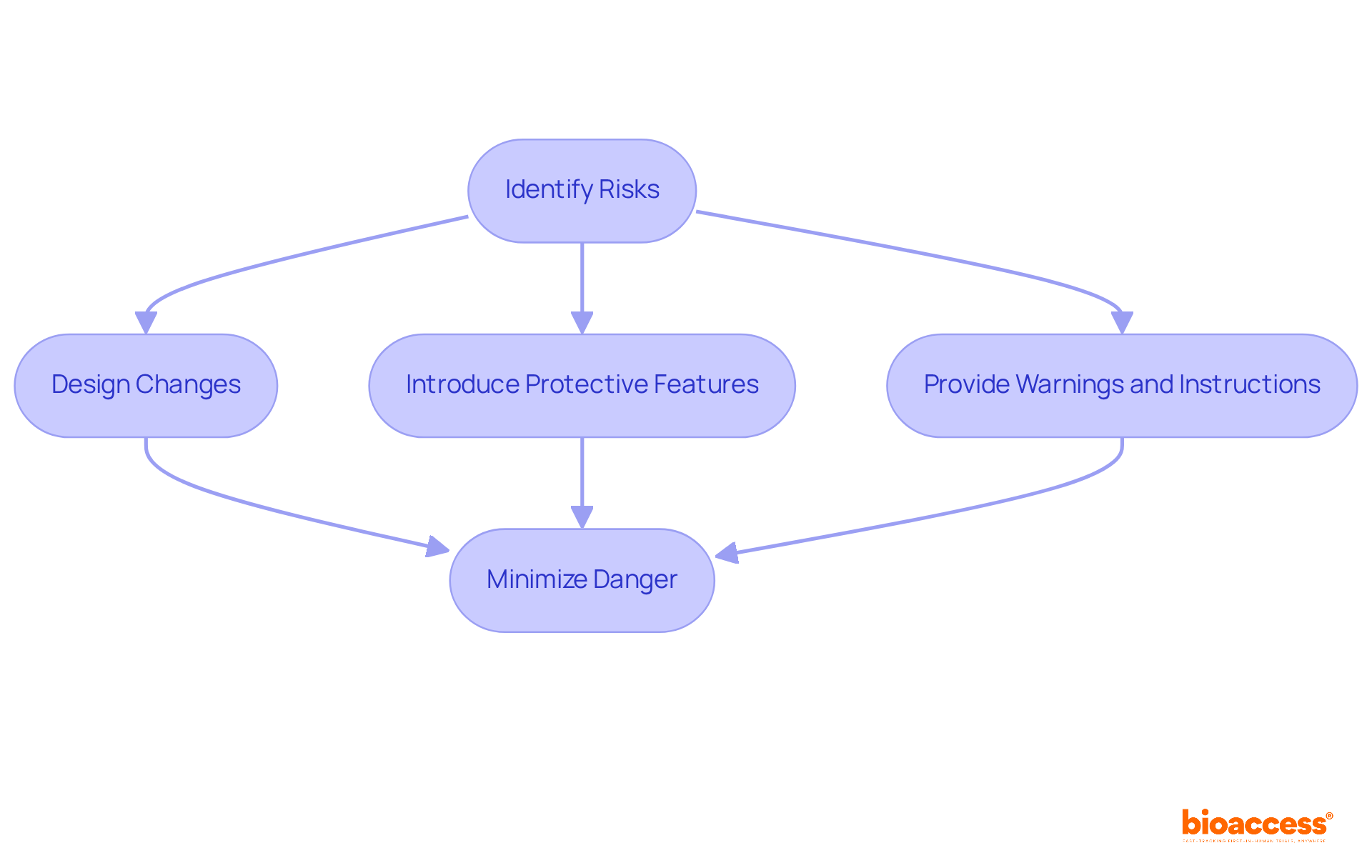
Once threats have been identified, it is critical to establish effective control measures. This may include:
The objective is to minimize danger to an acceptable level while preserving the device's functionality. Regular reviews and updates of these measures are essential to ensure ongoing compliance and safety.
After implementing control measures, it is imperative to assess any residual uncertainties that may remain. This evaluation must determine whether the outstanding uncertainties are acceptable, considering medical device risk management and the benefits provided by the medical instrument. Documenting this assessment is vital for regulatory compliance and offers essential clarity to stakeholders regarding medical device risk management and the safety of the equipment.

Conducting regular evaluations of potential issues is crucial for the continuous improvement of medical equipment reliability and effectiveness. These assessments should examine the efficacy of risk control measures, identify new risks that may emerge, and ensure that the medical device risk management process aligns with current regulations and standards, such as those established by INVIMA, the Colombia National Food and Drug Surveillance Institute.
INVIMA plays a pivotal role in overseeing the marketing and production of health products, including medical devices, ensuring compliance with quality and effectiveness standards. This proactive strategy supports the maintenance of high security standards and adaptation to any shifts in the clinical landscape.
For instance, the FDA's recent initiative to release daily adverse event data enables organizations to more effectively identify trends and emerging issues that may impact product performance. Additionally, the case study of the Nuffield Orthopaedic Centre illustrates how effective hazard oversight practices can be implemented in real-world scenarios, ensuring patient safety and minimizing disruptions during transitions.
Furthermore, engaging in upcoming webinars, such as those focusing on impurity profiling and sterility testing, can provide valuable insights and strategies for enhancing safety practices. Ultimately, the ongoing enhancement of hazard oversight is not merely a regulatory obligation; it is a strategic imperative for medical device risk management that can significantly influence the success of Medtech innovations.
Keeping thorough records of all management activities is essential for compliance with regulatory requirements. This includes documenting evaluations, control measures, assessments, and reviews. Experts such as Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, emphasize that proper documentation not only bolsters regulatory submissions but also acts as a valuable resource for future projects.
By leveraging insights gained from past experiences, organizations can enhance their current practices, particularly in the areas of medical products and cannabis regulation in Colombia. Moreover, meticulous documentation practices are vital for demonstrating adherence to industry standards and mitigating potential compliance issues. Organizations with robust documentation often encounter significantly fewer regulatory challenges.
Incorporating medical device risk management through hazard assessment into the design and development processes of medical devices is essential for guaranteeing safety from the outset. This integration involves embedding medical device risk management concepts into design evaluations, prototyping, and testing stages. By addressing potential challenges early, manufacturers can enhance medical device risk management, develop safer products, and reduce the likelihood of costly redesigns later in the development cycle.
Leveraging the expertise of specialists such as John Myklusch, who has over 20 years of experience in financial strategy and oversight, and Katherine Ruiz, a regulatory expert in medical products, it is imperative to adopt a holistic approach that not only identifies potential challenges but also aligns financial planning with security objectives and regulatory standards.
This strategic integration can enhance financial performance and improve product security, ultimately benefiting both manufacturers and consumers.
Carrying out post-production management activities is essential for guaranteeing the continuous protection of medical instruments, particularly in relation to medical device risk management and adherence to the regulatory standards established by INVIMA, the Colombia National Food and Drug Surveillance Institute. This encompasses:
By actively implementing medical device risk management after the product is available, manufacturers can swiftly address any emerging safety issues and maintain compliance with INVIMA's standards, which are crucial for the safety, efficacy, and quality of medical equipment. Experts such as Katherine Ruiz, who specialize in regulatory affairs for medical devices and in vitro diagnostics in Colombia, offer invaluable guidance in navigating these complex regulations.

Effective medical device risk management is a multifaceted process that demands stringent adherence to established standards and a commitment to continuous improvement. By integrating comprehensive risk management strategies, manufacturers not only enhance patient safety but also build trust with regulatory bodies and stakeholders. The systematic approach outlined here emphasizes the importance of understanding ISO 14971:2019, implementing a structured six-step process, and maintaining thorough documentation throughout the product lifecycle.
Key insights from this discussion highlight the necessity of:
Collaboration with expert clinical research services, such as those provided by bioaccess®, exemplifies how specialized knowledge can streamline compliance and enhance safety oversight. Furthermore, integrating risk management into the design and development phases significantly reduces potential hazards and improves overall product quality.
Ultimately, the ongoing commitment to medical device risk management is not merely a regulatory requirement; it is a strategic imperative that can influence the success of innovations in healthcare. By prioritizing safety and compliance, manufacturers can ensure that their products not only meet market demands but also contribute positively to patient outcomes. Embracing these essential steps will pave the way for safer medical devices and a more resilient healthcare landscape.
What services does bioaccess® provide for medical device innovators?
bioaccess® offers specialized clinical research services that enhance safety oversight, ensuring compliance with regulatory standards during early-phase clinical studies.
How does bioaccess® ensure safety in clinical research?
bioaccess® ensures safety by meticulously selecting research locations and principal investigators while adhering to country-specific regulatory requirements.
What is the significance of hazard mitigation strategies in medical device risk management?
Robust hazard mitigation strategies minimize potential risks and foster a culture of compliance and accountability, leading to safer medical products.
Can you provide an example of bioaccess®'s successful collaboration?
An example is bioaccess®'s collaboration with Avantec Vascular for their first-in-human clinical study in Latin America, showcasing the effective application of their services.
What is ISO 14971:2019?
ISO 14971:2019 is the global standard for medical device risk management that outlines a systematic approach to identifying hazards, estimating and evaluating risks, and implementing effective controls.
Why is understanding ISO 14971:2019 important for manufacturers?
Understanding this standard is crucial for manufacturers as it enhances patient safety and builds trust with regulatory bodies and stakeholders.
What are the six steps in the risk management process for medical devices?
The six steps are: 1. Risk Analysis 2. Risk Assessment 3. Threat Management 4. Evaluation of Residual Hazards 5. Risk Oversight Evaluation 6. Risk Management Report
How does the six-step process align with ISO 14971 standards?
The six-step process aligns with ISO 14971 by promoting systematic risk management and fostering a culture of safety and continuous improvement throughout the product lifecycle.
What should be included in the Risk Management Report?
The Risk Management Report should summarize all risk management activities, including benefit-risk analyses, to support regulatory submissions before the medical device is marketed.
What elements are covered in the Management Plan for risk management?
The Management Plan outlines the scope of activities, intended use of products, roles and responsibilities, and acceptability criteria for the risk management process.