


The landscape of Investigational Device Exemption (IDE) trials is rapidly evolving, propelled by technological advancements and regulatory reforms that aim to streamline the clinical research process. As innovators strive to introduce groundbreaking medical devices to the market, grasping the essential strategies that underpin successful IDE trials is crucial. This article explores ten critical approaches that not only enhance the efficiency of these trials but also ensure compliance and data integrity.
What challenges hinder success in IDE trials, and how can organizations utilize expert guidance to navigate these obstacles?
bioaccess® specializes in providing tailored support for IDE trials, drawing on extensive expertise in regulatory frameworks and diverse patient demographics across Latin America, the Balkans, and Australia. This strategic approach enables rapid ethical approvals within 4-6 weeks and enhances experimental processes, including setup and project management. By empowering innovators to expedite their clinical studies while adhering to regulatory standards and addressing market needs, bioaccess® not only shortens timelines but also elevates the integrity and quality of the data collected. The result? Faster market access for groundbreaking medical devices.
As we look ahead to 2025, the landscape of IDE trials is undergoing significant transformation, with a strong focus on integrating digital technologies and adaptive study designs. These advancements are reshaping evaluation methods, allowing for real-time data monitoring and adjustments based on interim results. Regulatory agencies are also streamlining their processes, which markedly boosts success rates for medical studies. For example, companies like Avantec Vascular have successfully executed first-in-human studies in Colombia, thanks to the support of bioaccess®, showcasing the effectiveness and feasibility of conducting clinical research in Latin America.
Industry leaders emphasize that the combination of regulatory speed and strategic support from organizations like bioaccess® is crucial for enhancing the efficiency of IDE trials. This collaboration not only drives innovation but also ensures that new medical devices can reach the market more quickly, ultimately benefiting patients and healthcare systems alike. Are you ready to explore how bioaccess® can help you navigate the complexities of clinical research and accelerate your path to market?
Understanding the regulatory framework for IDE trials is crucial for anyone embarking on clinical research. This framework, established by the FDA and other relevant authorities, delineates the types of devices that require IDEs, the necessary documentation for submission, and the criteria that differentiate significant risk (SR) from non-significant risk (NSR) devices. For example, the FDA requires sponsors to submit a comprehensive IDE application, which includes prior investigations and an investigational plan that outlines the study's protocol and objectives.
Familiarity with these regulations not only aids in crafting a robust IDE application but also equips sponsors to navigate potential challenges during the approval process. Did you know that approximately 80% of clinical studies experience delays due to recruitment issues? This statistic underscores the importance of engaging with regulatory experts early on. By involving these specialists in the planning phase, sponsors can significantly enhance their chances of a successful submission, ensuring compliance and expediting the approval process.
In summary, understanding the IDE trials framework and collaborating with regulatory experts is essential for navigating the complexities of clinical research. Taking proactive steps now can lead to smoother approvals and ultimately, more successful studies.
Choosing the appropriate group of individuals is vital for the success of IDE trials. This process necessitates the establishment of specific eligibility standards that align with the study's objectives. Key factors to consider include:
By leveraging data analytics, researchers can significantly improve the identification of potential participants who meet these criteria, thus streamlining the recruitment process.
Moreover, collaborating with local healthcare providers can broaden access to a diverse array of individuals, ensuring that the study accurately reflects the varied demographics of the population expected to utilize the device. This multifaceted approach not only bolsters the credibility of IDE trials but also enhances participant recruitment efficiency, addressing common challenges faced in clinical research.
In the ever-evolving Medtech landscape, the importance of strategic partnerships and data-driven recruitment cannot be overstated. As you consider your own clinical research challenges, reflect on how these strategies could transform your recruitment efforts. The next steps involve embracing collaboration and utilizing analytics to ensure your study's success.

Effective communication strategies are crucial for the success of IDE trials. A comprehensive communication plan must detail how information will be disseminated among stakeholders, including sponsors, investigators, regulatory bodies, and patients. Frequent updates and clear reporting of project progress are vital for sustaining stakeholder involvement. By addressing concerns promptly, we foster trust and collaboration, which are essential in this landscape.
Leveraging digital platforms for real-time communication streamlines interactions, ensuring all parties are informed of any changes or developments. This proactive strategy not only reduces misunderstandings but also cultivates a cooperative atmosphere. Ultimately, this approach improves the success rates of IDE trials, reinforcing the importance of collaboration in clinical research.
Creating robust information management practices is essential for the success of ide trials and clinical studies. A comprehensive information management plan must outline clear procedures for data collection, storage, analysis, and ide trials. Did you know that ide trials utilizing Electronic Data Capture (EDC) systems can reduce operational expenses by up to 30%? This not only lowers costs but also significantly enhances accuracy and integrity during ide trials by minimizing human error associated with manual input.
Frequent audits and oversight are vital to ensure compliance with Good Clinical Practice (GCP) guidelines, which are crucial for maintaining the integrity of study information in ide trials. Furthermore, bioaccess employs advanced security measures, including encryption and role-based access controls, to safeguard information related to ide trials. This commitment to security enhances the trustworthiness of the data for regulatory submissions, reinforcing the credibility of the research.
In addition to data management, bioaccess offers extensive management services for ide trials and studies, such as:
As the landscape of medical research evolves, leveraging EDC systems alongside ide trials will be pivotal in achieving effective and compliant study operations. Collaboration in this area is not just beneficial; it is essential for navigating the complexities of clinical research.

Training clinical staff is not just important; it’s essential for ensuring compliance and maintaining high standards in clinical research. This thorough instruction must cover all aspects of clinical conduct, including regulatory adherence, information management, and participant interaction. Regular training sessions and workshops are vital for keeping staff informed about the latest regulations and best practices.
But how can we ensure that our staff is truly prepared? Incorporating simulation-based training significantly enhances practical skills, allowing staff to navigate real-world scenarios effectively. For instance, programs that utilize role-playing and interactive workshops have shown to improve staff engagement and knowledge retention dramatically.
A highly skilled group does more than just improve efficiency in testing; they play a crucial role in enhancing safety for subjects and ensuring information integrity. This ultimately leads to more dependable outcomes. In the ever-evolving landscape of clinical research, investing in comprehensive training is not merely a choice; it’s a necessity.
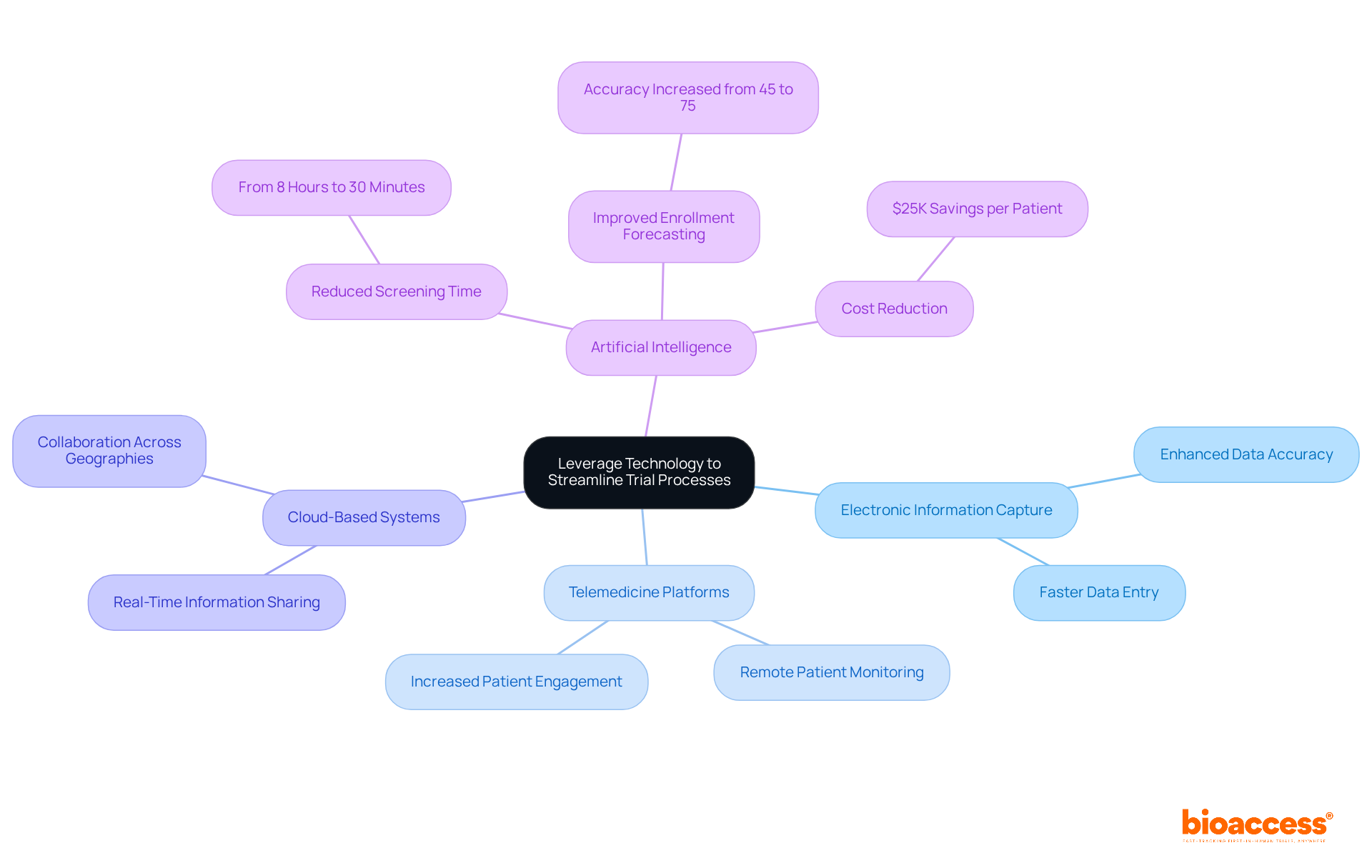
Integrating advanced technology into ide trials is essential for enhancing efficiency and outcomes. Solutions range from electronic information capture systems to telemedicine platforms that facilitate remote patient monitoring. The adoption of cloud-based systems allows for real-time information sharing and collaboration among research teams, breaking down geographical barriers.
Artificial intelligence (AI) is pivotal in data analysis, significantly accelerating the identification of trends and insights that inform decision-making. For instance, AI can reduce screening time for individuals from an average of eight hours to just 30 minutes, marking a remarkable 94% reduction in time spent on this critical task. Furthermore, AI-driven tools enhance enrollment forecasting accuracy from 45% to 75%, enabling more effective resource allocation.
By utilizing these technologies, study sponsors can streamline operations, reduce expenses, and ultimately enhance the results of ide trials. With bioaccess®, you can achieve patient enrollment 50% faster than Western sites and realize $25K savings per patient through FDA-ready data-no rework, no delays. Incorporating bioaccess®'s accelerated clinical research solutions, including expertise in medical device clinical studies, allows sponsors to improve their competitive advantage in an increasingly challenging environment.
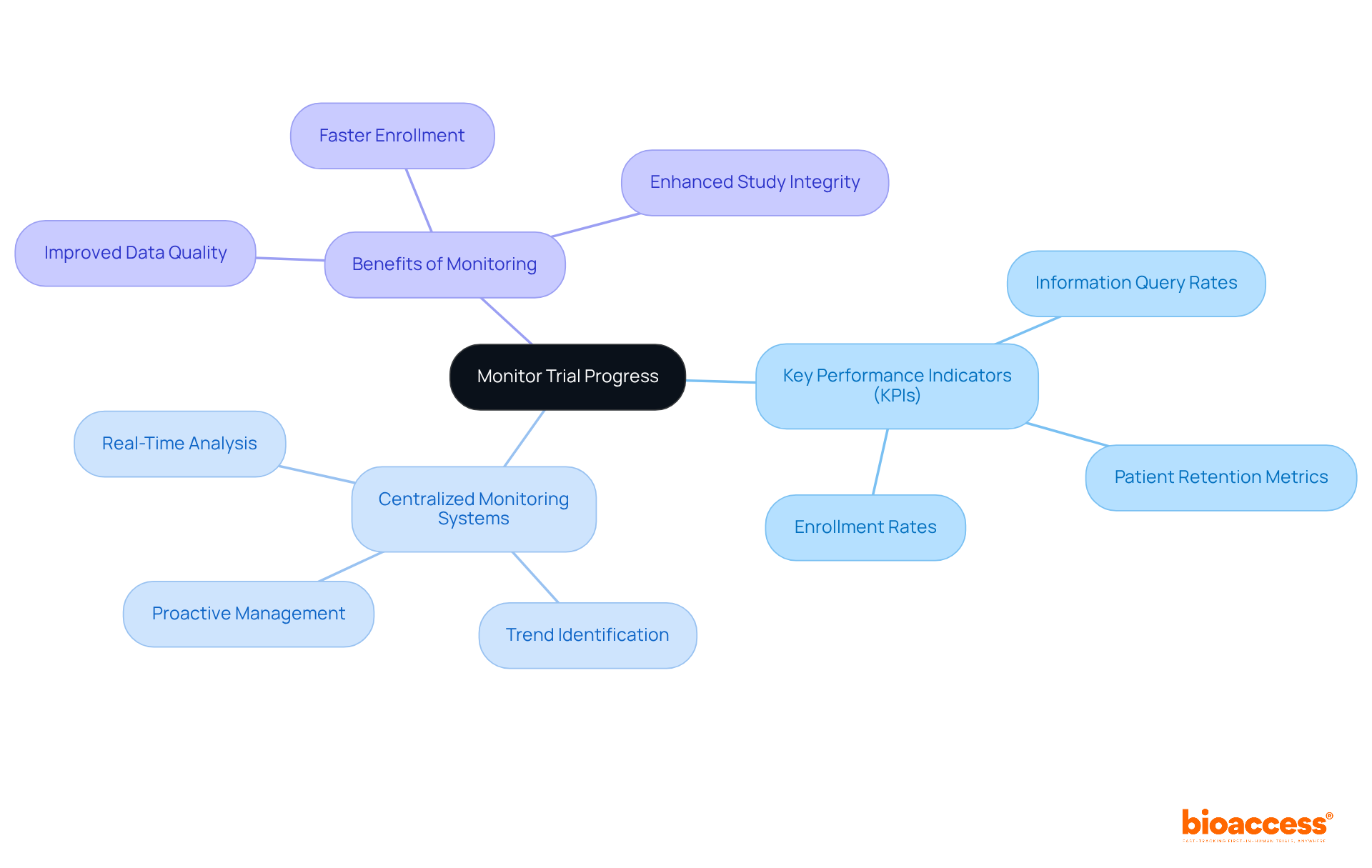
Effective monitoring of progress in experiments is essential for achieving successful outcomes in clinical research. This involves systematic evaluations of recruitment rates, information gathering, and adherence to established protocols. Key performance indicators (KPIs) are vital metrics for tracking progress and identifying areas needing improvement. For instance, KPIs can include:
Collectively, these provide a comprehensive overview of study health.
Centralized monitoring systems significantly enhance oversight by consolidating information from various locations, allowing for real-time analysis and swift interventions when issues arise. These systems help identify trends and anomalies, enabling proactive management of potential challenges. Regular communication with site staff and stakeholders is crucial, fostering alignment and collaborative problem-solving. This approach not only promotes transparency but also bolsters the overall integrity of the examination.
Research shows that studies employing robust KPI frameworks and centralized monitoring systems yield better outcomes, such as faster enrollment and improved data quality. By establishing KPIs tailored to the specific needs of the study, organizations can effectively measure performance and drive continuous improvement, ultimately leading to more successful research outcomes.
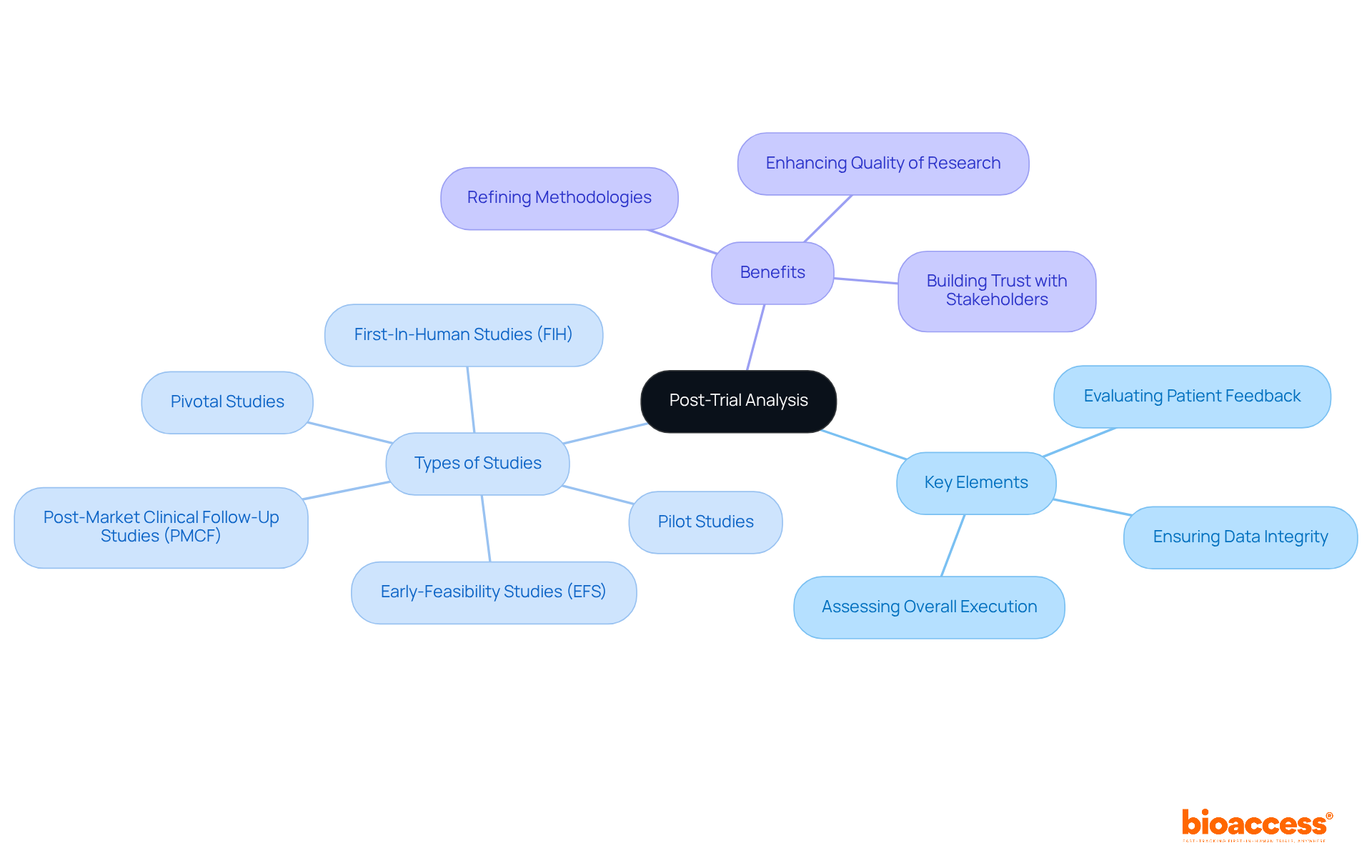
Conducting a thorough post-experiment analysis is essential for evaluating the effectiveness of clinical studies, especially within the scope of the various investigations managed by bioaccess®. This process involves a careful examination of all collected data to measure outcomes against established objectives. Key elements of this analysis include:
With bioaccess®'s expertise in:
Researchers can tap into specialized knowledge to refine methodologies and inform future study designs. By identifying lessons learned, researchers can enhance the quality of medical research. Moreover, sharing these insights with stakeholders not only fosters transparency but also builds trust, paving the way for future collaborations and increasing funding opportunities.
This iterative approach to learning from past experiences is crucial for driving innovation and improving the success rates of subsequent studies. As the Medtech landscape evolves, the importance of collaboration and continuous improvement cannot be overstated. What challenges are you facing in your clinical research efforts? Engaging with experts like bioaccess® can provide the support needed to navigate these complexities effectively.
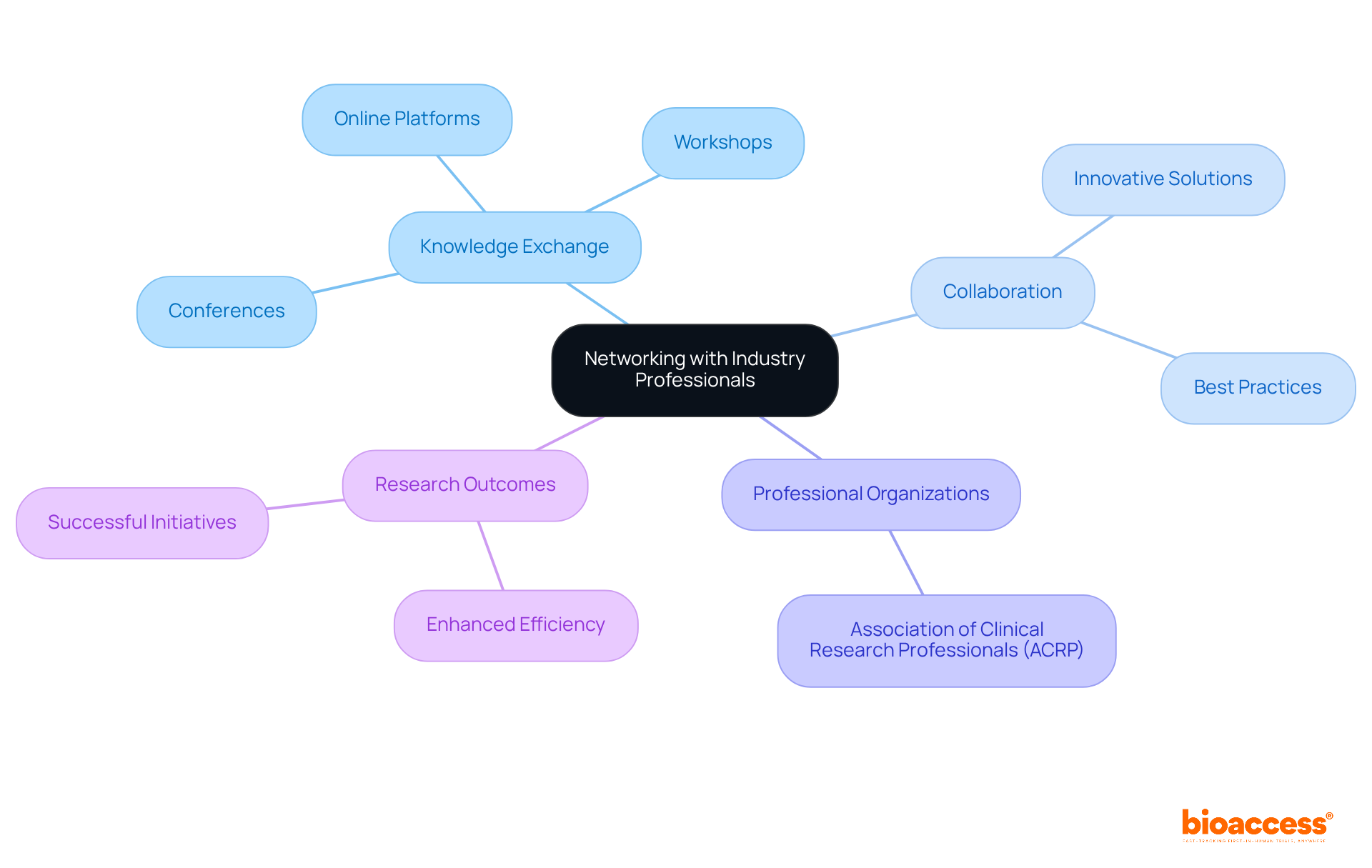
Establishing a strong network with industry experts is crucial for navigating the complexities of clinical studies. Engaging with colleagues at conferences, workshops, and online platforms fosters a culture of knowledge exchange and teamwork, significantly enhancing outcomes. Professional organizations, such as the Association of Clinical Research Professionals (ACRP), provide invaluable resources, training, and networking opportunities that empower study sponsors. By actively participating in these networks, sponsors gain access to a wealth of knowledge and support, ultimately boosting the efficiency and success of their research initiatives.
Moreover, collaborations within the industry often lead to innovative solutions and the sharing of best practices. This reinforces the necessity of strong connections in advancing ide trials. As you consider your own challenges in clinical research, reflect on how building a robust network could transform your approach and outcomes.
The successful execution of IDE trials relies on a blend of strategic planning, regulatory insight, and effective communication. By embracing the essential strategies outlined, innovators can navigate the complexities of clinical research more efficiently, ultimately leading to faster market access for groundbreaking medical devices. Organizations like bioaccess® play a pivotal role in this process, offering tailored support that accelerates timelines while ensuring compliance with regulatory standards.
Key insights from this exploration underscore the significance of:
Effective communication with stakeholders and robust data management practices further enhance the quality and integrity of the research. Moreover, continuous training for clinical staff and thorough post-trial analysis are vital for fostering innovation and improving future studies.
In conclusion, the landscape of IDE trials is evolving, and embracing these strategies is crucial for success. As the industry approaches 2025, the integration of digital technologies and adaptive study designs will transform how clinical research is conducted. Engaging with experts and building strong networks will empower sponsors to overcome challenges and drive advancements in medical technology. Taking proactive steps today can pave the way for a brighter future in clinical research, ultimately benefiting patients and healthcare systems worldwide.
What is bioaccess® and what services does it provide for IDE trials?
bioaccess® specializes in offering tailored support for IDE trials, leveraging extensive expertise in regulatory frameworks and diverse patient demographics across Latin America, the Balkans, and Australia. They facilitate rapid ethical approvals within 4-6 weeks and enhance experimental processes, including setup and project management.
How does bioaccess® help accelerate clinical studies?
bioaccess® empowers innovators to expedite their clinical studies while adhering to regulatory standards and addressing market needs, which shortens timelines and improves the integrity and quality of the data collected, leading to faster market access for medical devices.
What changes are expected in IDE trials by 2025?
By 2025, IDE trials are anticipated to undergo significant transformations with a focus on integrating digital technologies and adaptive study designs, allowing for real-time data monitoring and adjustments based on interim results. Regulatory agencies are also streamlining their processes, which enhances success rates for medical studies.
Why is understanding the regulatory framework for IDE trials important?
Understanding the regulatory framework is crucial as it outlines the types of devices that require IDEs, necessary documentation for submission, and the criteria for significant risk (SR) versus non-significant risk (NSR) devices. This knowledge aids in crafting robust IDE applications and navigating potential challenges during the approval process.
What common issues do clinical studies face, and how can they be addressed?
Approximately 80% of clinical studies experience delays due to recruitment issues. Engaging with regulatory experts early in the planning phase can significantly enhance the chances of successful submissions and expedite the approval process.
How should researchers select the right patient population for their IDE trials?
Researchers should establish specific eligibility standards aligned with the study's objectives, considering demographic characteristics, disease specifics, and previous treatment histories. Utilizing data analytics and collaborating with local healthcare providers can improve participant identification and recruitment efficiency.
What role do strategic partnerships play in IDE trials?
Strategic partnerships and data-driven recruitment are essential in the Medtech landscape. They help broaden access to diverse populations and enhance the credibility and efficiency of participant recruitment in clinical research.