


The world of clinical research relies heavily on the effectiveness of clinical labels, which act as vital communication tools among researchers, healthcare providers, and patients. With nearly 80% of research studies failing to meet enrollment targets, the urgency for precise and compliant labeling has never been greater.
What strategies can organizations adopt to ensure their clinical labels not only adhere to regulatory standards but also bolster patient safety and understanding? This article explores ten essential tips that illuminate the path to crafting effective clinical labels, ultimately driving successful research outcomes.
bioaccess® is dedicated to accelerating the clinical marking process, ensuring that clinical labels adhere strictly to regulatory standards across LATAM, Eastern Europe, and Australia. With deep expertise in navigating complex regulatory landscapes, bioaccess® empowers Medtech, Biopharma, and Radiopharma companies to achieve faster approvals while enhancing patient safety through the accurate use of clinical labels for identification. This approach merges local insights with global regulatory requirements, enabling the development of Master English Label Text (MELT) tailored to diverse packaging types.
This strategic alignment not only expedites the process of creating clinical labels but also addresses the critical need for accurate translations that meet regional compliance. Given that nearly 80% of research studies fail to meet their initial enrollment targets, effective clinical labels are essential for enhancing recruitment and retention rates. By leveraging advanced technologies like AI-driven simulation modeling, bioaccess® enhances trial efficiency, significantly reducing timelines and costs.
Their commitment to maintaining high-quality standards while executing projects faster than traditional timelines positions bioaccess® as a crucial partner in medical research, ensuring that innovative therapies reach the market swiftly and securely. Furthermore, bioaccess® provides comprehensive acceleration for global trials, activating over 50 pre-qualified networks in under eight weeks and delivering FDA/EMA/MDR-ready datasets with centralized monitoring.

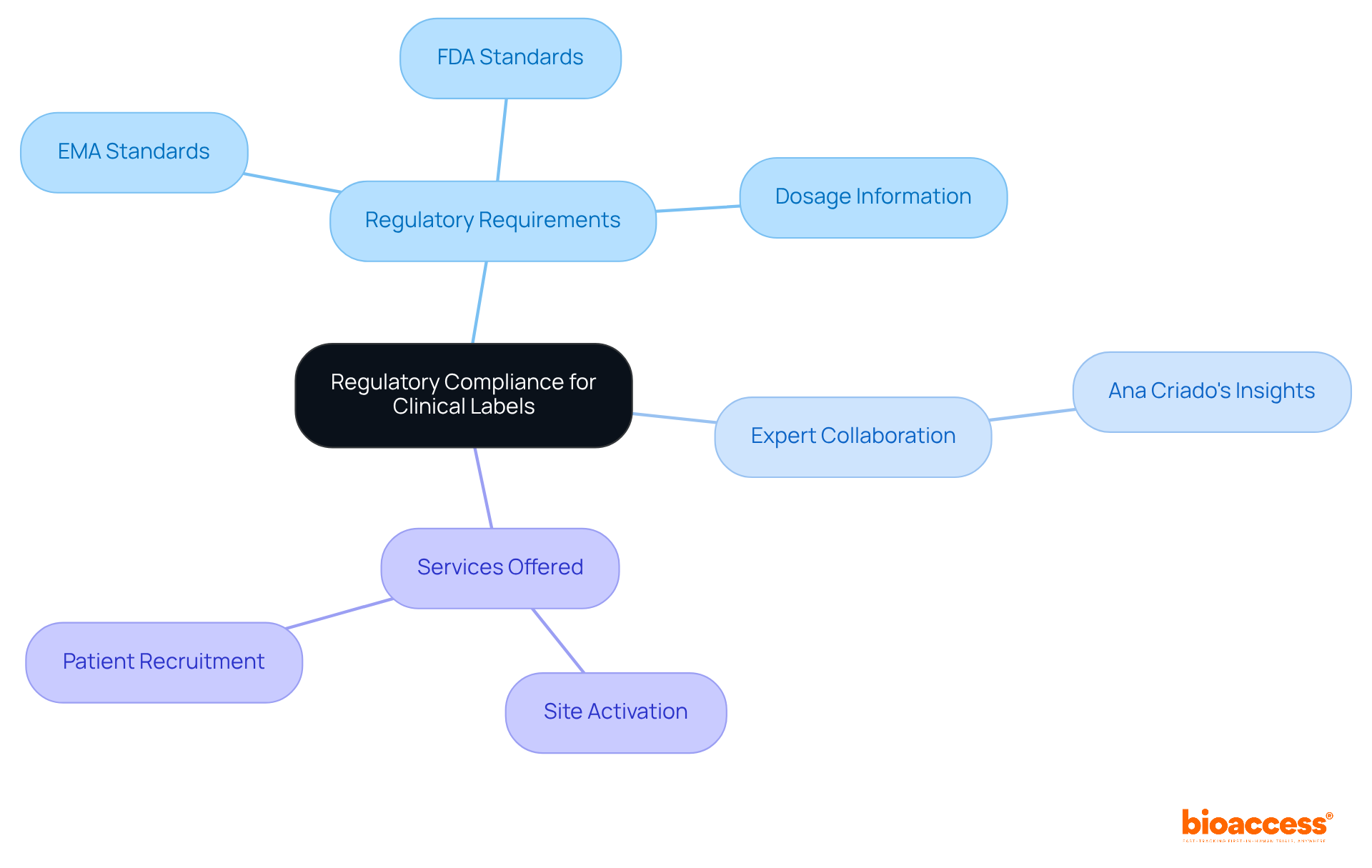
To ensure compliance with regulations, the stringent requirements established by agencies like the FDA and EMA must be adhered to in clinical labels. This involves accurately representing clinical labels that include product information, dosage, and administration instructions. Regular updates and audits of clinical labels are crucial for maintaining standards and adapting to any regulatory changes.
Engaging with regulatory experts, such as Ana Criado, our Director of Regulatory Affairs, brings invaluable insights into best practices. With her extensive experience in biomedical engineering and regulatory compliance, she can help mitigate risks associated with non-compliance. This collaboration is essential in navigating the complexities of the Medtech landscape.
Additionally, bioaccess® offers accelerated site activation and patient recruitment services, ensuring that clinical trials are conducted efficiently and in accordance with FDA, EMA, and MDR standards. By prioritizing these elements, we can enhance the effectiveness of clinical research and drive successful outcomes.
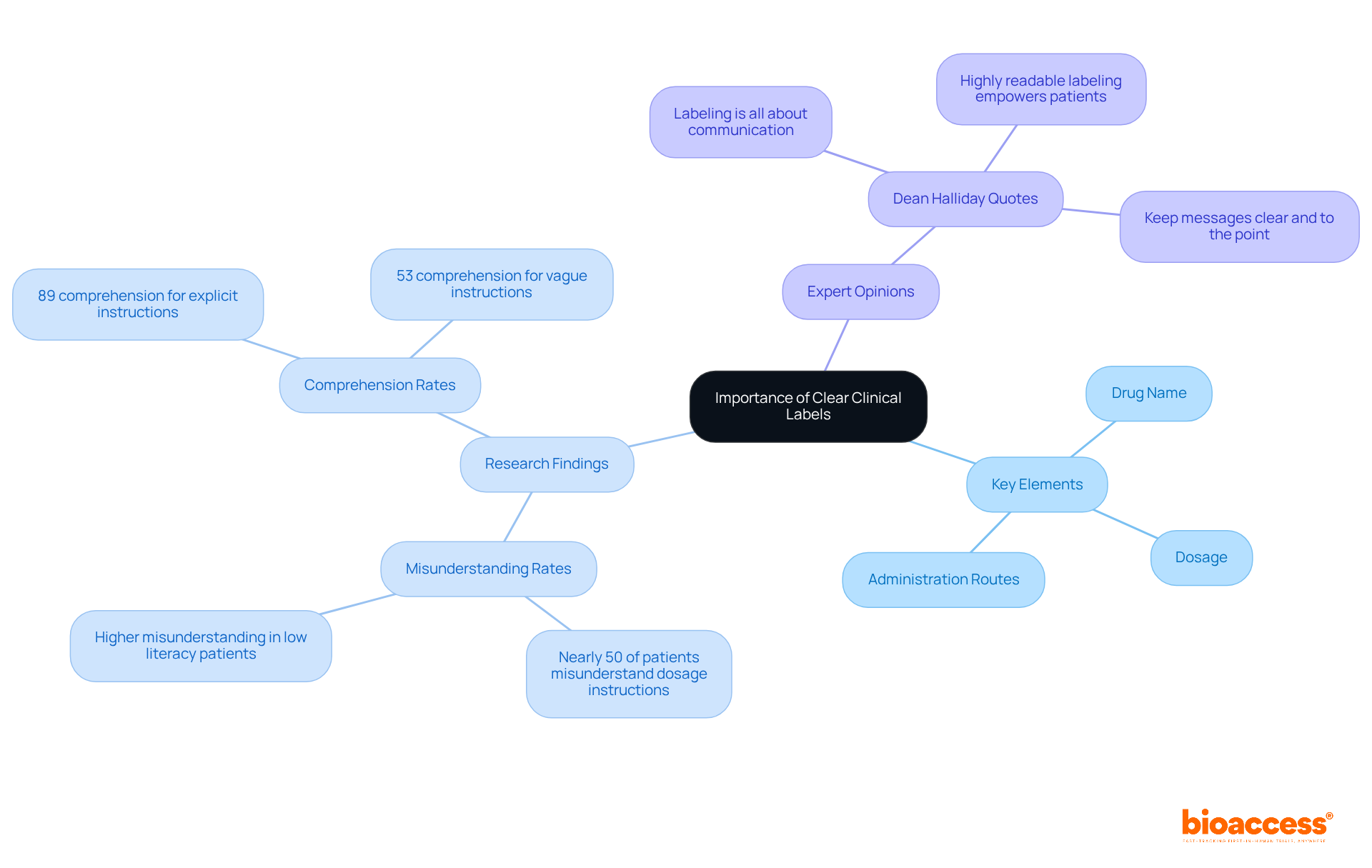
Clinical labels must convey information clearly and concisely, avoiding technical jargon that could confuse patients or healthcare providers. Key elements such as the drug name, dosage, and administration routes should be prominently displayed to facilitate quick understanding. Research involving 359 adult participants across multiple locations indicates that nearly half of primary care patients misunderstand common dosage instructions, highlighting the critical need for clarity.
Utilizing standardized symbols and formats not only enhances readability but also significantly improves patient safety and adherence to treatment protocols. For instance, explicit instructions using specific time periods have shown a comprehension rate of 89%, compared to just 53% for vague instructions, with an adjusted relative risk ratio of 0.42 for the latter. This underscores the significance of efficient identification using clinical labels in reducing medication errors and promoting better patient outcomes.
As Dean Halliday aptly states, "Labeling is all about communication," and he further emphasizes, "Highly readable labeling empowers patients by helping them understand their treatments." This reinforces that clear messaging is essential for patient safety.
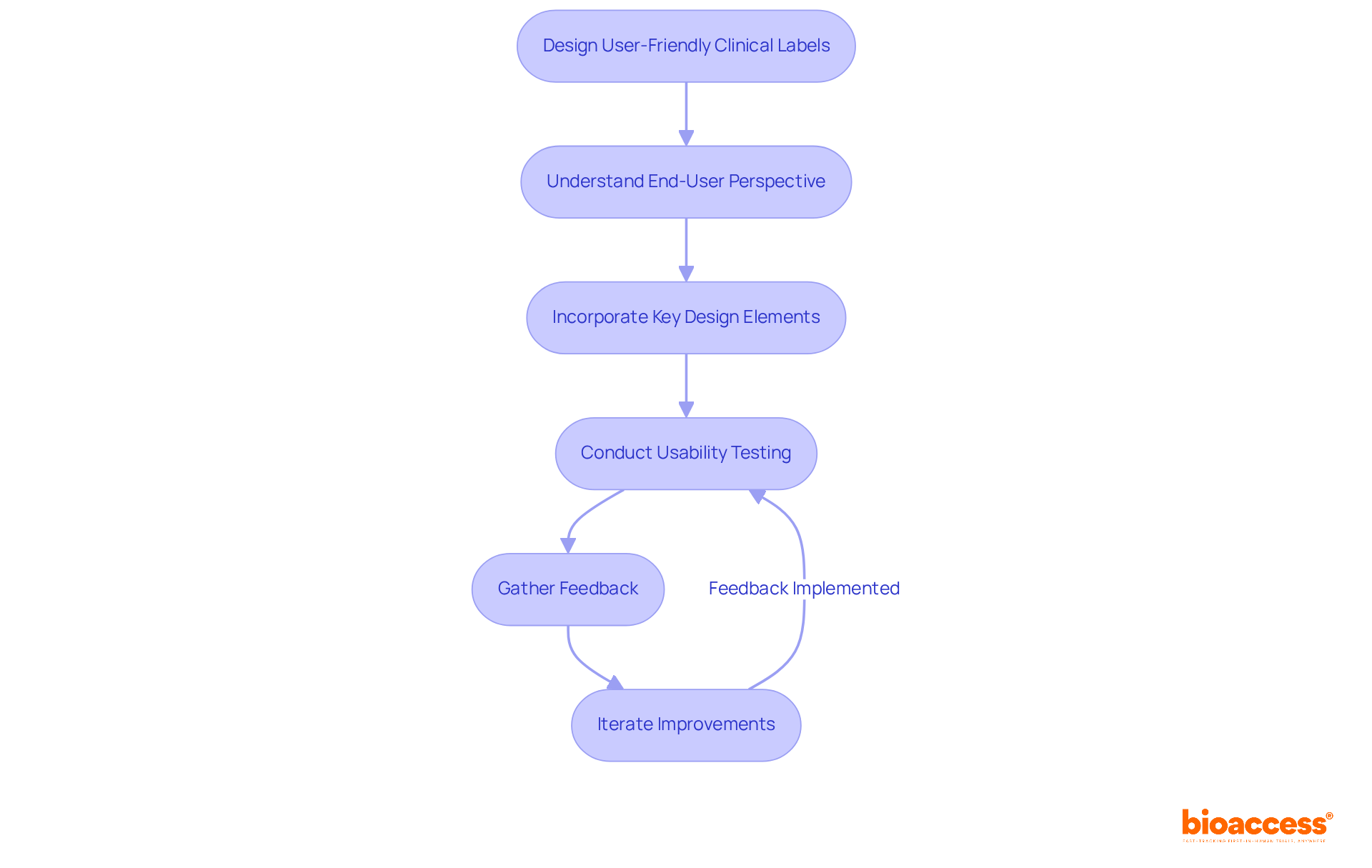
Creating user-friendly clinical labels is essential for enhancing interactions between patients and healthcare professionals. Understanding the end-user's perspective is crucial; labels must not only be visually appealing but also logically structured to facilitate easy navigation through information. Key design elements - such as appropriate font size, high color contrast, and effective use of graphics - play a vital role in enhancing readability.
Usability testing with actual users is indispensable. It provides critical insights into how patients and healthcare professionals engage with tags. For instance, research shows that nearly half of Americans taking prescription drugs do not adhere to their prescribed regimen properly, underscoring the need for effective packaging design. In one notable case, usability testing revealed that simplifying label layouts resulted in a 25% increase in user comprehension.
Moreover, incorporating feedback from usability tests helps identify common pain points, enabling iterative improvements that enhance the clarity of clinical labels and usability. As usability experts emphasize, investing in thorough testing not only improves user experience but also fosters trust and compliance, ultimately leading to better health outcomes. As Steve Krug aptly stated, 'If you want a great site, you’ve got to test.' This highlights the necessity of continuous evaluation and adaptation in clinical research.
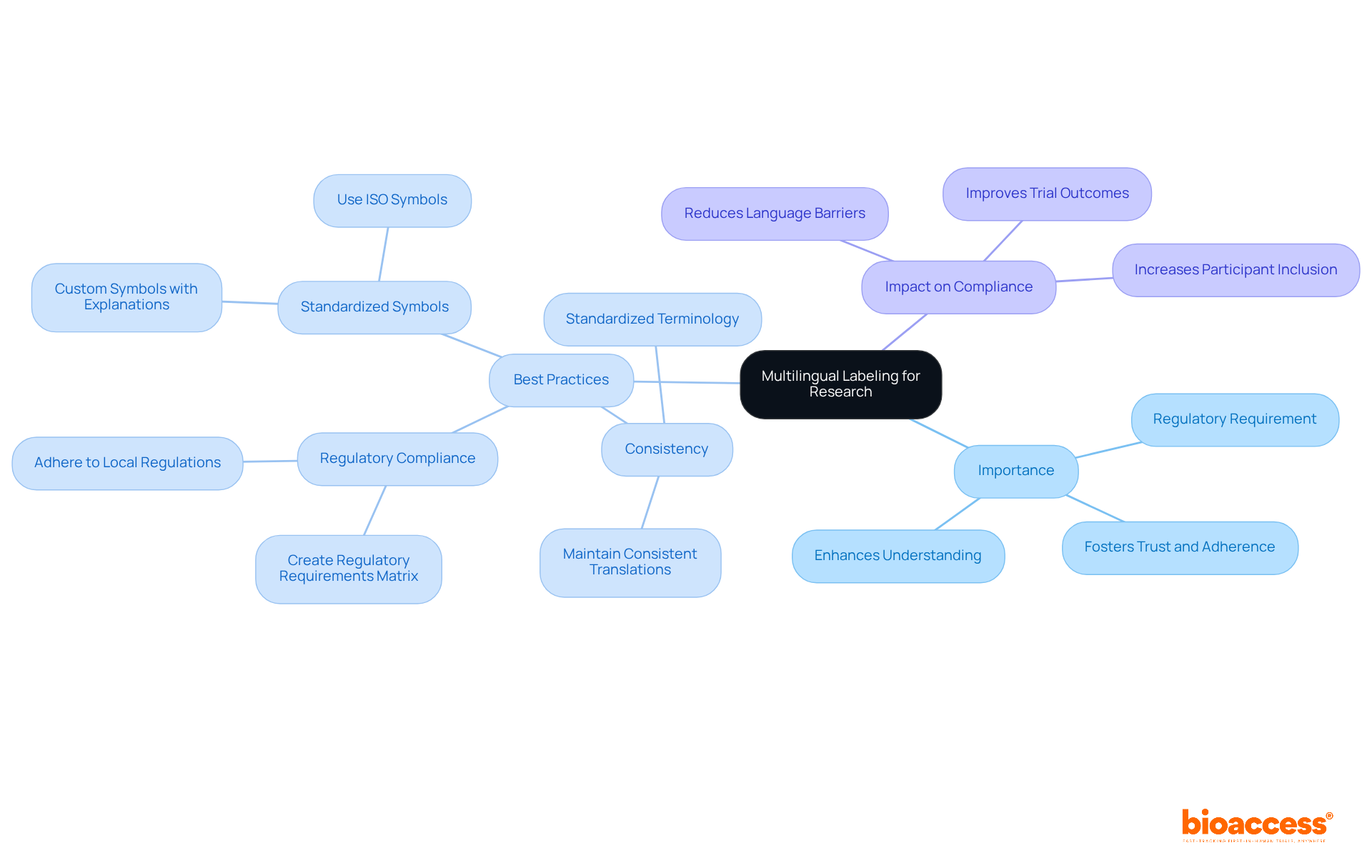
Including multilingual tags is crucial for research studies, especially in diverse language environments. Accurate translations are essential to convey the nuances of each language, ensuring that all participants fully understand the instructions and warnings. Professional translation services, particularly those with expertise in clinical research, significantly enhance the clinical labels process. For example, utilizing culturally relevant adaptations not only improves comprehension but also fosters trust and adherence among participants.
Best practices in this area include:
The impact of language barriers on patient compliance cannot be overstated; studies indicate that inadequate language support can lead to misunderstandings, ultimately affecting trial outcomes. Therefore, investing in effective translation services is not just a regulatory requirement but a vital step toward inclusive and successful research in the medical field, particularly when considering the importance of clinical labels.
Collecting feedback from healthcare personnel and patients is essential for understanding the efficiency of clinical labels in clinical designations. By utilizing surveys, focus groups, and direct interviews, organizations can pinpoint common issues or misunderstandings related to clinical labels content. This feedback loop is not just beneficial; it is crucial for ongoing enhancement. It empowers organizations to refine their categorization strategies, ensuring they better meet user needs and ultimately improve overall trial success.
How effectively are you gathering feedback to drive your clinical research forward?
Incorporating technology into the process of identifying clinical labels is crucial for enhancing both efficiency and accuracy. Solutions like automated tagging systems, digital display tags, and electronic tagging not only streamline the creation and management of tags but also significantly reduce the risk of human error.
Have you considered how these technologies could transform your clinical trials? They enable real-time updates and compliance monitoring, ensuring that tags remain current and precise throughout the trial. This integration of technology is not just a trend; it's a necessary evolution in the Medtech landscape.
Training on the importance of clinical labels is vital for all parties involved in clinical trials. Effective training programs must encompass:
By fostering an environment of adherence and understanding, organizations can ensure that every team member comprehends their responsibilities related to tagging. This understanding is essential for enhancing the outcomes of clinical labels and ultimately improving the integrity of medical research.
For instance, programs that incorporate practical workshops and real-world case studies have shown a notable increase in adherence rates among clinical research personnel. However, it’s concerning that only 10% of employees report that compliance training has impacted their work practices, underscoring the necessity for effective training design. Moreover, emphasizing informed consent practices within these training sessions can lead to better engagement and a deeper understanding of clinical labels.
Recent studies highlight that organizations prioritizing comprehensive training not only meet regulatory standards but also cultivate an atmosphere of accountability and ethical decision-making, which is crucial for successful trials. This raises an important question: Is online research training effective? After all, no training can be deemed effective if participants do not complete it. The challenges in training adherence are further illustrated by the statistic that only 13% of participants finished the training within a reasonable timeframe.
Therefore, it is imperative that training programs are meticulously designed to ensure high completion rates and meaningful engagement. By addressing these challenges, organizations can significantly enhance the effectiveness of their training initiatives.
Conducting regular audits of clinical labels is essential for maintaining compliance and ensuring accuracy in clinical research. These audits must assess not only the content and format but also adherence to regulatory requirements. This is a key aspect of bioaccess's comprehensive clinical trial management services, which include feasibility studies and reporting. By identifying discrepancies and areas for improvement, organizations can take corrective actions to enhance their labeling processes.
Furthermore, incorporating compliance reviews and project management practices into the audit process significantly boosts the effectiveness of these audits. A systematic audit schedule ensures that clinical labels are kept up-to-date and compliant throughout the trial lifecycle. This proactive approach not only mitigates risks but also fosters a culture of continuous improvement, ultimately leading to more successful clinical outcomes.
Collaborating with regulatory organizations is crucial for staying abreast of best practices and evolving standards in medical documentation. Engaging in discussions and attending workshops not only provides valuable insights but also enhances categorization strategies. For instance, industry forums have proven effective in sharing knowledge and elevating practice standards, fostering a community of learning and adaptation. By building strong connections with regulatory bodies, organizations can align their product information practices with current expectations, ultimately boosting the success of research trials.
To remain informed about clinical labels standards, a proactive approach is essential. This includes participating in relevant workshops and forums that emphasize best practices and emerging trends. Are you ready to take the necessary steps to ensure your organization meets these evolving standards? By prioritizing collaboration and continuous learning, you can navigate the complexities of the Medtech landscape and address key challenges effectively.
Effective clinical labeling stands as a cornerstone of successful medical research, ensuring that critical information is conveyed accurately and efficiently. By emphasizing regulatory compliance, clarity, user-friendly design, and the integration of technology, organizations can significantly enhance the quality and effectiveness of clinical labels. This comprehensive approach not only aids in meeting regulatory standards but also fosters better patient understanding and adherence, ultimately leading to improved trial outcomes.
The article outlines essential strategies for achieving effective clinical labels, including:
It highlights the role of technology in streamlining labeling processes and underscores the necessity of training programs to equip all team members with the knowledge and skills required to maintain high standards.
As the landscape of medical research continues to evolve, prioritizing these best practices in clinical labeling is more crucial than ever. Organizations are encouraged to embrace these strategies not only to comply with regulatory requirements but also to enhance patient safety and engagement. By investing in effective clinical labeling practices, the potential for improved recruitment and retention rates in clinical trials becomes a reality, paving the way for innovative therapies to reach those in need more swiftly and securely.
What is bioaccess® and what services do they provide?
bioaccess® is dedicated to accelerating the clinical marking process, ensuring that clinical labels comply with regulatory standards in LATAM, Eastern Europe, and Australia. They provide expert services to Medtech, Biopharma, and Radiopharma companies to achieve faster approvals while enhancing patient safety through accurate clinical labels.
How does bioaccess® help in the clinical labeling process?
bioaccess® merges local insights with global regulatory requirements to develop Master English Label Text (MELT) tailored to various packaging types. This strategic alignment expedites the creation of clinical labels and ensures accurate translations that meet regional compliance.
Why are effective clinical labels important in research studies?
Effective clinical labels are crucial because nearly 80% of research studies fail to meet their initial enrollment targets. Accurate labeling enhances recruitment and retention rates in clinical trials.
What technologies does bioaccess® utilize to improve trial efficiency?
bioaccess® leverages advanced technologies like AI-driven simulation modeling to enhance trial efficiency, significantly reducing timelines and costs associated with clinical trials.
How does bioaccess® ensure regulatory compliance for clinical labels?
bioaccess® adheres to stringent requirements established by agencies like the FDA and EMA, ensuring clinical labels accurately represent product information, dosage, and administration instructions. Regular updates and audits are conducted to maintain compliance with any regulatory changes.
Who can provide insights into regulatory compliance at bioaccess®?
Ana Criado, the Director of Regulatory Affairs at bioaccess®, brings invaluable insights into best practices for regulatory compliance, helping to mitigate risks associated with non-compliance.
What role does clarity play in clinical labels?
Clinical labels must convey information clearly and concisely, avoiding technical jargon. Key elements like drug name, dosage, and administration routes should be prominently displayed to facilitate quick understanding and improve patient safety.
How does the clarity of clinical labels impact patient understanding?
Research shows that nearly half of primary care patients misunderstand common dosage instructions, highlighting the need for clarity. Standardized symbols and formats enhance readability and significantly improve patient safety and adherence to treatment protocols.
What is the significance of readable labeling according to Dean Halliday?
Dean Halliday emphasizes that "Labeling is all about communication" and that "Highly readable labeling empowers patients by helping them understand their treatments," underscoring the importance of clear messaging for patient safety.