


Understanding the complexities of clinical research is essential for anyone contemplating participation in a study. Participants have the opportunity to contribute to groundbreaking medical advancements, placing them in a unique position that carries both responsibilities and rights. This article presents ten essential tips aimed at empowering individuals, enabling them to navigate the clinical research landscape with confidence and clarity.
How can prospective participants prepare themselves to enhance their experience while also making a meaningful contribution to the research process?
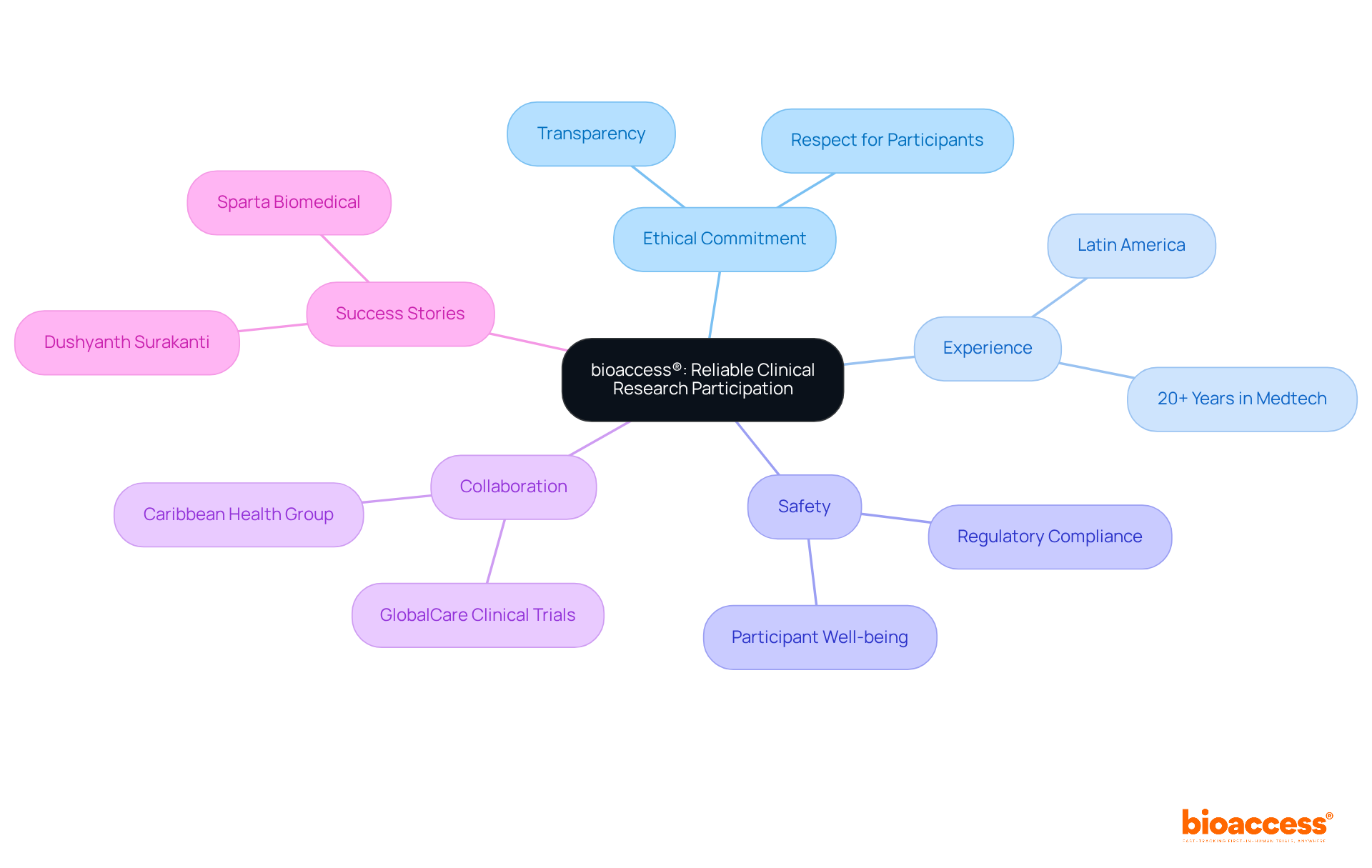
bioaccess® stands out due to its unwavering commitment to ethical medical studies, ensuring that individuals are treated with the utmost respect and transparency. With extensive experience managing research studies across Latin America, bioaccess® fosters a trustworthy environment for study participants. Their rigorous focus on regulatory compliance significantly enhances safety for study participants, making bioaccess® a preferred choice for those considering participation in trials.
Recent initiatives, such as the collaboration with Caribbean Health Group to position Barranquilla as a key hub for research studies, underscore their dedication to creating positive experiences for research volunteers. Success stories, like that of Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during his first human trial in Colombia, highlight bioaccess®'s effectiveness in navigating complex regulatory landscapes while prioritizing the well-being of study participants. This reinforces their reputation as a leader in ethical clinical investigations.
Before enrolling in a clinical study, it's crucial for individuals to grasp the study's objectives fully. This includes understanding the specific aims, the hypotheses being tested, and the significance of the study participant's involvement in advancing medical knowledge. Engaging proactively with the study group to clarify these aspects not only enriches the individual's experience but also fosters a deeper commitment to the project.
Effective communication plays a vital role in this process. Study groups should utilize clear, jargon-free language and visual aids to convey complex information. This approach not only clarifies the investigative process but also empowers individuals, making them feel valued and informed. By emphasizing transparency in communication, research groups can significantly enhance involvement and satisfaction, ultimately contributing to the success of the medical study.
With bioaccess®'s expertise in managing various studies, including Early-Feasibility and First-In-Human Studies, individuals can be confident that their participation is part of a well-organized and professionally managed research process. This assurance enhances their overall experience and underscores the importance of collaboration in clinical research.

Eligibility requirements for research studies are crucial for determining individual suitability and upholding the integrity of the investigation. These criteria typically include factors such as age, health status, and medical history. Prospective study participants must carefully review these requirements to evaluate their fit as a study participant. This proactive approach not only streamlines the enrollment process but also significantly enhances the likelihood of successful participation.
Research shows that around 80% of medical trials encounter delays or closures due to recruitment challenges, often stemming from eligibility issues. By comprehending and adhering to these criteria, individuals can help reduce dropout rates, which are frequently linked to misunderstandings about study requirements. As one investigator noted, "A thorough eligibility review is essential for maintaining the quality and reliability of medical studies."
Ultimately, a clear understanding of these criteria empowers prospective study participants to make informed choices, thereby improving their involvement in the clinical study process.

As a study participant in clinical research, you hold several essential rights that are crucial for your experience. These include:
Understanding these rights is not just beneficial; it’s vital for ensuring a positive and ethical experience. As a study participant, you should feel empowered to ask questions and seek clarification about your rights throughout the study, reinforcing your role in this important process.
Consider this: how often do participants fully grasp their rights? Many may not realize the significance of being informed and the power they possess to influence their involvement as a study participant. By familiarizing yourself with these rights, you not only protect yourself but also contribute to the integrity of the research process. Remember, your voice matters, and your questions are not just welcomed - they're encouraged.

Study participants should feel empowered to ask questions about their role in the study. Inquiries should cover expectations, procedures, and the use of their data. Notably, studies show that only 32% of patients mention their doctors providing details about clinical studies, highlighting a significant communication gap. Open communication with the research group not only fosters a cooperative atmosphere but also ensures that individuals are well-informed and comfortable with their engagement.
Effective communication strategies can bridge this gap. A striking 93.6% of patients with chronic conditions emphasize the importance of knowing they can complete the study. By clarifying expectations and addressing concerns, researchers can enhance understanding and involvement, ultimately leading to more successful studies. This collaboration is essential for advancing clinical research and ensuring that study participants feel valued and informed.

Before agreeing to participate in a research study, individuals must carefully assess the potential risks and benefits involved. This assessment should include a clear understanding of possible side effects, the likelihood of receiving a placebo, and the chance to contribute to significant medical advancements. Engaging in open conversations with the study participants is crucial, as it can provide valuable insights and help individuals make informed decisions.
Significantly, 92.7% of oncology patients emphasize the importance of discussing research studies with their physicians before participating, highlighting the need for open communication. Furthermore, recognizing that 73% of patients prefer to learn about research opportunities from their healthcare providers can aid participants in obtaining essential information. It is also important to note that only 32% of patients reported that their physicians had ever provided them with information regarding clinical studies, underscoring the necessity for proactive communication.
Lastly, 21% of patients expressed a desire to learn about trials through advertisements, indicating that multiple channels of communication should be considered. By actively engaging in these discussions, individuals can better weigh their options and feel more confident in their choices.

Clear channels of communication with the study group are vital for individuals participating in clinical research. Promptly reporting side effects, asking questions, and providing health status updates are essential components of this communication. A collaborative relationship with researchers not only enhances the overall experience but also ensures that any issues are swiftly addressed. Research shows that:
By actively engaging with the study participants, they contribute to a more efficient and adaptive evaluation process. Notably, 84% of respondents indicated that the team consistently paid close attention to their needs. This interaction not only improves health outcomes but also advances the progress of medical science. In summary, fostering clear communication channels is essential for enhancing the clinical research experience and achieving successful outcomes.

Participants play a vital role in enhancing the quality of clinical research by providing feedback on their experiences. Insights regarding study procedures, communication with researchers, and overall satisfaction are essential for refining future trials. For instance, a recent survey revealed that while 85-95% of respondents felt respected and heard, only 60% felt entirely prepared by the consent procedure. This indicates significant room for improvement in how studies engage with individuals involved.
Involving individuals in the feedback process not only fosters a sense of collaboration but also leads to more relevant and effective research outcomes. Strategies such as engaging individuals in the creation of informed consent forms and providing lay summaries of trial results can greatly enhance transparency and satisfaction. In fact, 91% of individuals expressed a desire to receive health check results, highlighting the importance of keeping them informed.
Moreover, studies show that organizations valuing patient involvement experience a 40% increase in recruitment and a 30% rise in retention rates. Additionally, 63% of individuals indicated that obtaining a summary of the study findings would be very significant for enrolling in a future study. By actively seeking and responding to feedback from study participants, researchers can cultivate a more inclusive environment that values the perspectives of those directly impacted by the study. This collaborative approach not only enhances the quality of research but also ensures it effectively meets the needs of the community.

Before joining a research study, it’s crucial for individuals to fully grasp the timeline, including significant milestones and their anticipated responsibilities. This understanding encompasses the study's duration, the frequency of visits, and any necessary follow-up assessments. Research indicates that a striking 90.4% of chronic illness patients deem it essential to communicate with study coordinators and nurses prior to participation, underscoring the importance of being well-informed.
Moreover, 73% of patients prefer to learn about clinical research opportunities through their doctor's office, highlighting the pivotal role healthcare providers play in engaging potential volunteers. By comprehending these elements, individuals can effectively organize their involvement, ensuring they meet their responsibilities and contribute to the project's success. Clinical researchers assert that clear communication regarding commitments is vital for fostering trust and enhancing retention rates throughout the study.
Significantly, 92.7% of oncology patients consider it essential to engage in dialogue with the physicians involved in studies before participating, emphasizing the necessity for open conversation. Additionally, it’s important to recognize that 80% of research studies face delays or shutdowns due to recruitment challenges, making commitment to involvement even more critical for the success of medical investigations.

Exchanging experiences among research volunteers is crucial for building a supportive community in clinical research. When individuals share their stories, they provide valuable insights into the clinical study process, helping to clarify it for potential participants. These narratives can significantly influence others' decisions to join studies, as they highlight the personal impact of research on health and wellness. Moreover, research shows that those who discuss their experiences often report higher satisfaction levels and a stronger sense of belonging. This communal support fosters trust and encourages more individuals to consider participation, ultimately enhancing recruitment efforts.
By creating an environment where narratives are shared, the medical community can deepen understanding of the studies' significance and their potential benefits for society. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, underscoring the importance of feedback from participants in shaping future research. Additionally, the collaboration between Bioaccess and Caribbean Health Group aims to position Barranquilla as a premier destination for clinical trials in Latin America, further emphasizing the value of study participant stories in advancing clinical research.
Participating in clinical research presents a unique opportunity for individuals to contribute to significant medical advancements. It is essential to navigate this landscape with a clear understanding of responsibilities and rights. This article underscores the importance of being well-informed and proactive throughout the research process, enabling participants to maximize both their experience and impact.
Key points include:
Effective communication with researchers, evaluating potential risks and benefits, and providing feedback are crucial elements that enhance the participant's experience and the overall quality of the research. By fostering a supportive community through shared experiences, individuals can empower one another and encourage broader participation in clinical trials.
Ultimately, the journey of a clinical research participant transcends individual involvement; it contributes to a larger mission of advancing health and wellness. By engaging actively, asking questions, and sharing experiences, participants can help shape the future of medical research. Embracing these essential tips not only enhances personal involvement but also strengthens the collective effort to improve healthcare for all.
Why should individuals choose bioaccess® for clinical research participation?
Individuals should choose bioaccess® for its commitment to ethical medical studies, respect for participants, transparency, and extensive experience managing research studies across Latin America, which fosters a trustworthy environment.
How does bioaccess® ensure the safety of study participants?
bioaccess® enhances safety through a rigorous focus on regulatory compliance, which significantly reduces risks associated with clinical trials.
What recent initiatives has bioaccess® undertaken to improve research participation?
bioaccess® has collaborated with Caribbean Health Group to position Barranquilla as a key hub for research studies, demonstrating their dedication to creating positive experiences for research volunteers.
Can you provide an example of bioaccess®'s effectiveness in clinical trials?
An example is Dushyanth Surakanti, the Founder & CEO of Sparta Biomedical, who successfully navigated his first human trial in Colombia with bioaccess®, highlighting their ability to manage complex regulatory landscapes while prioritizing participant well-being.
What should individuals understand before enrolling in a clinical study?
Individuals should understand the study's objectives, specific aims, hypotheses being tested, and the significance of their involvement in advancing medical knowledge.
How can effective communication enhance participation in clinical studies?
Effective communication using clear, jargon-free language and visual aids helps clarify the investigative process, empowers individuals, and fosters a sense of value and information, enhancing involvement and satisfaction.
What are the eligibility criteria for participating in research studies?
Eligibility criteria typically include factors such as age, health status, and medical history, which help determine individual suitability and uphold the integrity of the investigation.
Why is it important for prospective participants to review eligibility criteria?
Reviewing eligibility criteria is crucial for determining fit as a study participant, streamlining the enrollment process, and reducing dropout rates linked to misunderstandings about study requirements.
What impact do eligibility issues have on medical trials?
Approximately 80% of medical trials face delays or closures due to recruitment challenges stemming from eligibility issues, making a thorough review essential for maintaining the quality and reliability of studies.