


Romania's clinical trial landscape is experiencing a significant transformation, fueled by recent legislative updates that align with EU standards and strengthen the regulatory framework for research studies. As the country positions itself as a competitive hub for international research, it becomes essential for sponsors and investigators to grasp the critical requirements for trial agreements. This understanding is not merely beneficial; it is crucial for ensuring compliance while maximizing the ethical and operational integrity of clinical trials.
However, navigating these complexities raises a pressing question: how can stakeholders ensure compliance while maximizing the ethical and operational integrity of their clinical trials? This inquiry invites a deeper exploration into the evolving Medtech landscape and the pivotal role that organizations like Bioaccess play in addressing key challenges. By fostering collaboration and leveraging expertise, stakeholders can effectively navigate the intricacies of clinical research, ensuring that their trials not only meet regulatory standards but also uphold the highest ethical principles.
Romania's research study landscape is significantly influenced by Law 95/2006 and the EU Research Regulations (EU No. 536/2014). These regulations establish a comprehensive framework for conducting clinical research, which includes the trial agreement requirements under Romanian legislation, outlining the rights of participants, the responsibilities of sponsors, and the procedural requirements for assessments. Familiarity with these regulations is essential for compliance and effective management. The National Agency for Medicines and Medical Devices (ANMDMR) oversees this regulatory framework, ensuring that all studies uphold ethical standards and maintain scientific integrity.
The recent introduction of Law 249/2022 mandates a 60-day approval period for new research study applications, aligning Romania's practices with EU standards. This legislation not only streamlines the regulatory process but also positions Romania as an attractive destination for international backers, who currently account for 91% of active research studies in the country. By 2025, Romania is expected to host over 500 clinical studies, with oncology leading the charge, showcasing a robust environment for clinical research.
Case studies, such as those from Cromos Pharma, exemplify the positive effects of these regulatory changes. Cromos Pharma has successfully reduced enrollment timelines in 95% of its studies, demonstrating the effectiveness of local expertise in navigating the regulatory landscape. The alignment with EU regulations is poised to further enhance Romania's competitiveness in the research arena, making it crucial for research professionals to stay updated on the trial agreement requirements under Romanian legislation.
Informed consent is essential in the enrollment of individuals in clinical trials, as stipulated by the trial agreement requirements under Romanian legislation. This essential process mandates the delivery of clear and comprehensive information about the project's purpose, procedures, risks, and benefits. The informed consent form (ICF) must be articulated in accessible language, ensuring that individuals fully grasp the details before affixing their signatures. Special attention is directed towards vulnerable populations, necessitating additional safeguards to uphold their rights and welfare.
Recent research reveals a concerning trend: comprehension of informed consent elements has stagnated over the past 30 years, with only 52.1% to 75.8% of individuals understanding critical aspects such as the project's purpose and their right to withdraw. As we look towards 2025, best practices emphasize the significance of employing closed-ended questions during the consent process, which have demonstrated effectiveness in enhancing comprehension rates. Furthermore, expert opinions underscore the necessity for tailored strategies aimed at improving understanding among diverse groups, thereby ensuring ethical adherence and preserving individual autonomy throughout the research process.
A comprehensive trial protocol is essential; it serves as the foundational blueprint for any clinical research. This document meticulously outlines research objectives, methodology, participant selection criteria, and statistical analysis plans, ensuring adherence to the trial agreement requirements under Romanian legislation and international standards. A well-crafted protocol streamlines the regulatory approval process and fosters alignment among all team members regarding the study's goals and procedures.
Research indicates that medical studies with detailed protocols achieve considerably better success rates, underscoring the significance of protocol adherence. Specialists assert that a clear and comprehensive protocol is the cornerstone of quality assurance in research studies, promoting reproducibility and integrity in results. To create an effective research protocol, it is crucial to include comprehensive details about the study design, such as the rationale, objectives, and methodologies, while also addressing all ethical considerations.
In 2025, the importance of a strong research protocol cannot be overstated; it remains a crucial component in advancing medical studies and attaining successful study results.
The qualified investigator is pivotal in the successful execution of clinical studies at the site. They oversee the entire process, ensuring strict adherence to the protocol and regulatory standards. To effectively fulfill these responsibilities, investigators must have the necessary qualifications, training, and experience. Statistics reveal that well-trained investigators significantly enhance case outcomes; research shows that those with extensive training are more likely to ensure compliance and prioritize safety.
In Romania, understanding the trial agreement requirements under Romanian legislation is essential for navigating the local healthcare landscape. Investigators must communicate effectively with contributors and regulatory bodies, fostering trust and transparency. Case studies illustrate instances where skilled researchers have adeptly navigated complex regulatory environments, leading to faster approval processes and improved participant engagement. The importance of proficient researchers cannot be overstated; industry experts emphasize that their expertise is vital for maintaining the integrity and success of studies. Influential figures assert that the most effective research encompasses not just data but also meaningful interactions with individuals, highlighting the human element in medical research.
Romanian laws mandate that sponsors secure insurance protection for individuals involved in research, effectively shielding them from potential injuries or adverse effects that may arise during the process. This insurance is not merely a formality; it is a critical component of the application process. Sponsors must provide proof of coverage to guarantee that individuals are financially protected throughout the study's duration. Notably, research studies submitted under previous legislation can continue until January 30, 2025, which is crucial for ongoing investigations.
Recent statistics indicate that participant injuries in research studies can lead to substantial financial obligations, underscoring the necessity of extensive insurance coverage. For instance, research studies insurance can encompass medical costs, legal expenses, and compensation for injuries, potentially saving organizations millions in liabilities. Furthermore, it is essential to recognize that regulatory bodies frequently mandate research study insurance, highlighting its significance in the testing process.
Case studies illustrate the effectiveness of coverage for individuals in Romania. For example, Trident Insurance has developed tailored policies that address the unique risks associated with research studies, ensuring that individuals receive adequate coverage. Their approach not only mitigates financial risks for sponsors but also fosters trust among participants, encouraging greater engagement in research. As Trident Insurance emphasizes, prioritizing the safety of those involved is vital for building trust in research studies and motivating more individuals to participate in significant medical investigations.
As the landscape of medical studies evolves, particularly with the introduction of advanced treatments and technologies, the importance of robust insurance coverage for subjects cannot be overstated. Expert opinions stress that prioritizing participant safety through comprehensive insurance is essential for maintaining the integrity of research studies and advancing medical exploration. Additionally, it is crucial to acknowledge that non-litigation-related issues, such as injuries from unrelated activities, are typically excluded from litigation insurance, further emphasizing the need for comprehensive coverage.
Data protection stands as a pivotal component of clinical studies, especially with the stringent enforcement of the General Data Protection Regulation (GDPR). It is imperative for sponsors to ensure that personal data collected during the study is processed in a manner that is lawful, transparent, and secure. This responsibility encompasses:
In the evolving Medtech landscape, the role of data protection cannot be overstated. Sponsors must navigate the complexities of compliance while addressing the key challenges that arise in clinical research. By prioritizing data protection, they not only fulfill legal obligations but also foster trust among participants, which is essential for the success of any clinical study.
In conclusion, the importance of collaboration in ensuring data protection cannot be overlooked. As clinical research continues to advance, sponsors must take proactive steps to align with GDPR requirements, ensuring that participant data is handled with the utmost care and respect. This commitment to data protection is not just a regulatory necessity; it is a fundamental aspect of ethical clinical research.
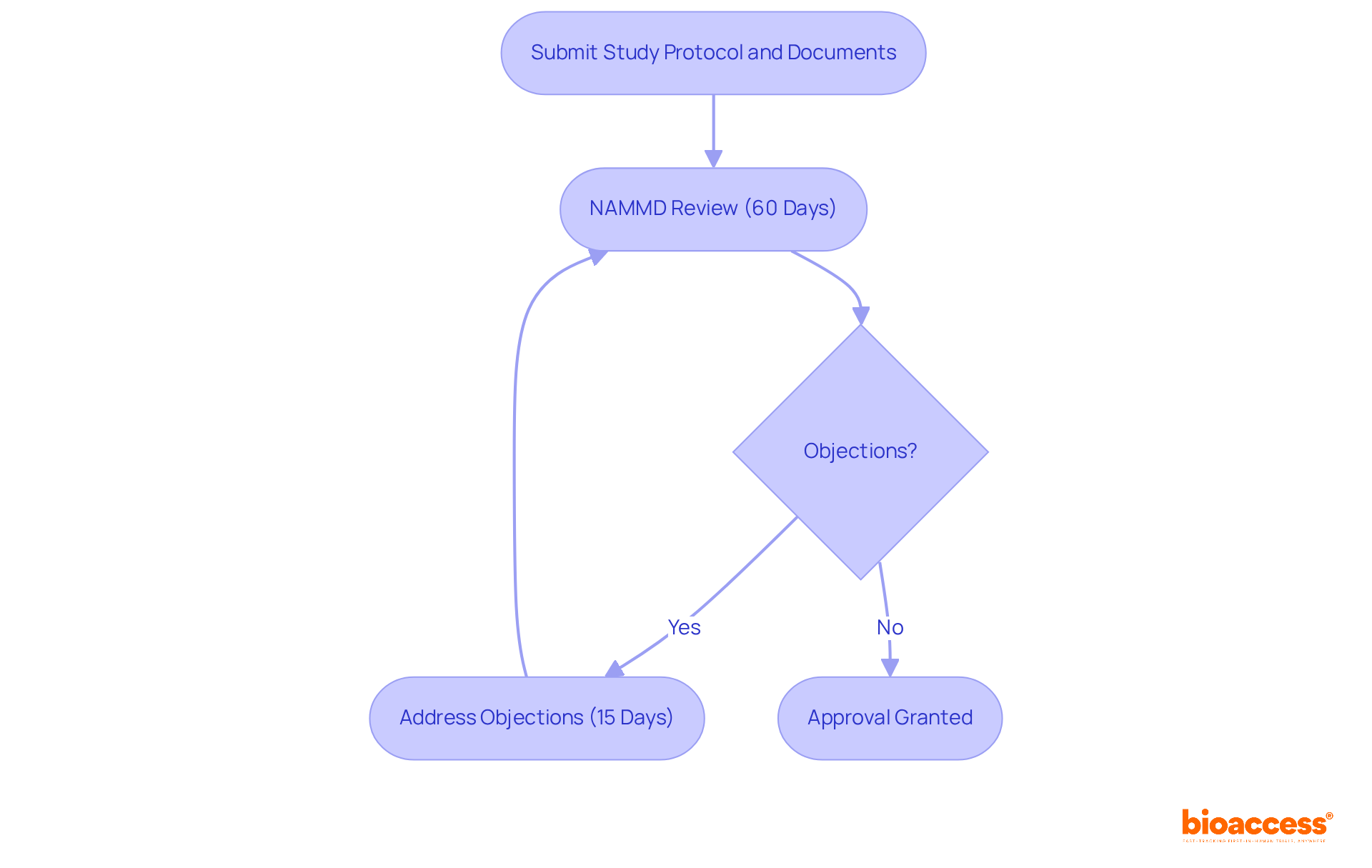
To initiate a research study in Romania, sponsors must secure authorization from the National Agency for Medicines and Medical Devices (NAMMD). This critical process involves submitting the study protocol, informed consent documents, and proof of insurance. The NAMMD conducts a thorough review of these submissions to ensure compliance with both national and EU regulations. Notably, the approval timeline is capped at 60 days, with the agency required to inform sponsors of any objections within 30 days of the initial submission. If objections arise, sponsors have 15 days to address them, fostering a responsive and collaborative approach to regulatory compliance.
This organized procedure enhances the predictability of study initiation and underscores Romania's commitment to maintaining high standards in medical research. Recent legislative updates have further streamlined these processes, making Romania an increasingly attractive destination for medical studies. With a centralized healthcare system and a population nearing 20 million, the country facilitates efficient patient recruitment, which is a significant advantage for sponsors.
As highlighted by bioaccess, their extensive trial management services - including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting - are essential for navigating these regulatory landscapes. The impact of medtech trials on local economies, such as job creation and healthcare improvements, emphasizes the importance of international collaboration in advancing research.
In summary, the revised regulatory processes and timelines position Romania as a compelling option for research studies in Europe. By leveraging the expertise of organizations like bioaccess, sponsors can effectively navigate the complexities of clinical research, ensuring successful outcomes and contributing to the growth of the medtech sector.
A comprehensive budget is essential for effectively managing the financial aspects of a clinical study. This budget must encompass all necessary costs, including:
By developing a detailed financial plan, sponsors can clearly outline anticipated expenses and identify potential funding sources. Regular budget reviews throughout the trial are crucial; they can uncover financial inefficiencies and enable timely adjustments to mitigate risks. For instance, high participant dropout rates can lead to unforeseen expenses, making it essential to monitor and adapt the budget proactively.
As Benjamin Franklin wisely stated, 'An investment in knowledge pays the best interest,' emphasizing the importance of informed financial planning in research. Moreover, statistics show that effective budget management can greatly improve the return on investment (ROI) in research studies, highlighting the importance of a strategic financial strategy. As we approach 2025 in Romania, addressing the trial agreement requirements under Romanian legislation will be more crucial than ever for creating a clear budget and financial strategy to navigate the changing regulatory environment and ensure successful outcomes.
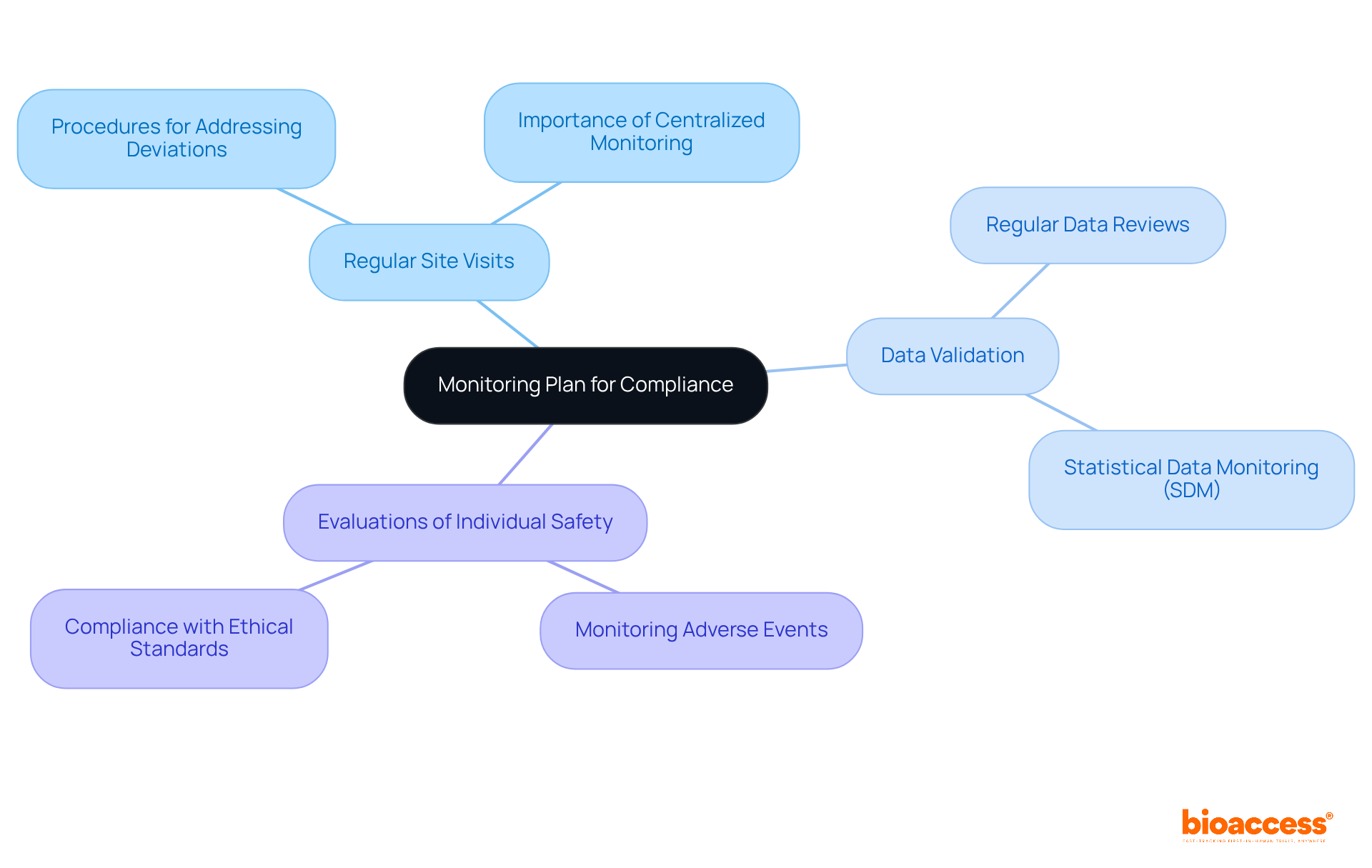
A comprehensive monitoring plan is essential for supervising the execution of clinical studies, ensuring compliance with both the protocol and regulatory requirements. This plan must include:
These elements align with Bioaccess's capabilities in setup and project management. It's crucial to outline procedures for addressing and documenting any deviations from the protocol. Effective monitoring not only protects the well-being of participants but also plays a vital role in maintaining the integrity of the trial.
Studies have shown that centralized monitoring can significantly enhance data quality. In fact, 83% of sites improved their Data Inconsistency Score (DIS) after addressing identified risk signals. Furthermore, compliance monitoring is increasingly recognized as a cornerstone of medical research, with regulatory bodies emphasizing its necessity to uphold ethical standards and ensure participant safety. As Paul Koziarz aptly noted, compliance is not merely an obligation but a strategic advantage that enhances overall performance and credibility.
In the evolving Medtech landscape, Bioaccess stands ready to address these key challenges. By fostering collaboration and implementing robust monitoring strategies, we can ensure that clinical trials not only meet regulatory standards but also prioritize participant safety and data integrity. The next steps involve engaging with stakeholders to refine these processes and enhance the overall effectiveness of clinical research.
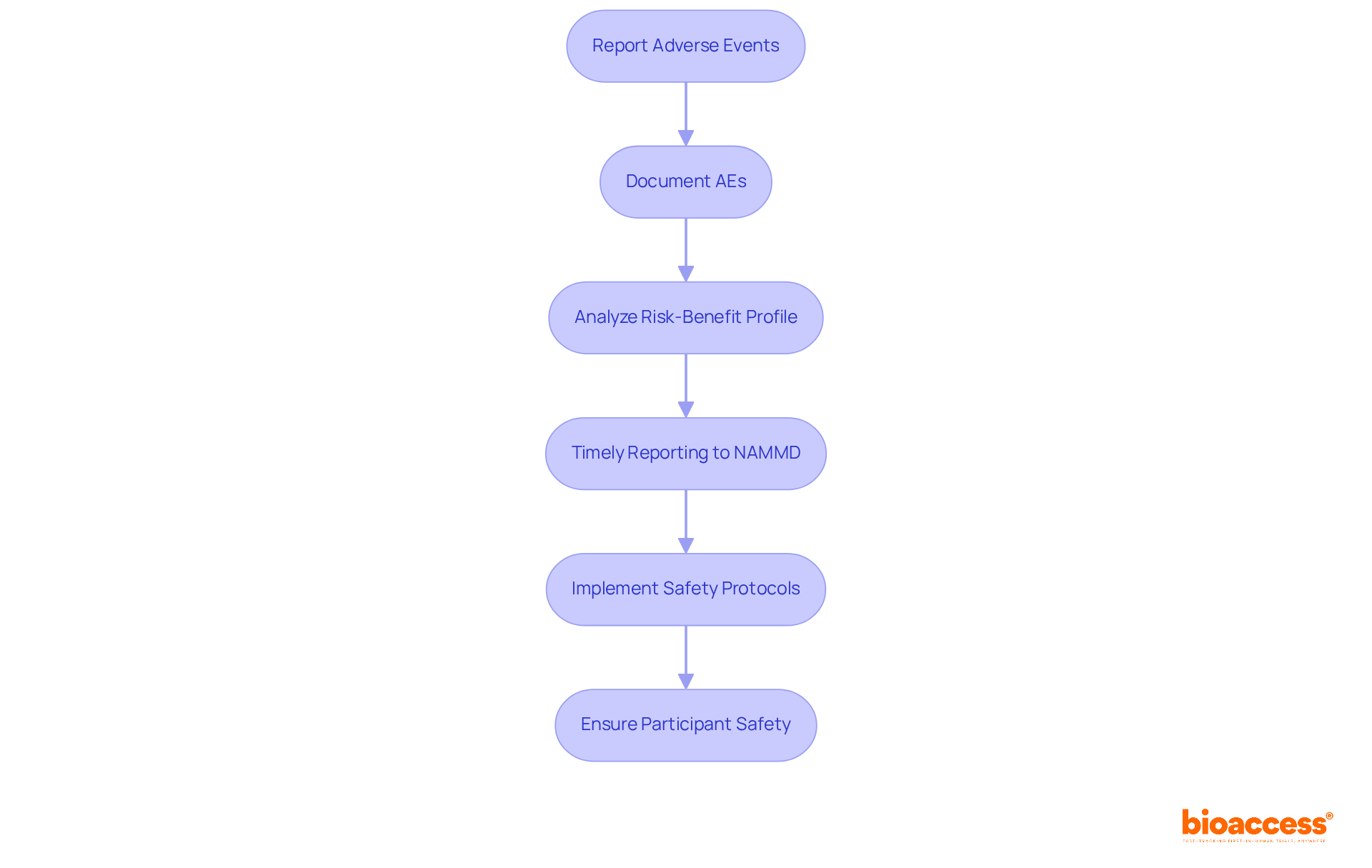
Sponsors and researchers must implement robust procedures for reporting adverse events (AEs) and serious adverse events (SAEs) during clinical studies. Timely reporting to the National Agency for Medicines and Medical Devices (NAMMD) is crucial, ensuring individuals receive necessary medical care when needed. The primary concern is participant safety; thus, all AEs must be meticulously documented and analyzed to assess the study's risk-benefit profile.
In 2025, ongoing discussions in Romania highlight the necessity for strict compliance with safety protocols. Recent research indicates that safety issues are a primary reason for failures in medical investigations, with 17% of unsuccessful phase 3 evaluations linked to patient safety problems. Effective monitoring and transparent communication are essential, as they foster public trust in medical research.
Extensive research management services, such as feasibility assessments, site selection, project initiation, and oversight provided by bioaccess, ensure that reporting practices are detailed and adhere to regulatory standards. Case studies demonstrate that comprehensive reporting practices, like those seen in ClinicalTrials.gov, enhance the reliability of safety data, ensuring that all adverse events are accounted for and addressed promptly.
By prioritizing participant safety, clinical trials not only protect individuals but also contribute to the integrity and success of medical research. This ultimately impacts local economies through job creation and healthcare improvement.
Understanding the trial agreement requirements under Romanian legislation is crucial for the successful execution of clinical studies. The comprehensive framework established by Law 95/2006 and the EU Research Regulations outlines essential elements that contribute to ethical research practices and participant protection. As Romania aligns its regulations with EU standards, the importance of adhering to these requirements cannot be overstated.
Key aspects discussed in this article include:
Each of these components plays a vital role in fostering trust, ensuring compliance, and enhancing the overall integrity of clinical research in Romania. Moreover, the streamlined approval processes and the focus on participant safety underscore the country's commitment to advancing medical research.
As Romania positions itself as a competitive hub for clinical trials, stakeholders must remain vigilant and proactive in navigating these evolving regulations. Emphasizing best practices and compliance will not only contribute to the success of individual studies but also enhance the reputation of the Romanian research landscape on the international stage. Ultimately, prioritizing these trial agreement requirements is essential for advancing medical innovation and ensuring the safety and rights of participants in clinical trials.
What laws govern clinical trials in Romania?
Clinical trials in Romania are primarily governed by Law 95/2006 and the EU Research Regulations (EU No. 536/2014), which provide a comprehensive framework for conducting clinical research.
What is the role of the National Agency for Medicines and Medical Devices (ANMDMR)?
The ANMDMR oversees the regulatory framework for clinical trials in Romania, ensuring that studies uphold ethical standards and maintain scientific integrity.
What is the significance of Law 249/2022 in the context of clinical trials?
Law 249/2022 mandates a 60-day approval period for new research study applications, streamlining the regulatory process and aligning Romania's practices with EU standards, making the country more attractive to international research backers.
How has the regulatory environment affected the number of clinical studies in Romania?
By 2025, Romania is expected to host over 500 clinical studies, with a significant focus on oncology, indicating a robust environment for clinical research driven by recent regulatory changes.
What is the importance of informed consent in clinical trials?
Informed consent is essential for enrolling individuals in clinical trials, requiring clear and comprehensive information about the study's purpose, procedures, risks, and benefits, ensuring participants fully understand before agreeing to participate.
What challenges exist regarding participant comprehension of informed consent?
Research indicates that comprehension of informed consent elements has stagnated, with only 52.1% to 75.8% of individuals understanding critical aspects such as the project's purpose and their right to withdraw.
What practices can improve understanding during the informed consent process?
Employing closed-ended questions during the consent process has been shown to enhance comprehension rates, along with tailored strategies aimed at improving understanding among diverse groups.
Why is a comprehensive trial protocol important?
A comprehensive trial protocol serves as the foundational blueprint for clinical research, outlining objectives, methodology, participant selection criteria, and statistical analysis plans, which ensures adherence to regulatory requirements.
How does a detailed protocol impact the success of medical studies?
Research indicates that medical studies with detailed protocols achieve significantly better success rates, highlighting the importance of protocol adherence for quality assurance and reproducibility of results.
What elements should be included in an effective research protocol?
An effective research protocol should include comprehensive details about the study design, rationale, objectives, methodologies, and address all ethical considerations.