


In the realm of oncology trials, ethical considerations are paramount, especially in regions like Serbia where regulatory landscapes are evolving rapidly. Addressing these ethical challenges not only safeguards participant rights but also enhances the credibility of research outcomes. As researchers navigate complex dilemmas - ranging from informed consent to the use of placebo controls - questions arise about how to balance scientific advancement with the imperative to protect patient welfare.
What strategies can be employed to uphold ethical integrity while ensuring that clinical trials contribute meaningfully to cancer research? This inquiry is crucial as it highlights the need for a robust framework that prioritizes both ethical standards and the advancement of medical knowledge. By fostering a culture of ethical vigilance, we can ensure that the rights of participants are respected while also paving the way for significant breakthroughs in oncology.
bioaccess® is dedicated to maintaining the highest moral standards in oncology studies, understanding that overcoming ethical challenges in oncology trials in Serbia is essential for achieving successful clinical outcomes. By leveraging a deep understanding of local regulations - including necessary approvals from institutional review boards (IRB), Colombia's regulatory agency (INVIMA), and import permits from the Ministry of Industry and Commerce (MinCIT) - bioaccess® guarantees that all research activities are conducted with integrity. This commitment includes:
Such measures not only safeguard participant rights but also tackle ethical challenges in oncology trials in Serbia, enhancing the credibility of research findings and fostering trust among stakeholders and participants. As industry experts emphasize, effective ethical practices are vital for advancing cancer research and ensuring that studies yield significant outcomes that benefit society.
Informed consent in oncology studies is not just a formality; it stands as a cornerstone of ethical research that upholds patient autonomy. Researchers must ensure that individuals are thoroughly informed about the study's nature, potential risks, benefits, and available alternatives. This requires clear communication, utilizing layman's terms to prevent misunderstandings. Moreover, establishing ongoing consent processes is crucial, allowing individuals the freedom to withdraw at any time without facing penalties.
Prioritizing informed consent fosters trust and enhances patient engagement, ultimately contributing to the ethical integrity and success of clinical trials. Recent analyses reveal that approximately 75% of individuals comprehend their rights and the study's purpose, yet gaps persist, particularly concerning complex concepts like randomization and placebo. How can we bridge these gaps? Utilizing best practices - such as employing closed-ended questions to evaluate understanding - can significantly enhance individuals' comprehension and ensure their autonomy is honored throughout the research process.
Enrolling individuals for oncology studies involves ethical challenges in oncology trials in Serbia, especially when trying to balance the urgency of recruitment with the need to protect subjects' rights and welfare. Researchers must adopt recruitment strategies that prioritize both efficiency and ethics to address the ethical challenges in oncology trials in Serbia, ensuring potential participants are well-informed and empowered to make decisions about their involvement. Effective strategies include:
Research shows that 92.7% of cancer patients deem it essential to discuss studies with their healthcare providers, underscoring the critical role of open communication.
Moreover, fostering an ethical recruitment environment can significantly enhance participant diversity and retention, which are essential for overcoming ethical challenges in oncology trials in Serbia. By taking a proactive approach-such as simplifying recruitment materials and ensuring they are easily understood-researchers can reduce barriers to participation. This not only helps achieve enrollment goals but also contributes to more robust results. Trials that implement patient-friendly protocols have reported improved retention rates, with some reaching as high as 95%. Additionally, nearly 80% of clinical studies fail to meet initial enrollment targets, highlighting the urgent need for effective recruitment strategies. This challenge is particularly pertinent for Medtech and Biopharma startups, which often struggle with site participation and patient eligibility. Ultimately, a commitment to principled recruitment methods helps address the ethical challenges in oncology trials in Serbia, adheres to regulatory standards, and builds trust within the community, laying the groundwork for future collaborations and advancements in cancer research.
Transparency in clinical study protocols is not just a best practice; it is essential for maintaining ethical research in oncology. This principle involves the open exchange of study frameworks, methodologies, and outcomes with all stakeholders, including subjects, regulatory authorities, and the public. By committing to transparency, researchers significantly enhance accountability and foster trust-two vital components for participant engagement and retention.
This commitment encompasses several key practices:
Such practices not only uphold ethical standards but also strengthen the overall integrity of the research process. Ultimately, this benefits the progress of cancer treatments, paving the way for advancements that can save lives.
In cancer research, protecting patient data is not just important; it’s essential due to the sensitive nature of the health information involved. Researchers must implement comprehensive data privacy measures to comply with regulations like GDPR and HIPAA. Key strategies include:
By prioritizing these practices, researchers not only meet legal standards but also foster trust with participants, enhancing their willingness to engage in clinical studies. Efficient data safeguarding transcends regulatory requirements; it embodies a fundamental moral duty that upholds the integrity of cancer research.
Ethics committees play a crucial role in upholding moral integrity in clinical studies, especially when addressing the ethical challenges in oncology trials in Serbia. They meticulously examine study designs, informed consent procedures, and risk-benefit evaluations to ensure adherence to ethical standards and the protection of participant rights. Recent statistics reveal that the median duration for governance approval in oncology studies is approximately 34 days, highlighting the ethical challenges in oncology trials in Serbia, with some studies experiencing prolonged activation periods due to complex ethical considerations.
Involving ethics boards early in the study design process is essential for identifying potential ethical concerns and streamlining approval workflows. Effective engagement strategies include fostering open dialogue and collaboration, which can significantly enhance the ethical oversight of studies. By viewing ethics committees as allies rather than obstacles, researchers can navigate the regulatory landscape more effectively, ensuring that their studies not only meet ethical standards but also contribute positively to the advancement of cancer research.
The implementation of placebo controls in oncology trials leads to ethical challenges in oncology trials in Serbia, especially when effective treatments are available. Researchers face the critical task of weighing the potential benefits of using a placebo against the ethical challenges in oncology trials in Serbia, especially regarding the risks to individuals who may be denied effective therapies. In the context of ethical challenges in oncology trials in Serbia, ethical guidelines dictate that placebo use is permissible only when no established effective treatment exists or when withholding treatment poses minimal risk to individuals.
Recent discussions highlight that when effective treatments are available, the justification for placebo use raises ethical challenges in oncology trials in Serbia, becoming increasingly tenuous. The European Medicines Agency has observed that while synthetic control arms are recommended in certain situations, they are discouraged when randomized studies can be ethically conducted. This raises important questions regarding the ethical challenges in oncology trials in Serbia: How do we balance the need for scientific advancement with the imperative to protect patient welfare?
Navigating the ethical challenges in oncology trials in Serbia is essential for researchers aiming to design trials that prioritize participant welfare while contributing to the advancement of scientific knowledge. By carefully considering these ethical implications, we can preserve the integrity of clinical research and uphold moral standards. Collaboration among stakeholders is crucial in addressing these challenges and ensuring that clinical trials are conducted ethically and effectively.
Conducting oncology research involving vulnerable populations presents ethical challenges in oncology trials in Serbia that demand heightened responsibilities. Researchers must ensure that these groups are not exploited, and that their participation is both voluntary and informed. This includes providing additional support and resources to help them grasp the trial's implications. Ethical guidelines dictate that researchers perform comprehensive risk-benefit assessments and establish safeguards to protect vulnerable individuals, addressing the ethical challenges in oncology trials in Serbia.
Recent discussions in the field, particularly a paper from Dana-Farber Cancer Institute, underscore the importance of preserving patient autonomy and dignity. As cancer patients increasingly engage with AI technologies for health monitoring and treatment guidance, the authors advocate for collaboration among medical societies, AI technologists, and government leaders. This collaboration is essential to ensure that AI not only preserves patient autonomy but also promotes healthcare equity.
By prioritizing these moral considerations, researchers can foster equitable participation and enhance the integrity of their studies. The call for action is clear: we must work together to navigate the complexities of oncology research and address the ethical challenges in oncology trials in Serbia, ensuring that ethical standards are upheld and that vulnerable populations are protected.
Researchers bear a crucial ethical responsibility to communicate the findings of oncology studies effectively, especially in light of the ethical challenges in oncology trials in Serbia, to both participants and the broader medical community. This responsibility encompasses providing timely information about study outcomes and their potential implications for patient care. Effective communication not only builds trust but also empowers patients to make informed decisions regarding their treatment options. Furthermore, researchers must actively advocate for the integration of study results into clinical practice, as this can significantly enhance patient outcomes.
In Latin America, particularly in Colombia, media coverage plays an essential role in disseminating legal outcomes and raising public awareness, which can further encourage patient involvement. Statistics reveal that organizations prioritizing patient engagement see a 30% increase in retention and achieve enrollment goals 25% faster. This underscores the positive impact of transparent communication on participation and patient care. By fulfilling these ethical obligations, researchers can help navigate the ethical challenges in oncology trials in Serbia and make substantial contributions to the advancement of cancer treatment.

The environment of moral standards is facing ethical challenges in oncology trials in Serbia, driven by technological advancements, regulatory changes, and shifting societal expectations. Researchers must stay alert to the ethical challenges in oncology trials in Serbia, particularly those related to data privacy, the integration of artificial intelligence, and the enhancement of patient engagement. Establishing acceptable risk levels for individuals in early-phase studies is a crucial moral concern that demands attention. By proactively addressing the ethical challenges in oncology trials in Serbia and adapting moral frameworks to fit new realities, researchers can uphold the integrity of oncology studies while prioritizing participant welfare.
Moreover, the moral obligation to ensure continued access to beneficial treatments after clinical trials underscores the importance of transparency and accountability in decision-making processes. Ongoing education and collaboration among all stakeholders will be vital in nurturing a robust culture of responsible research in this dynamic field. As Heinz R. Pagels aptly stated, "Science cannot resolve moral conflicts, but it can help to more accurately frame the debates around these conflicts." This highlights the necessity for continuous dialogue regarding the ethical challenges in oncology trials in Serbia.
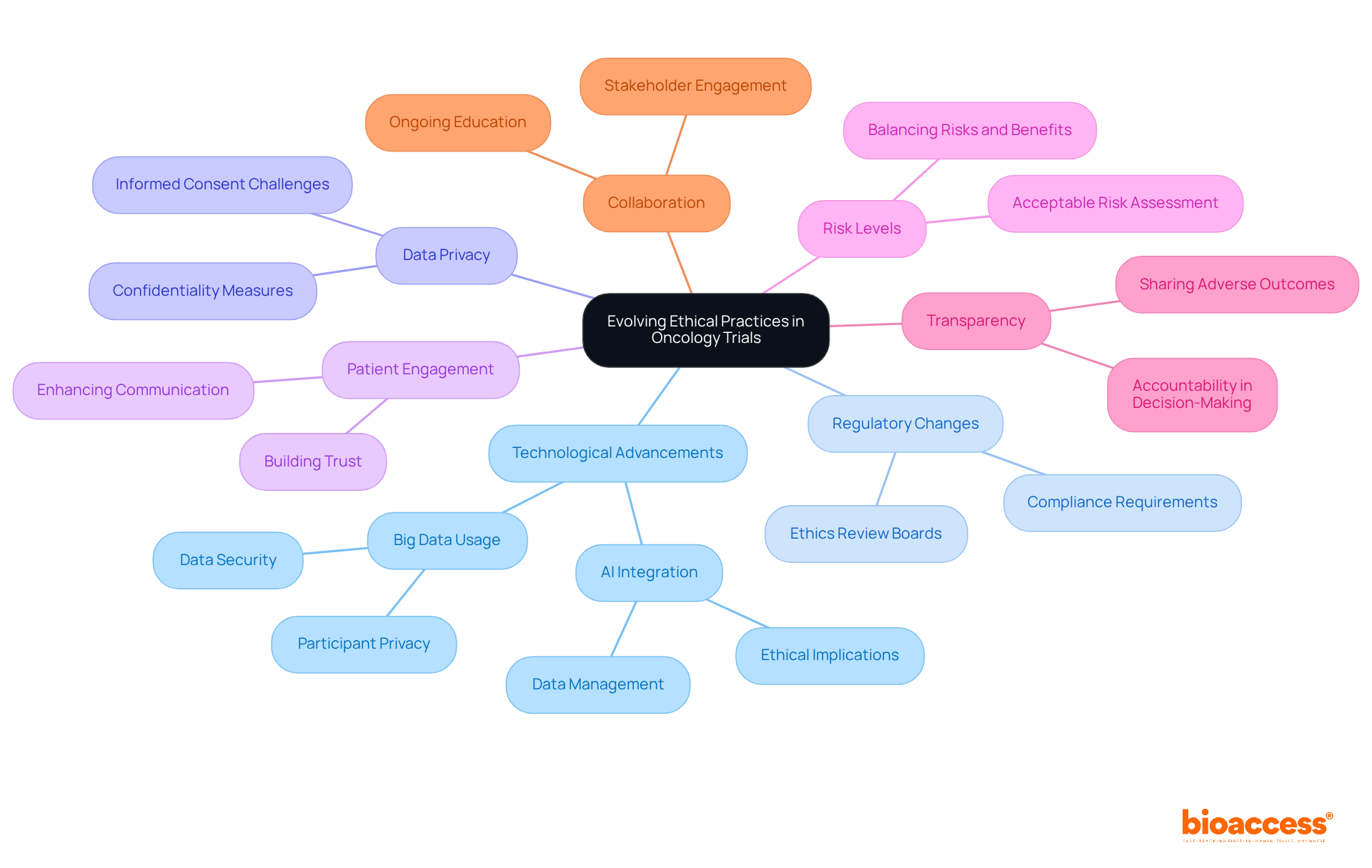
Addressing the ethical challenges in oncology trials in Serbia is not just important; it is essential for ensuring that research adheres to high moral standards and effectively contributes to advancements in cancer treatment. The commitment to ethical practices - such as informed consent, transparency, and the protection of vulnerable populations - forms the backbone of responsible research. By prioritizing these principles, researchers can foster trust and enhance the credibility of their studies, ultimately benefiting society as a whole.
Key areas of focus include:
Furthermore, the discussion highlights the need for transparency in trial protocols and robust data privacy measures to protect sensitive patient information. Each of these elements plays a vital role in navigating the complex landscape of oncology research and addressing the unique challenges faced in Serbia.
As the field of oncology continues to evolve, it is crucial for researchers, regulatory bodies, and stakeholders to engage in ongoing dialogue and collaboration. This collective effort will not only enhance ethical practices but also ensure that the rights and welfare of participants remain at the forefront of clinical trials. Embracing these ethical considerations is not merely a regulatory requirement; it is a moral obligation that can lead to more effective and equitable cancer treatments, ultimately improving outcomes for patients and fostering trust in the research community.
What is bioaccess® and its role in oncology trials?
bioaccess® is dedicated to maintaining high moral standards in oncology studies, particularly in Serbia. It ensures ethical compliance by understanding local regulations, obtaining necessary approvals, and conducting research activities with integrity.
What measures does bioaccess® take to ensure ethical compliance in oncology trials?
bioaccess® implements comprehensive training for personnel on ethical practices, conducts regular evaluations of research protocols, and engages proactively with ethics committees to address potential issues.
Why is informed consent important in oncology studies?
Informed consent is crucial as it upholds patient autonomy by ensuring individuals are thoroughly informed about the study's nature, potential risks, benefits, and alternatives. It fosters trust and enhances patient engagement in clinical trials.
How can researchers improve understanding of informed consent among participants?
Researchers can improve understanding by using layman's terms, establishing ongoing consent processes, and employing best practices such as closed-ended questions to evaluate comprehension.
What are the ethical challenges in patient recruitment for oncology studies?
Ethical challenges include balancing the urgency of recruitment with the need to protect participants' rights and welfare, ensuring that potential participants are well-informed and empowered to make decisions about their involvement.
What strategies can enhance ethical patient recruitment in oncology trials?
Effective strategies include collaborating with community organizations, providing clear and accessible information about risks and benefits, and utilizing patient-centered communication methods.
How does communication with healthcare providers impact patient recruitment for oncology studies?
Research indicates that 92.7% of cancer patients find it essential to discuss studies with their healthcare providers, highlighting the importance of open communication in the recruitment process.
What are the benefits of implementing patient-friendly protocols in clinical trials?
Patient-friendly protocols can improve participant diversity and retention rates, with some trials reporting retention as high as 95%. They also help reduce barriers to participation and contribute to more robust research results.
What challenges do Medtech and Biopharma startups face in patient recruitment?
Medtech and Biopharma startups often struggle with site participation and patient eligibility, making effective recruitment strategies particularly critical for their success in oncology trials.