


The article titled "10 FDA Guidance Design Control Tips for Clinical Research Leaders" provides essential strategies for clinical research leaders to effectively navigate FDA design control regulations. It underscores that the implementation of structured design control practices not only ensures compliance with FDA standards but also significantly enhances the quality and efficiency of clinical studies. This, in turn, expedites product development and approval processes, making it a crucial read for those in the Medtech landscape.
Navigating the complexities of FDA regulations presents a formidable challenge for clinical research leaders, particularly within the dynamic realms of Medtech and Biopharma. Mastering the intricacies of design control transcends mere regulatory obligation; it serves as a strategic advantage that can streamline product development and bolster compliance.
How can leaders ensure that their processes not only satisfy regulatory standards but also promote innovation and efficiency? This article explores ten essential tips for implementing effective FDA guidance design control practices, empowering clinical research leaders to expedite their projects while upholding the highest quality and safety standards.
bioaccess® excels in guiding Medtech, Biopharma, and Radiopharma innovators through the intricacies of FDA compliance by implementing effective FDA guidance design control practices. By specializing in early-phase clinical research, bioaccess® leverages its extensive knowledge of regulatory frameworks to assist clients in meeting stringent safety and efficacy standards. This expertise is crucial for clinical research leaders who aim to expedite product development timelines while ensuring adherence to FDA regulations.
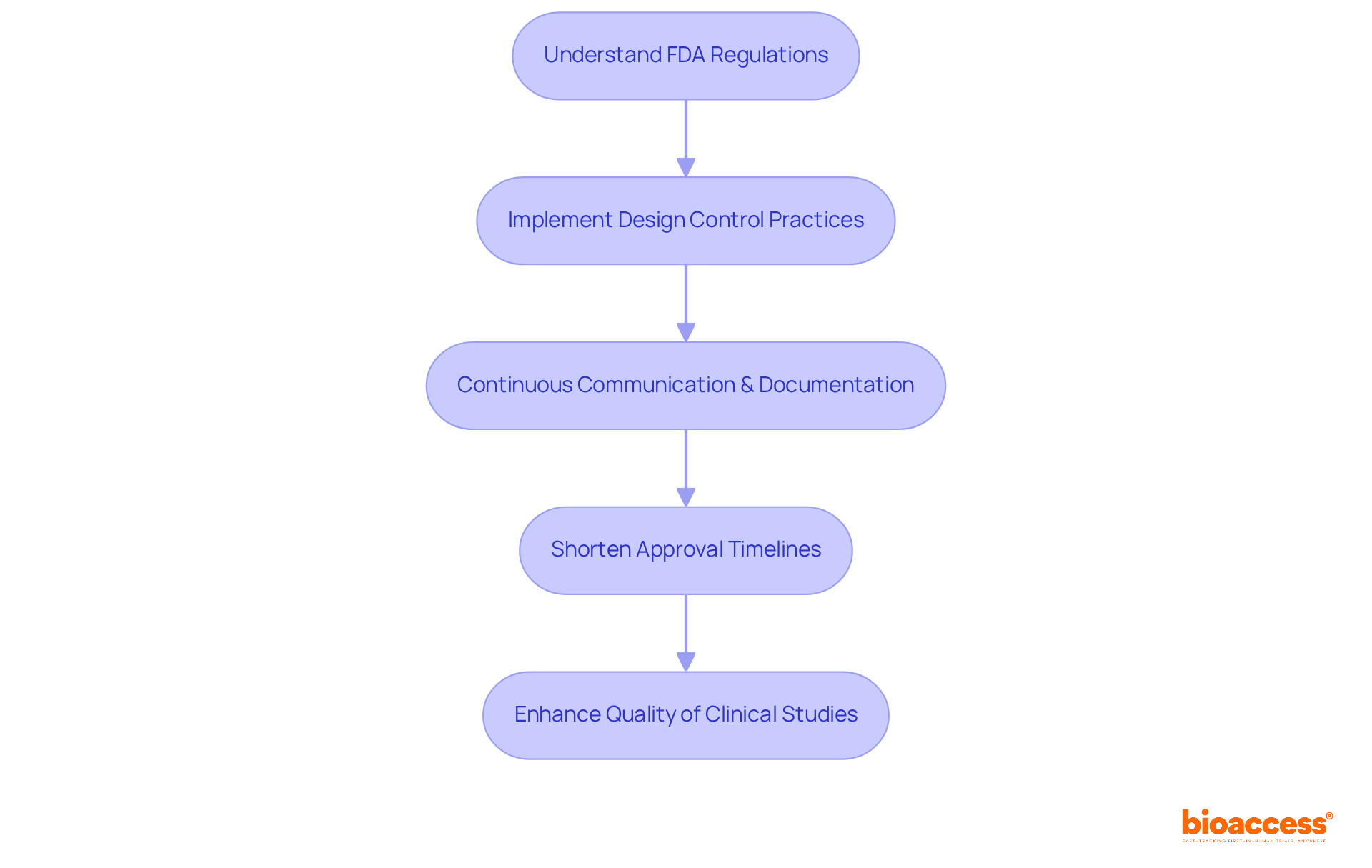
Implementing strong control practices aligned with FDA guidance design control enables organizations to significantly shorten approval timelines, simplify workflows, and enhance the overall quality of their clinical studies. Furthermore, bioaccess® emphasizes the importance of continuous communication and documentation throughout the research process. These elements are essential for maintaining compliance and achieving successful outcomes in clinical trials.
In the evolving landscape of Medtech, collaboration with a knowledgeable partner like bioaccess® not only addresses key challenges but also fosters innovation and efficiency in clinical research. As the industry continues to face regulatory complexities, the role of bioaccess® becomes increasingly vital in navigating these challenges effectively.
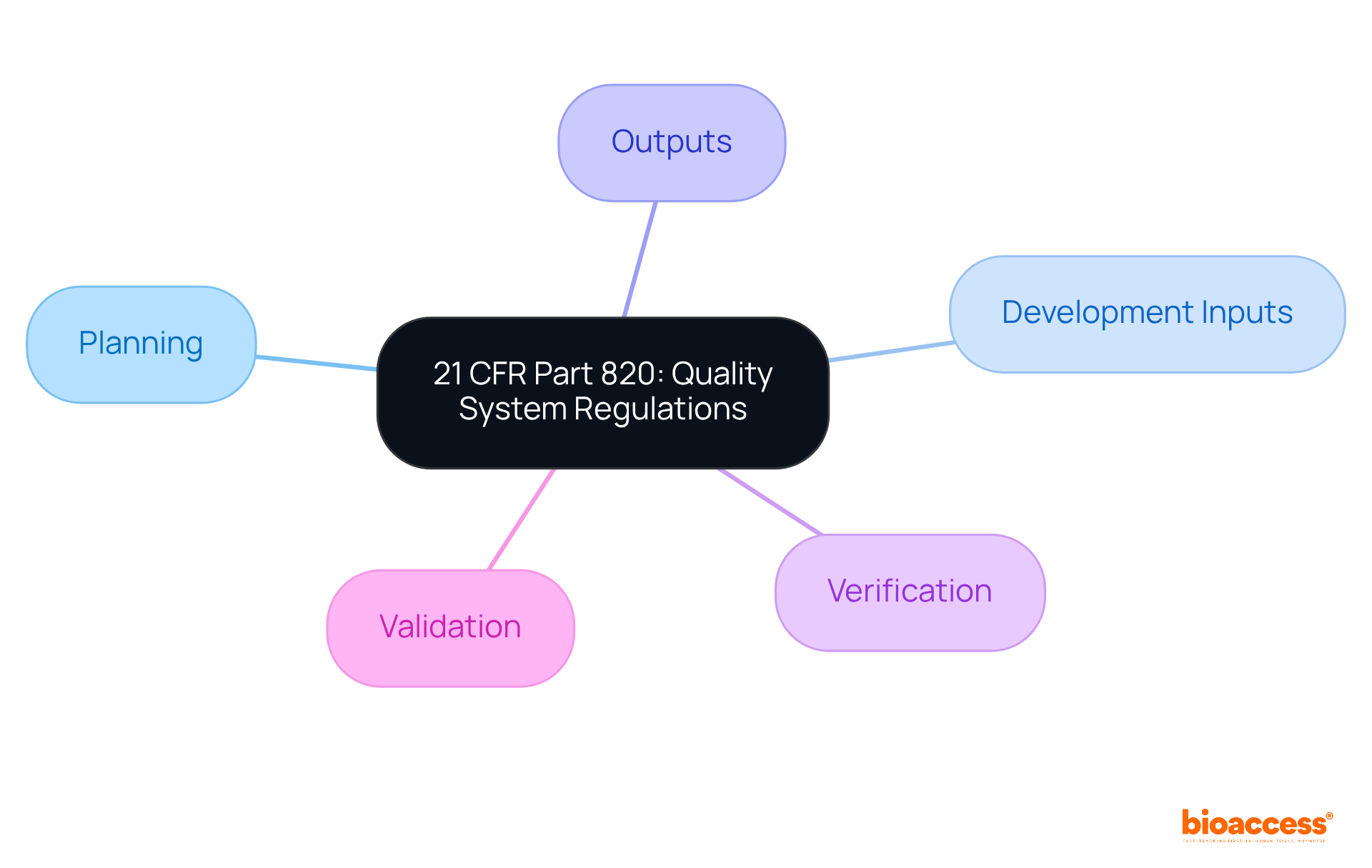
21 CFR Part 820 establishes the quality system regulations (QSR) that medical device manufacturers must adhere to, encompassing every aspect of device development. This includes essential components such as:
For clinical research leaders, mastering the FDA guidance design control regulations is crucial to ensure compliance throughout the planning process, significantly mitigating the risk of regulatory challenges during product development. A comprehensive understanding of these requirements is vital for establishing a robust quality management system, which plays a key role in facilitating successful FDA submissions.
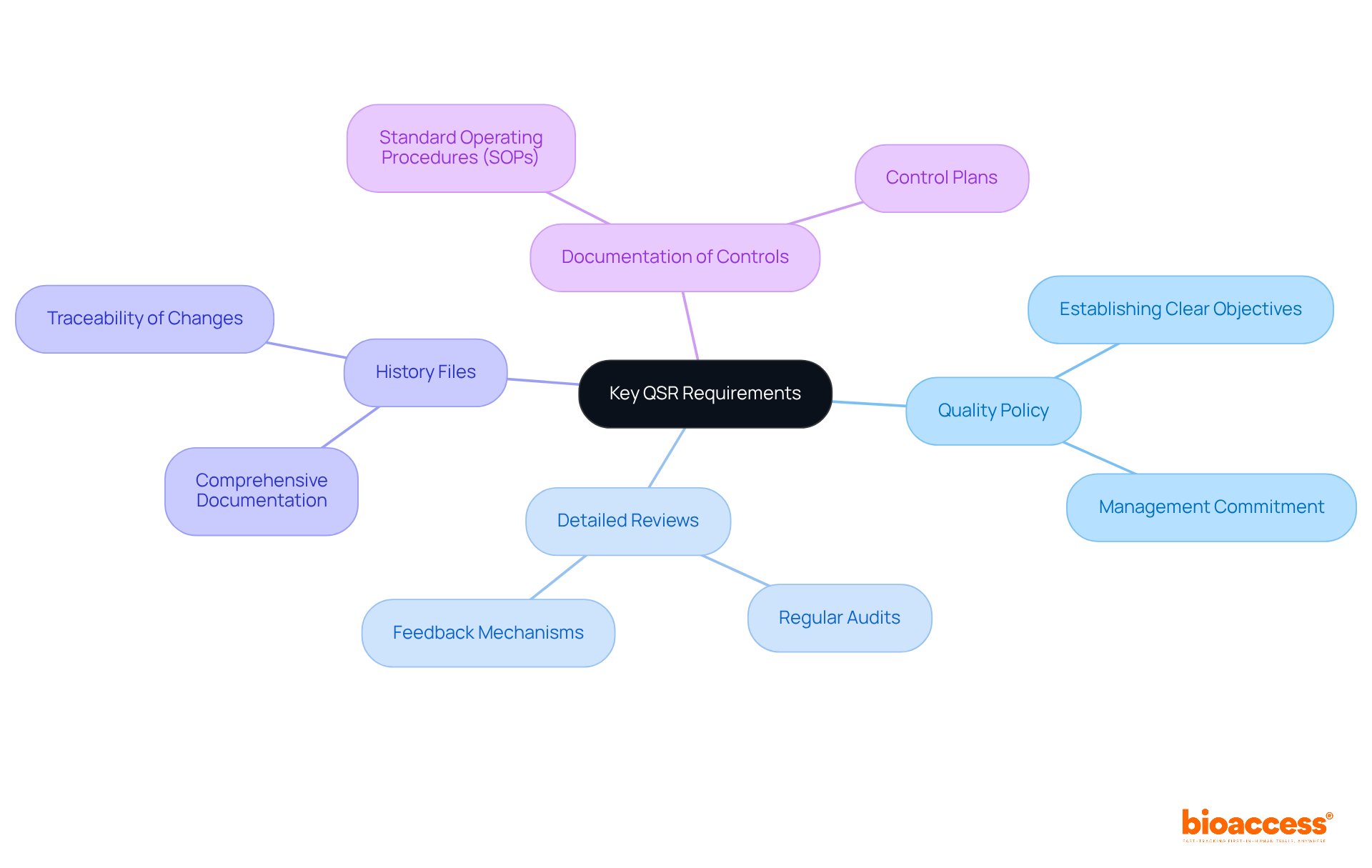
Key requirements of the Quality System Regulation (QSR) encompass several foundational elements that are crucial for ensuring quality in medical product development. A strong quality policy, detailed reviews, extensive history files, and precise documentation of controls are vital practices. These components not only demonstrate compliance with FDA guidance design control but also play a pivotal role in ensuring the safety and effectiveness of medical devices. By prioritizing these requirements, clinical research leaders can cultivate a culture of quality within their organizations. This approach ultimately facilitates successful product development and expedites market entry.
Carrying out crucial evaluation reviews is vital in the control system, serving as a key practice at significant milestones during the development cycle. These evaluations are designed to assess development progress, identify potential issues, and ensure adherence to user needs and regulatory standards. A structured review process that adheres to FDA guidance design control not only enhances collaboration among team members but also facilitates informed decision-making, ultimately improving the quality of the medical device being developed.
Statistics reveal that numerous submissions encounter delays or rejections due to insufficient documentation and weak traceability between input and output elements. For instance, the EU COMBINE project highlighted that inadequate coordination and organized documentation in review processes resulted in considerable setbacks in trial approvals. Furthermore, a 2023 FDA warning letter stressed the importance of involving independent reviewers in evaluations, as their absence can lead to significant oversights, exemplified by the Class II recall of a U.S. medical device due to a software malfunction.
To conduct effective evaluations, clinical research leaders should establish a formal review process with scheduled milestones and standardized templates. This approach ensures that all necessary elements, including risk management and FDA guidance design control, are consistently addressed. Cross-functional participation is essential; involving experts from quality assurance, regulatory affairs, and manufacturing allows for a comprehensive evaluation of the product from multiple perspectives.
Implementing standardized checklists that follow FDA guidance design control and ISO requirements can further enhance the review process, ensuring that all critical aspects are covered. Each review of the layout should conclude with recorded action items, designated owners, and deadlines, fostering accountability and demonstrating a commitment to ongoing enhancement. Follow-up reviews are crucial to confirm the completion of these actions, reinforcing the team's responsiveness to regulatory expectations.
By viewing assessments as strategic checkpoints rather than casual gatherings, organizations can mitigate risks and improve compliance. The organized documentation of decisions and the rationale behind them not only aids in audits but also facilitates future development iterations. Ultimately, efficient evaluations of products are not merely mandatory obligations; they are essential instruments for ensuring the safety, effectiveness, and compliance of medical devices.

Efficient preparation during the creation and development stages is crucial for meeting regulatory obligations and addressing user needs. This process necessitates the clear definition of project timelines, effective resource allocation, and the implementation of robust risk management strategies.
Clinical research leaders must develop a comprehensive blueprint and development plan that articulates objectives, deliverables, and milestones, thereby laying a solid foundation for the project. Such thorough preparation not only streamlines workflows but also significantly enhances the probability of successful submissions following FDA guidance design control.
Industry leaders emphasize that a well-structured strategy can reduce the typical time spent on creation and development in Medtech, ultimately accelerating the path to market for innovative medical products.

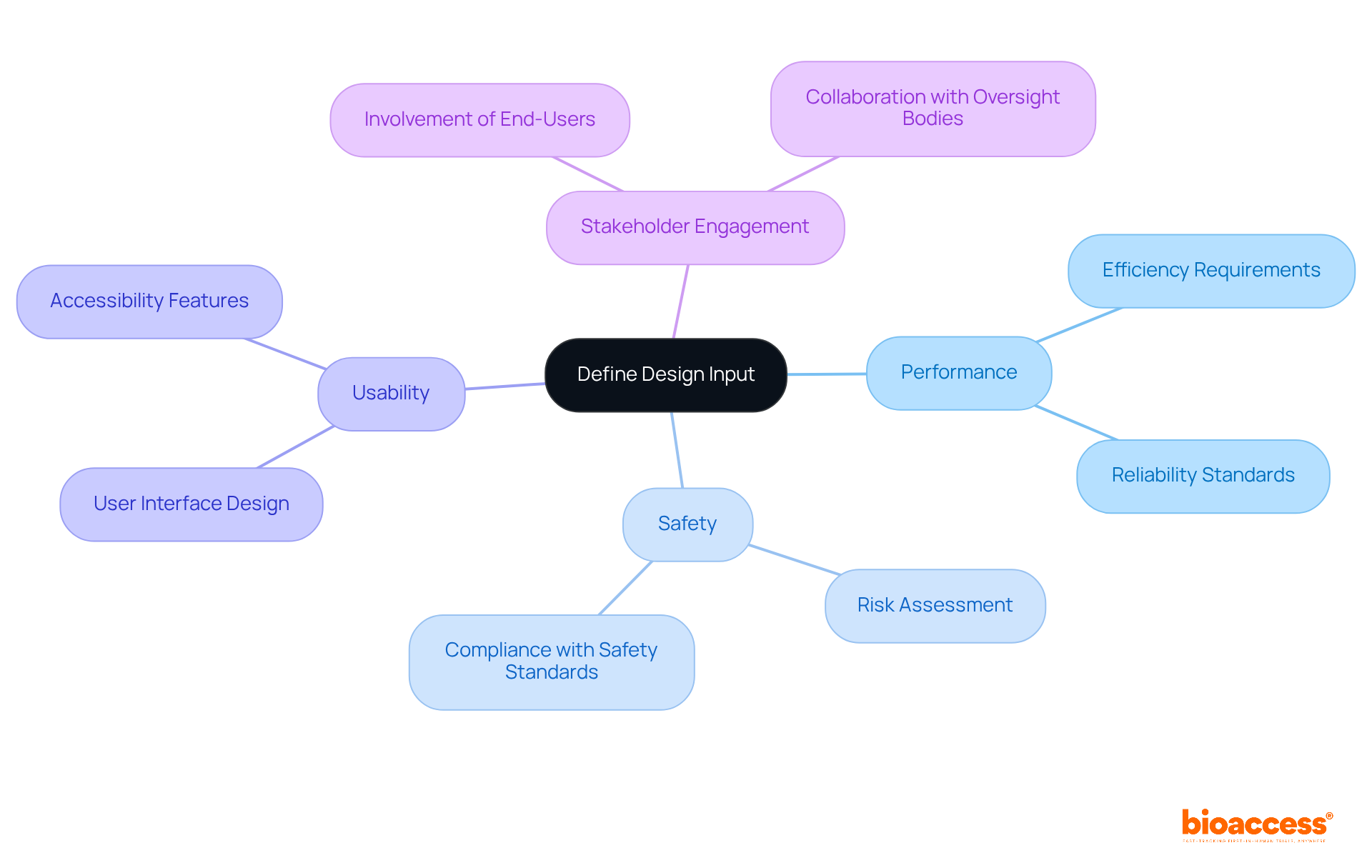
Defining design inputs is a crucial step in the medical equipment development process. These inputs articulate the specific requirements that the device must meet, encompassing performance, safety, and usability criteria. To effectively gather comprehensive input that reflects the needs and expectations of the target population, clinical research leaders should engage stakeholders, including end-users and oversight bodies. By establishing clear and measurable inputs for creation, organizations can align their efforts with user needs and regulatory requirements, ultimately enhancing the development process in compliance with FDA guidance design control.
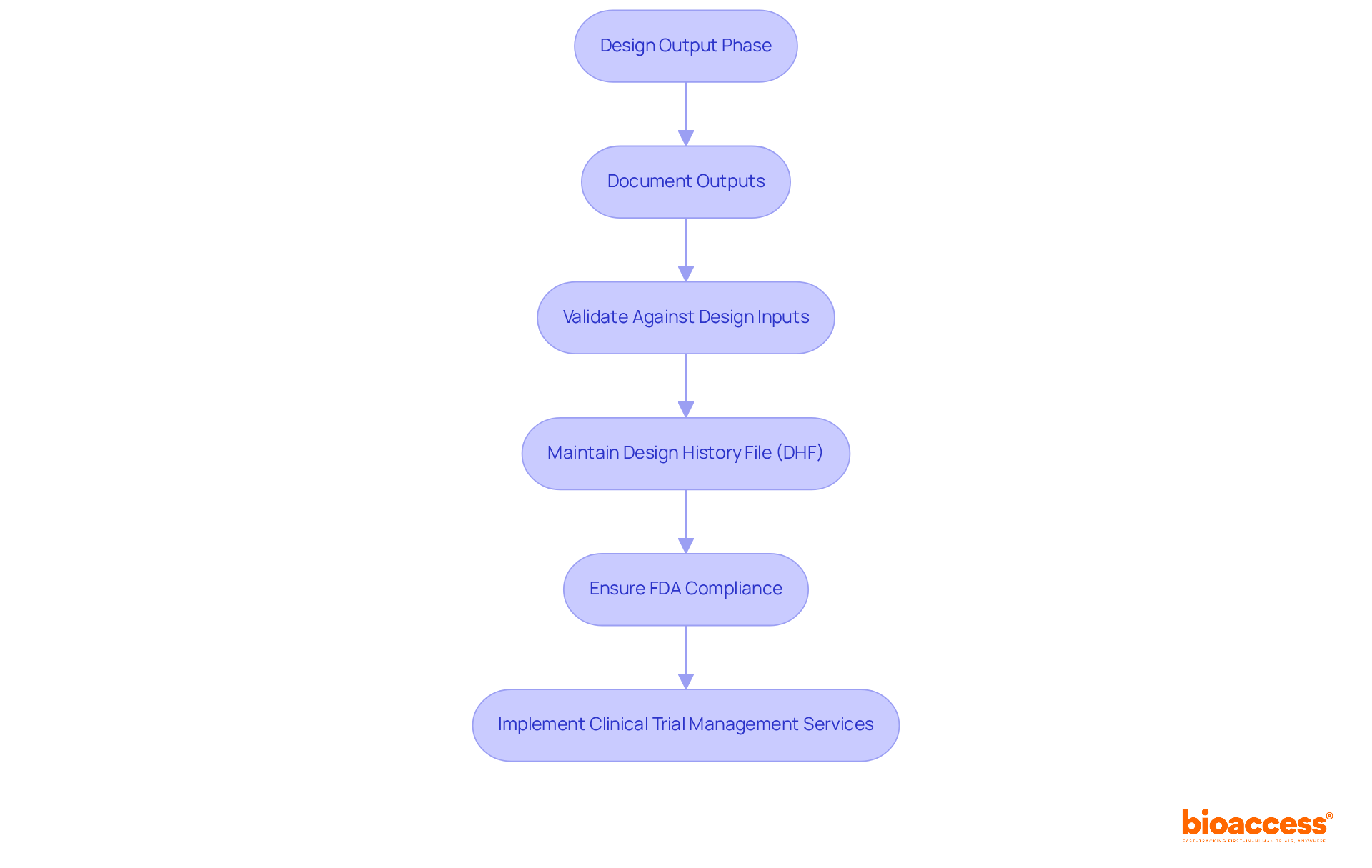
Ensuring that creation outputs align with established specifications is crucial for the success of any medical device. The outputs from the creation phase comprise all elements, assemblies, and documentation produced throughout development. Clinical research leaders must meticulously document and validate these outputs against the design inputs to mitigate risks and enhance product quality.
Statistics indicate that 85% of failures in medical device development stem from deficiencies in systems and procedures, underscoring the significance of robust documentation practices. Maintaining a well-organized Design History File (DHF) not only facilitates compliance with FDA guidance design control but also enhances the credibility of the development process.
Industry leaders assert that comprehensive documentation is not merely a compliance necessity; it serves as a strategic asset that can significantly improve the likelihood of successful FDA guidance design control and expedite market entry. By prioritizing quality and compliance during the design output stage, organizations can more effectively navigate the complexities of the oversight environment in accordance with FDA guidance design control, thereby enhancing their chances of delivering safe and effective medical devices.
Non-compliance with FDA guidance design control can lead to substantial penalties and product recalls, making it imperative for clinical research leaders to adopt thorough documentation practices. Furthermore, leveraging extensive clinical trial management services—including feasibility studies, site selection, compliance reviews, and project management—can optimize workflows. With specialists like Ana Criado, who possesses extensive experience in compliance matters and biomedical engineering, organizations can ensure that their clinical studies adhere to required standards.
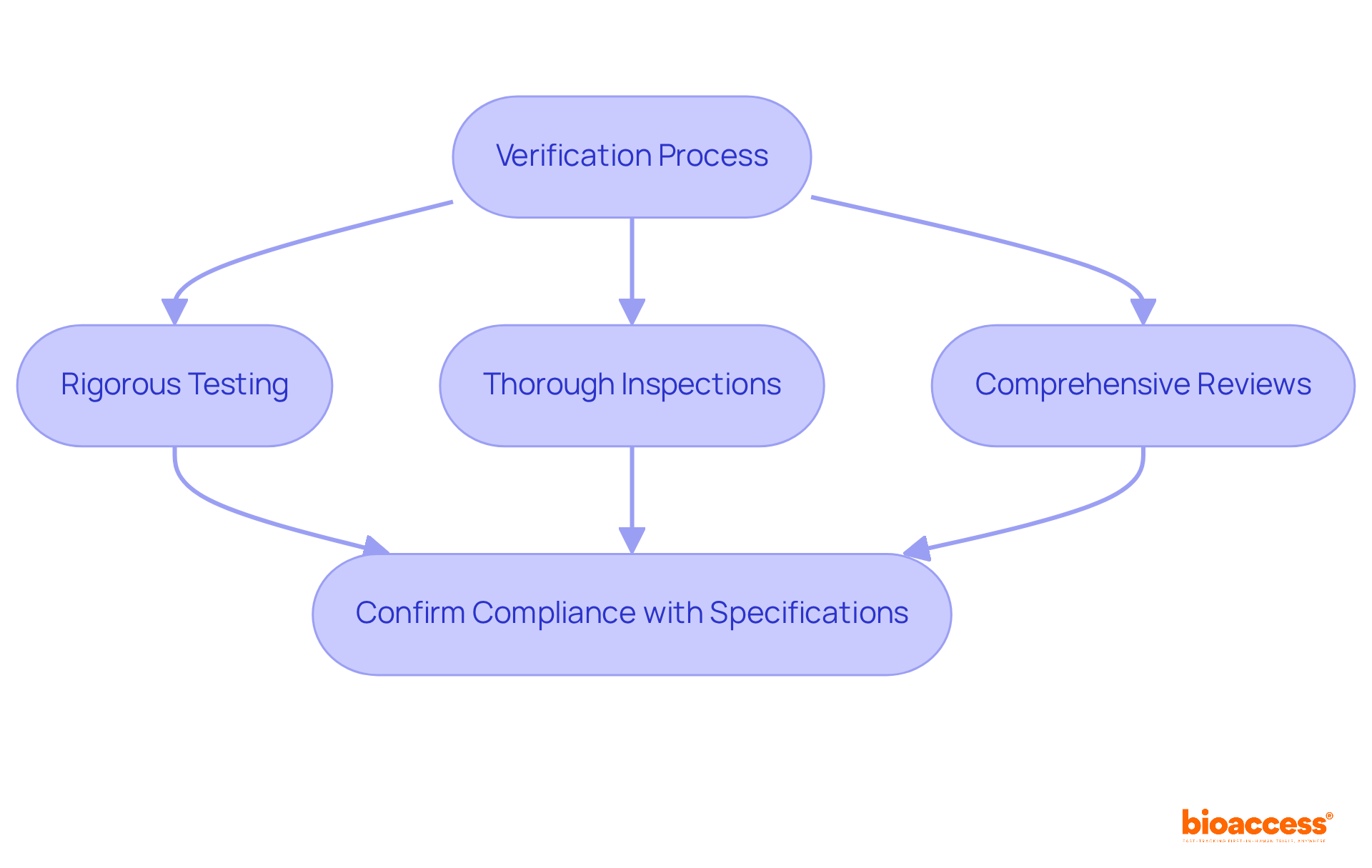
Verifying design outputs is a crucial component of the design control system, as outlined in the FDA guidance design control, ensuring alignment with the specifications detailed in the design inputs. Clinical research leaders must adopt a structured verification process that includes:
to confirm compliance with regulatory standards. This proactive approach addresses potential discrepancies early, significantly enhancing the overall quality and safety of medical devices. Statistics indicate that discrepancies discovered during verification can lead to costly delays and compliance issues, underscoring the importance of meticulous verification practices. Organized verification procedures have been shown to reduce the likelihood of post-market issues, as they ensure that all outputs meet the required specifications prior to product release. Expert insights reveal that effective verification, aligned with FDA guidance design control, not only protects user safety but also streamlines the journey to regulatory approval, making it an essential practice in the Medtech industry.
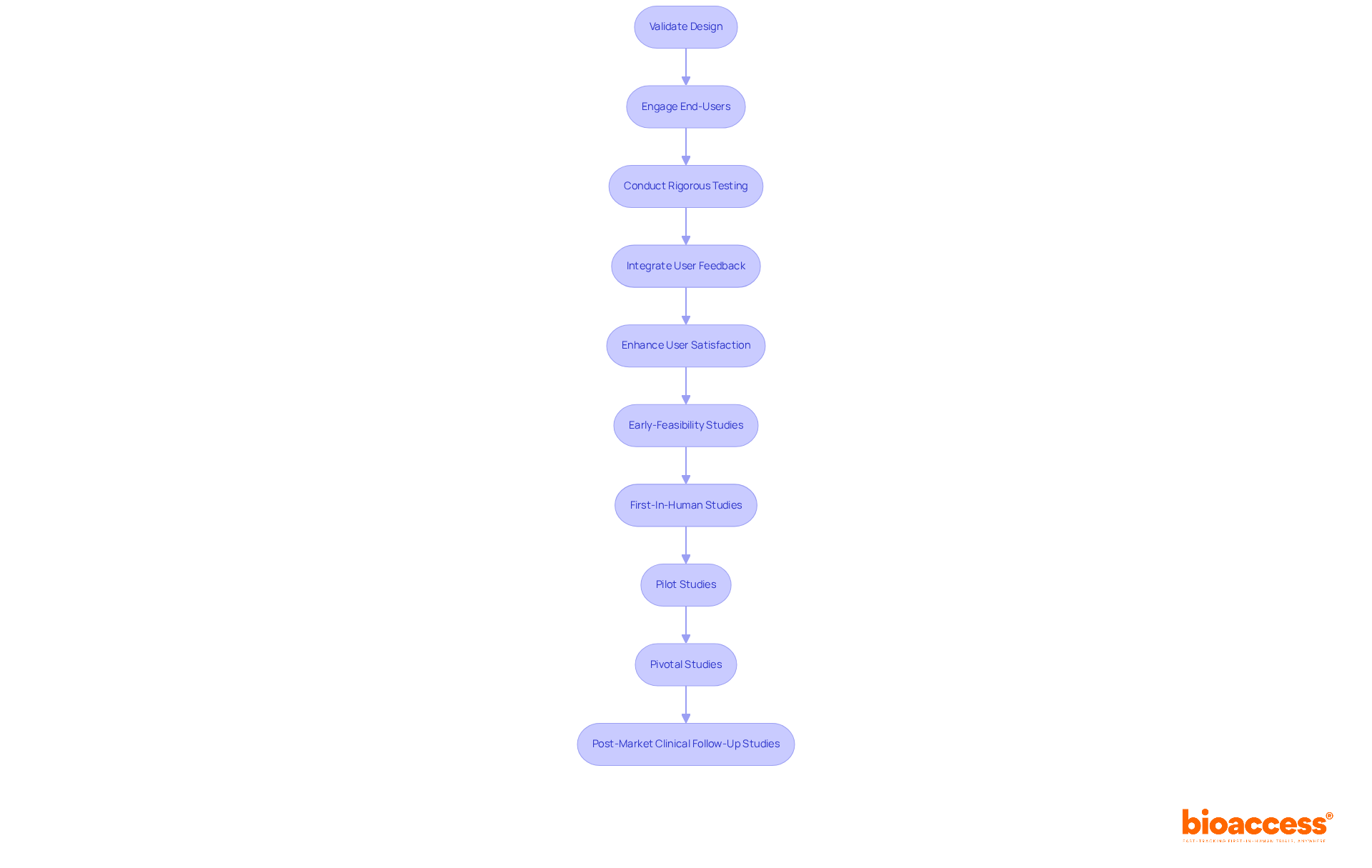
Validating the blueprint of a medical device is essential for confirming that it meets user needs and intended applications. This process necessitates rigorous testing in real-world scenarios to assess both performance and safety. Engaging end-users during validation not only provides valuable insights but also fosters design improvements based on actual user experiences. Research indicates that tools integrating user feedback during validation experience a notable rise in user satisfaction, with studies demonstrating satisfaction rates associated with effective validation efforts reaching up to 85%.
At bioaccess®, we leverage over 20 years of expertise in managing medical apparatus clinical trials across Latin America, encompassing:
Our comprehensive clinical trial management services include:
By prioritizing thorough validation in accordance with FDA guidance design control, organizations can enhance compliance with regulatory standards while ensuring that their products deliver optimal user experiences.
Recording the development history of a medical device is not merely important; it is essential for compliance and traceability. The Design History File (DHF) acts as a comprehensive record of all design and development activities, encompassing design inputs, outputs, verification, and validation processes. Clinical research leaders must ensure that the DHF is meticulously maintained and updated throughout the product development lifecycle. This diligence enables organizations to provide compelling evidence of compliance with FDA guidance design control, ultimately facilitating smoother regulatory submissions.
Implementing effective FDA guidance design control practices is paramount for clinical research leaders striving to navigate the complexities of medical device development. By focusing on robust quality management systems and adhering to established regulations, organizations can significantly enhance their chances of successful product submissions while ensuring compliance with safety standards.
Key insights from the article highlight the importance of:
Each step—from defining design inputs to validating outputs—plays a vital role in fostering a culture of quality and accountability. The emphasis on collaboration, continuous communication, and structured processes underscores the need for a comprehensive approach to FDA compliance.
As the Medtech landscape continues to evolve, leveraging expert guidance and adhering to best practices will be crucial for organizations aiming to expedite their development timelines and deliver safe, effective medical devices to the market. Embracing these principles not only mitigates regulatory risks but also paves the way for innovation and improved patient outcomes in the healthcare industry.
What is bioaccess® and what services does it provide?
bioaccess® specializes in guiding Medtech, Biopharma, and Radiopharma innovators through FDA compliance by implementing effective FDA guidance design control practices, particularly in early-phase clinical research.
How does bioaccess® help clients with FDA compliance?
bioaccess® leverages its extensive knowledge of regulatory frameworks to assist clients in meeting safety and efficacy standards, thereby expediting product development timelines while ensuring adherence to FDA regulations.
What are the benefits of implementing strong control practices aligned with FDA guidance?
Implementing strong control practices can significantly shorten approval timelines, simplify workflows, and enhance the overall quality of clinical studies.
Why is continuous communication and documentation important in clinical research?
Continuous communication and documentation are essential for maintaining compliance and achieving successful outcomes in clinical trials.
What is 21 CFR Part 820 and why is it important?
21 CFR Part 820 establishes the quality system regulations (QSR) that medical device manufacturers must follow, covering all aspects of device development, which is crucial for ensuring compliance and mitigating regulatory challenges.
What are the key requirements of the Quality System Regulation (QSR)?
Key requirements of the QSR include a strong quality policy, detailed reviews, extensive history files, and precise documentation of controls, all of which demonstrate compliance and ensure the safety and effectiveness of medical devices.
How can clinical research leaders cultivate a culture of quality within their organizations?
By prioritizing the key requirements of the QSR, clinical research leaders can establish a robust quality management system that facilitates successful product development and expedites market entry.
Turning Phase III Failure into Success: A Case Study in Innovative Imaging Analysis for Rare Disease Trials | IAG (https://ia-grp.com/case_studies/cloud-enabled-real-time-image-assessment-allowed-achieving-statistical-significance-of-the-treatment-efficacy-in-a-rare-disease-trial-faster)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
Validation & Verification in Medical Device Design for Patient Safety | Attract Group (https://attractgroup.com/blog/validation-verification-in-medical-device-design-for-patient-safety)
Case Studies (https://sebokwiki.org/wiki/Case_Studies)
Medical Device Design Verification and Design Validation – What They Are and Why They Are Important - Arrotek | Medical Device Innovation (https://arrotek.com/medical-device-design-verification-and-design-validation-what-they-are-and-why-they-are-important)
STAT-04: Statistical Techniques for Design Verification - Taylor Enterprises (https://variation.com/stat-04-statistical-techniques-for-design-verification)