


Early Access Programs (EAPs) are transforming the biopharma landscape in Australia, serving as a crucial lifeline for patients seeking innovative treatments. These programs not only provide access to investigational therapies but also generate a wealth of real-world data that can significantly enhance drug development and regulatory submissions. Yet, as the complexity of navigating regulatory pathways increases, biopharma companies face the challenge of effectively leveraging EAPs to maximize their benefits and improve patient outcomes.
This article explores the key advantages of EAPs, underscoring their strategic importance for biopharma firms and the profound impact they have on patient care in Australia. By understanding how to navigate these programs, companies can not only fulfill their commitment to innovation but also address the pressing needs of patients in a rapidly evolving healthcare environment.
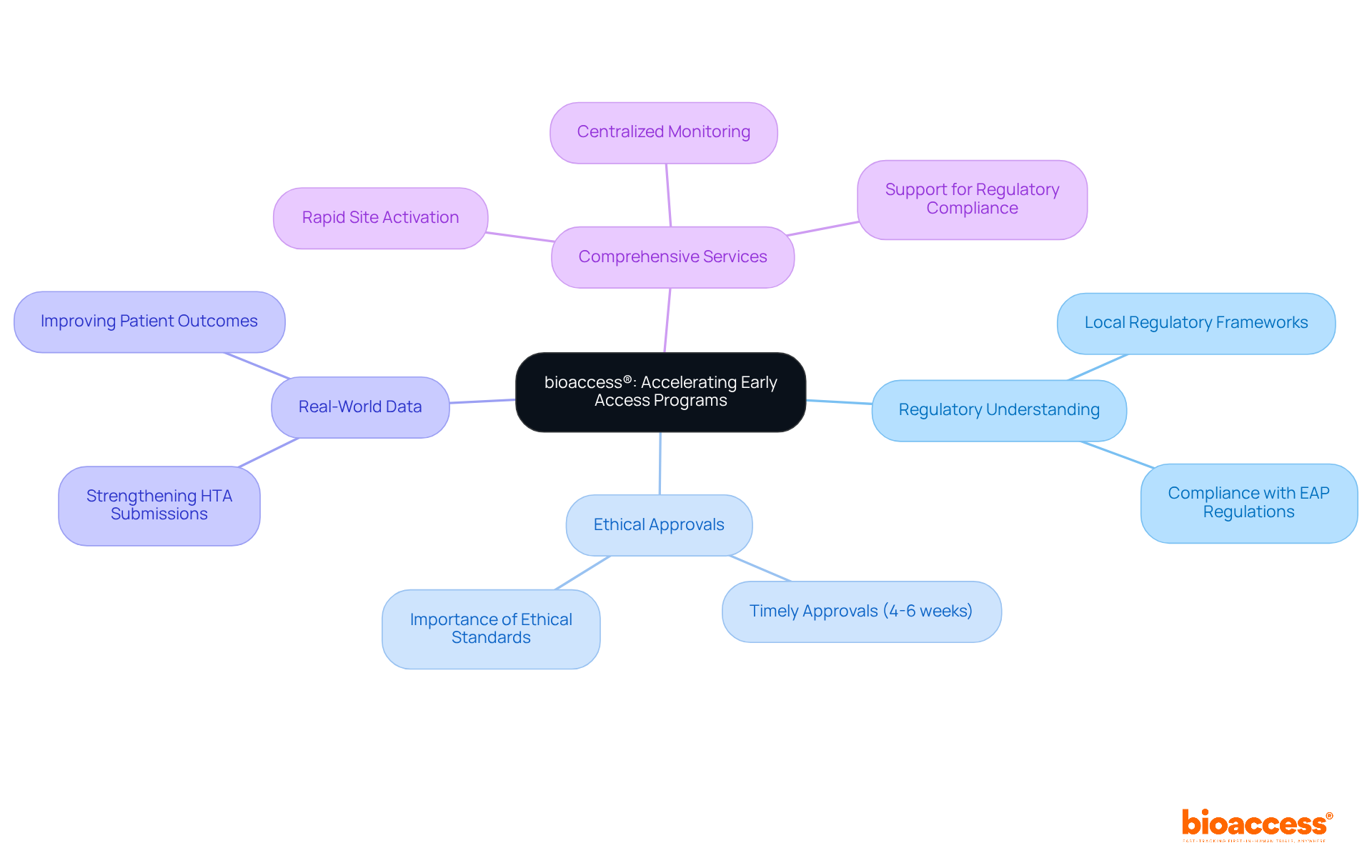
bioaccess® excels in accelerating early access programs (eaps) for biopharma in Australia by leveraging its deep understanding of local regulatory frameworks and diverse demographics. This expertise enables bioaccess® to expedite ethical approvals, typically securing them within 4-6 weeks. In a landscape where timely access to innovative therapies can dramatically enhance patient outcomes and drive market success, this capability is crucial.
Expanded access programs not only provide individuals with access to experimental treatments but also generate valuable real-world data that can strengthen Health Technology Assessment (HTA) submissions. Successful programs in Australia have shown significant improvements in patient health, underscoring the vital role these initiatives play in bridging the gap between clinical research and patient care. With a steadfast commitment to navigating the complexities of early access programs (eaps) for biopharma in Australia, bioaccess® has positioned itself as a leader in this domain, ensuring that biopharma innovators can effectively bring their groundbreaking therapies to market.
Additionally, bioaccess® offers comprehensive services, including:
This end-to-end approach ensures that clinical trials are conducted efficiently and effectively, paving the way for successful outcomes.
Australia's regulatory framework is strategically designed to expedite access to innovative therapies through streamlined pathways. The Therapeutic Goods Administration (TGA) has established expedited review processes that allow biopharma firms to speed up their market entry. In 2025, it is projected that 90% of worthy products could be listed on the Pharmaceutical Benefits Scheme (PBS) within six months of TGA registration, a significant improvement over the current 22-month timeframe.
By partnering with bioaccess®, companies can effectively navigate these regulatory pathways, ensuring compliance while minimizing delays. This collaboration not only enhances speed to market but also facilitates patient access to life-saving treatments. Early access programs (EAPs) for biopharma in Australia provide a strategic advantage for firms by allowing them to swiftly address unmet medical needs.
With bioaccess®'s end-to-end services, including rapid site activation - over 50 sites activated in less than 8 weeks - and compliance with FDA/EMA/MDR, biopharma companies can optimize their clinical trial processes. This approach not only streamlines operations but also positions firms to meet the growing demand for innovative therapies in a timely manner.

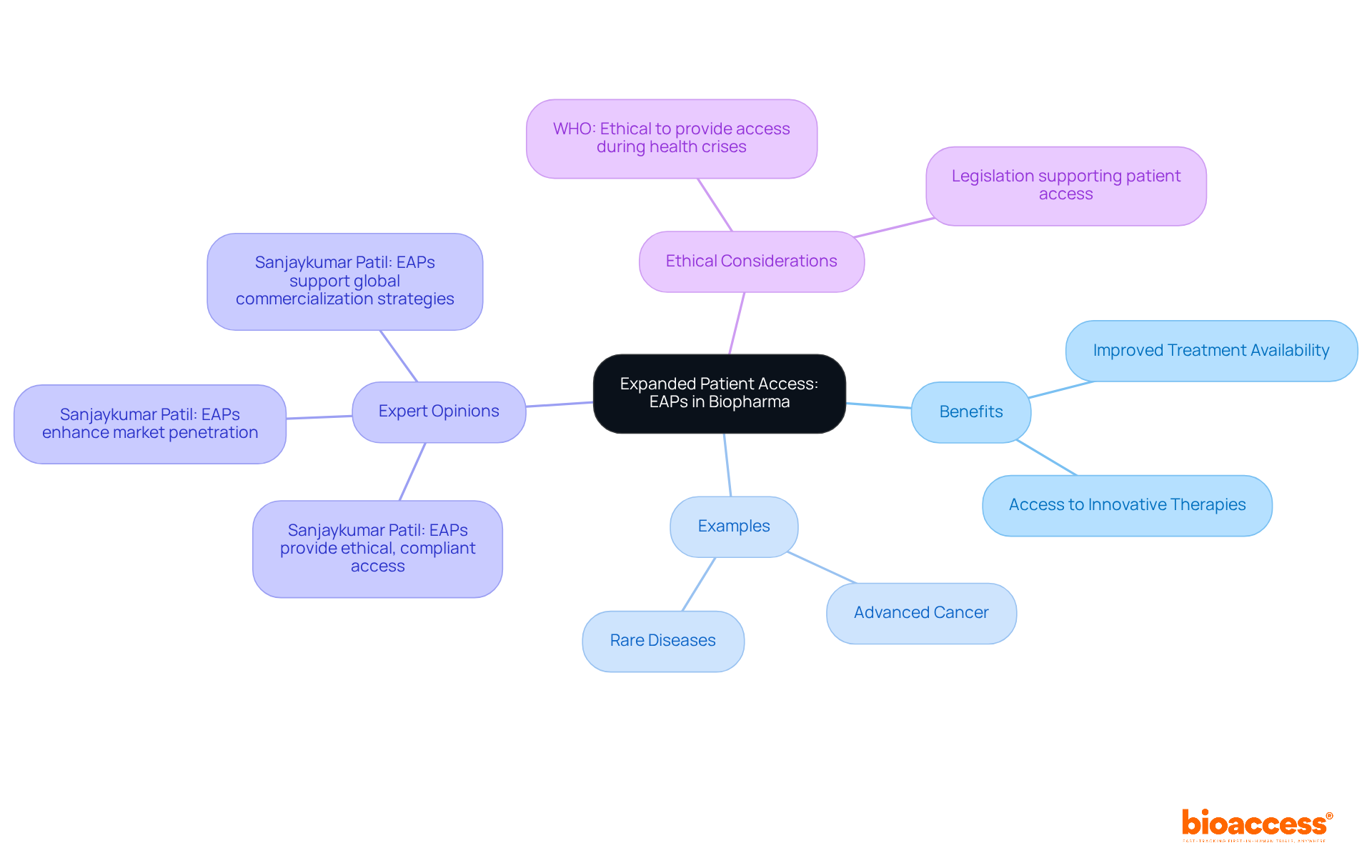
Early access programs (EAPs) for biopharma in Australia are vital for enhancing access to innovative treatments, particularly for individuals facing severe or life-threatening conditions. These early access programs (EAPs) for biopharma in Australia enable access to investigational medications prior to regulatory approval, effectively filling critical gaps in treatment availability. In Australia, where certain therapies may not yet be accessible through traditional pathways, the importance of early access programs (EAPs) for biopharma in Australia is paramount. As we look to 2025, more individuals are reaping the benefits of these programs, with reports indicating that thousands have utilized therapies through employee assistance programs, significantly broadening their treatment options.
Healthcare professionals recognize the transformative effect of employee assistance programs on treatment availability. Sanjaykumar Patil, a Subject Matter Expert in Risk Based Monitoring, states, "Expanded Access Programs provide ethical, compliant, and regulated methods for obtaining investigational drugs outside of the clinical trial environment and prior to the commercial release of the drug, for patients with life-threatening illnesses lacking available treatment options." This perspective resonates throughout the medical community, underscoring the essential role these programs play in bridging treatment gaps.
For instance, early access programs (EAPs) for biopharma in Australia have successfully facilitated access to therapies for conditions such as advanced cancer and rare diseases, where conventional clinical trials may not be feasible. By connecting biopharma companies with eligible patients, bioaccess® ensures that those in urgent need can access cutting-edge treatments sooner, ultimately improving patient outcomes and fostering a more responsive healthcare environment. Additionally, the ethical considerations surrounding employee assistance programs are significant; the World Health Organization has affirmed that it is ethical to provide access to investigational drugs during health crises, reinforcing the critical nature of these initiatives in urgent situations.
Collecting real-world evidence through early access programs (EAPs) for biopharma in Australia provides critical insights into the safety and efficacy of investigational therapies. This data not only strengthens regulatory submissions but also fuels future innovations in drug development. By analyzing outcomes and treatment responses in real-world settings, biopharma companies can refine their products and strategies, ultimately leading to improved therapies.
Regulatory agencies are increasingly recognizing the importance of real-world evidence (RWE) in drug development, especially in the context of early access programs (EAPs) for biopharma in Australia. The USFDA has noted a growing need to integrate RWE into decision-making processes. Companies like Clene exemplify how RWE can expedite access to therapies, showcasing its ability to guide clinical practices and enhance outcomes for patients.
bioaccess® plays a pivotal role in the systematic collection of RWE, enabling biopharma innovators to leverage this information effectively. This capability not only enhances their offerings but also meets market demands. With bioaccess®'s ability to enroll treatment-naive cardiology or neurology groups 50% faster than Western sites and achieve $25K savings per individual with FDA-ready data, the organization significantly optimizes clinical trials and addresses recruitment challenges.

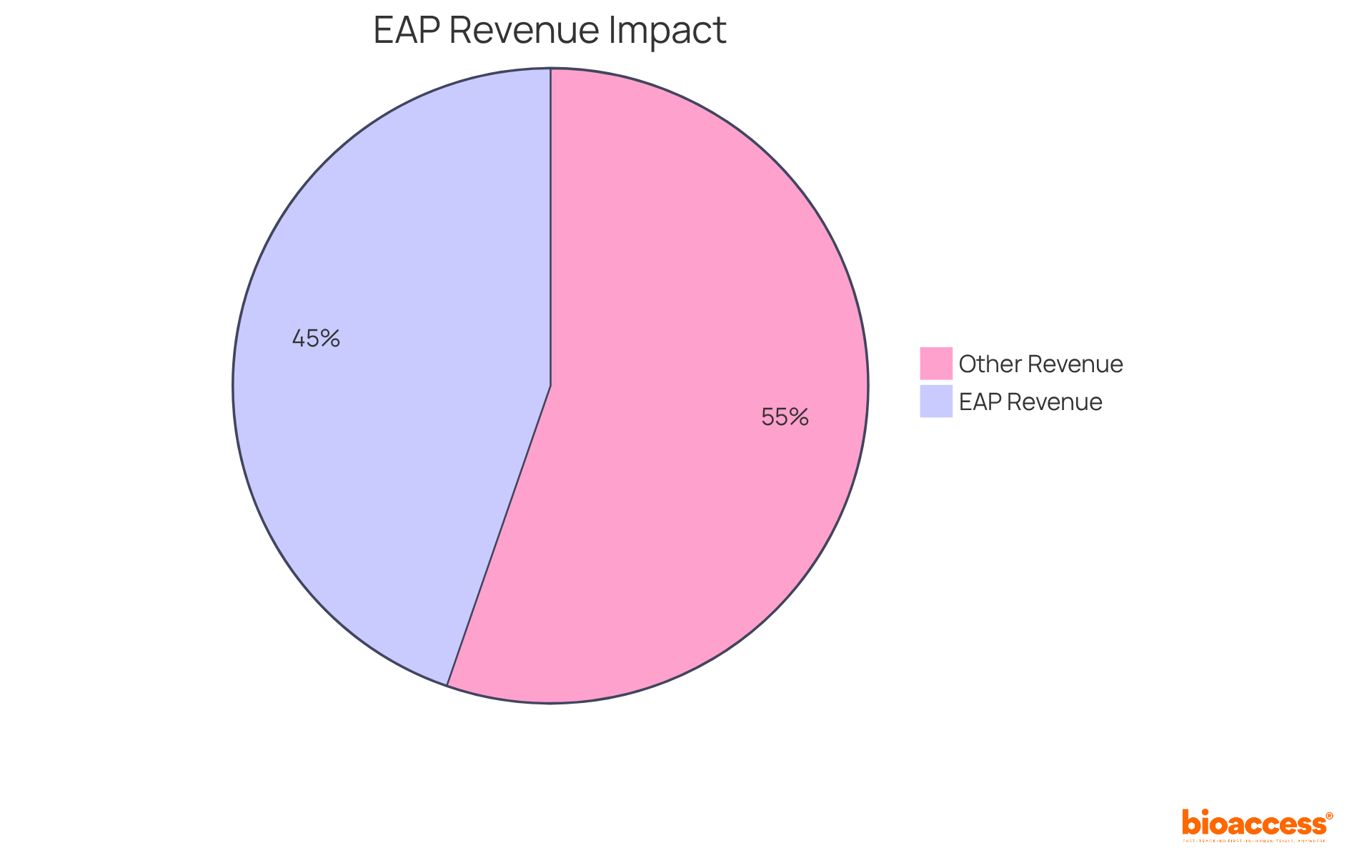
Engaging in early access programs (EAPs) for biopharma in Australia provides startups with a crucial pathway for generating early revenue, allowing them to monetize their innovations before achieving full market approval. By providing investigational treatments through expanded access programs, organizations can secure revenue that fuels ongoing research and development efforts. This financial edge is particularly vital for startups, which frequently face significant funding challenges.
In 2023, sales of drugs billed under early availability and compassionate programs in France soared by 44.7%, reaching €1.6 billion. This statistic underscores the substantial revenue opportunities available through these initiatives. Furthermore, industry experts emphasize that expanded access programs not only facilitate patient access to essential treatments but also allow organizations to gather real-world data that can enhance their market positioning.
bioaccess® plays a pivotal role in guiding these organizations through the complexities of early access programs (EAPs) for biopharma in Australia, ensuring they can effectively capitalize on initial revenue while adhering to regulatory standards. As the landscape of clinical research evolves, collaboration and strategic engagement become paramount for success.
In the competitive biopharma landscape, leveraging early access programs (eaps) for biopharma in Australia provides a distinct advantage. Organizations can set themselves apart from slower-moving rivals by offering early access programs (eaps) for biopharma in Australia. This proactive strategy not only garners attention from healthcare providers and patients but also positions the organization as a leader in innovation.
Marketing specialists emphasize that early access programs (eaps) for biopharma in Australia, when well-executed, can significantly enhance an organization's market share, especially in crowded markets where differentiation is crucial. For instance, biopharma companies that effectively engage with healthcare providers and key opinion leaders through early access programs (eaps) for biopharma in Australia can foster advocacy and strengthen relationships, ultimately leading to improved product adoption.
bioaccess® plays a vital role in assisting biopharma firms in developing and executing effective early access programs (eaps) for biopharma in Australia. With its expertise in overseeing clinical trials - including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies - bioaccess® ensures that clients capture market share and maintain a competitive edge. This comprehensive approach underscores the importance of collaboration in navigating the complexities of clinical research.
Early access programs (EAPs) for biopharma in Australia offer unique collaboration opportunities among biopharma companies, healthcare providers, and regulatory bodies. These partnerships are essential for enhancing the effectiveness of early access programs (EAPs) for biopharma in Australia, ensuring that individuals gain timely access to innovative therapies. Engaging with healthcare professionals not only aids in participant recruitment but also streamlines data collection, amplifying the program's overall impact. As a pivotal facilitator, bioaccess® connects stakeholders, empowering biopharma firms to establish successful partnerships crucial for the implementation of early access programs (EAPs) for biopharma in Australia.
Insights from industry leaders underscore that effective collaboration can significantly improve outcomes and expedite the delivery of new treatments. For instance, collaborations in educational assistance programs have shown to enhance the credibility of clinical information and foster trust among individuals receiving care and their providers. Looking ahead to 2025, the landscape of employment assistance programs in Australia continues to evolve, with successful partnerships paving the way for innovative solutions that meet consumer needs and regulatory expectations.
Early access programs (EAPs) for biopharma in Australia are designed to enhance health outcomes by providing access to groundbreaking treatments that may not yet be available through traditional channels. These early access programs (EAPs) for biopharma in Australia facilitate access to investigational medications, empowering individuals to benefit from advanced therapies that can significantly improve their quality of life. In Australia, the impact of early access programs (EAPs) for biopharma is particularly noteworthy, with research indicating that 89% of health outcomes improved following the adoption of evidence-based practices. This underscores the critical role these programs play in practical healthcare settings.
Healthcare professionals have acknowledged the transformative potential of early access programs (EAPs) for biopharma in Australia. A recent evaluation highlighted that compassionate use programs, such as those for ipilimumab in malignant melanoma, not only provided essential treatment access but also demonstrated significant improvements in individual well-being. This aligns with the perspective of industry leaders who emphasize that when individuals feel respected and supported, positive health outcomes are more likely to occur.
Moreover, early access programs (EAPs) for biopharma in Australia have been instrumental in enhancing the quality of life for individuals with conditions like hereditary transthyretin amyloidosis. Real-world experiences shared through these initiatives have shown substantial clinical advancements compared to conventional treatment methods. As the healthcare landscape evolves, Bioaccess remains dedicated to implementing effective early access programs (EAPs) for biopharma in Australia, ensuring that innovative treatments reach individuals promptly. By advancing medical technologies through innovation and quality, Bioaccess not only contributes to improved health outcomes but also fosters job creation, economic growth, and international collaboration within the healthcare sector.
Specifically, these programs have been demonstrated to stimulate local economies by generating jobs in clinical research and related fields, further highlighting the significance of such initiatives within the broader healthcare ecosystem.

Participating in early access programs (eaps) for biopharma in Australia significantly enhances a biopharma organization’s brand reputation, demonstrating a genuine commitment to care and innovation. Organizations can build trust among healthcare providers, patients, and regulatory bodies by offering early access programs (eaps) for biopharma in Australia to potentially life-saving therapies. This proactive approach not only elevates the organization's image but also cultivates loyalty and support from stakeholders, which is essential for long-term success.
As the pharmaceutical landscape evolves, organizations that prioritize ethical practices and transparency are likely to see a marked improvement in stakeholder trust. bioaccess® plays a pivotal role in guiding biopharma companies through the complexities of expanded access programs, ensuring they effectively communicate their dedication to improving health outcomes and bolstering their brand reputation. Participating in early access programs (eaps) for biopharma in Australia allows firms to create a reservoir of goodwill that mitigates potential trust deficits, ultimately positioning themselves as leaders in ethical healthcare innovation.
Early access programs (EAPs) for biopharma in Australia provide a compelling strategic value proposition for biopharma firms. They not only expedite access to innovative therapies but also generate early revenue and collect essential real-world evidence. Statistics reveal that organizations adopting employee assistance programs can experience a 1.362 times increase in spending efficiency before commercial launch, underscoring the financial advantages of these initiatives. Notably, biopharma companies engaged in expanded access programs often report enhanced competitive positioning and stronger collaborations with healthcare providers, leading to improved outcomes for individuals.
For instance, organizations like Astellas have successfully leveraged employee assistance programs to forge early connections with doctors, fostering loyalty and facilitating smoother market entry. Industry leaders assert that the optimal timing for EAP implementation is typically 12-18 months prior to product launch, allowing for sufficient preparation and alignment with market needs. This proactive approach not only enhances access for individuals but also enables organizations to capitalize on significant revenue opportunities through early access programs (EAPs) for biopharma in Australia’s multi-billion dollar healthcare sector.
bioaccess® is committed to empowering biopharma innovators in navigating the complexities of the regulatory landscape associated with expanded access programs. By offering tailored support and strategic insights, bioaccess® ensures that companies can effectively maximize the benefits of EAPs, ultimately delivering substantial value to both patients and stakeholders.

Early access programs (EAPs) for biopharma in Australia represent a pivotal shift in healthcare, enabling faster access to innovative therapies for patients in urgent need. By effectively bridging the gap between clinical research and patient care, these programs not only enhance treatment availability but also empower biopharma companies to adeptly navigate regulatory complexities, ensuring timely delivery of life-saving medications.
The article underscores several key benefits of EAPs, including:
With organizations like bioaccess® at the forefront, biopharma firms can seize early revenue opportunities while gathering valuable real-world evidence that informs future drug development. This collaborative approach fosters partnerships among stakeholders, ultimately enhancing the overall effectiveness of healthcare delivery.
As the biopharma landscape evolves, the importance of early access programs cannot be overstated. They serve as a crucial lifeline for patients facing severe health challenges and position biopharma companies as leaders in innovation and ethical practices. Embracing the potential of EAPs is essential for stakeholders aiming to improve health outcomes and drive advancements in the biopharmaceutical sector. The call to action is clear: prioritize the implementation of early access programs to ensure that patients receive the therapies they desperately need while simultaneously advancing the future of drug development.
What is bioaccess® and what role does it play in early access programs for biopharma in Australia?
bioaccess® is a leader in accelerating early access programs (EAPs) for biopharma in Australia. It leverages its understanding of local regulatory frameworks and diverse demographics to expedite ethical approvals, typically securing them within 4-6 weeks, thereby enhancing patient access to innovative therapies.
How do early access programs benefit patients and biopharma companies?
Early access programs provide individuals with access to experimental treatments, which can significantly improve patient health outcomes. For biopharma companies, these programs generate valuable real-world data that can strengthen Health Technology Assessment (HTA) submissions and help address unmet medical needs.
What services does bioaccess® offer to support clinical trials?
bioaccess® offers comprehensive services including rapid site activation, centralized monitoring, and support for regulatory compliance. This end-to-end approach ensures that clinical trials are conducted efficiently and effectively.
How does Australia's regulatory framework facilitate faster access to innovative therapies?
Australia’s regulatory framework, particularly through the Therapeutic Goods Administration (TGA), has established expedited review processes that allow biopharma firms to speed up market entry. By 2025, it is projected that 90% of worthy products could be listed on the Pharmaceutical Benefits Scheme (PBS) within six months of TGA registration.
What is the significance of employee assistance programs in the context of early access programs?
Employee assistance programs play a vital role in enhancing treatment availability by providing ethical, compliant methods for obtaining investigational drugs for patients with life-threatening illnesses prior to commercial release. They help bridge critical gaps in treatment availability, especially for severe conditions.
What types of conditions can benefit from early access programs in Australia?
Early access programs have successfully facilitated access to therapies for conditions such as advanced cancer and rare diseases, where conventional clinical trials may not be feasible.
What ethical considerations are associated with early access programs?
The World Health Organization has affirmed that it is ethical to provide access to investigational drugs during health crises, reinforcing the critical nature of early access programs in urgent situations.