


This article provides critical insights into Class 1 medical devices as regulated by the FDA, underscoring their low-risk nature and streamlined regulatory requirements. These devices, which encompass items such as bandages and surgical gloves, are typically exempt from premarket notifications, facilitating a quicker path to market. Moreover, it is essential to highlight the significance of adhering to:
These are vital for ensuring safety and efficacy.
The landscape of Class 1 medical devices is evolving rapidly, driven by the imperative for innovation and compliance within the healthcare sector. As low-risk products that are integral to patient care, a comprehensive understanding of the regulatory framework surrounding these devices is essential for manufacturers seeking to navigate the complexities of market entry.
However, with numerous compliance pitfalls and evolving FDA guidelines, how can companies ensure they meet all necessary requirements while efficiently bringing their products to market?
This article explores ten key insights from the FDA that every stakeholder in the medical device industry should be aware of, providing a strategic roadmap to success in this critical field.
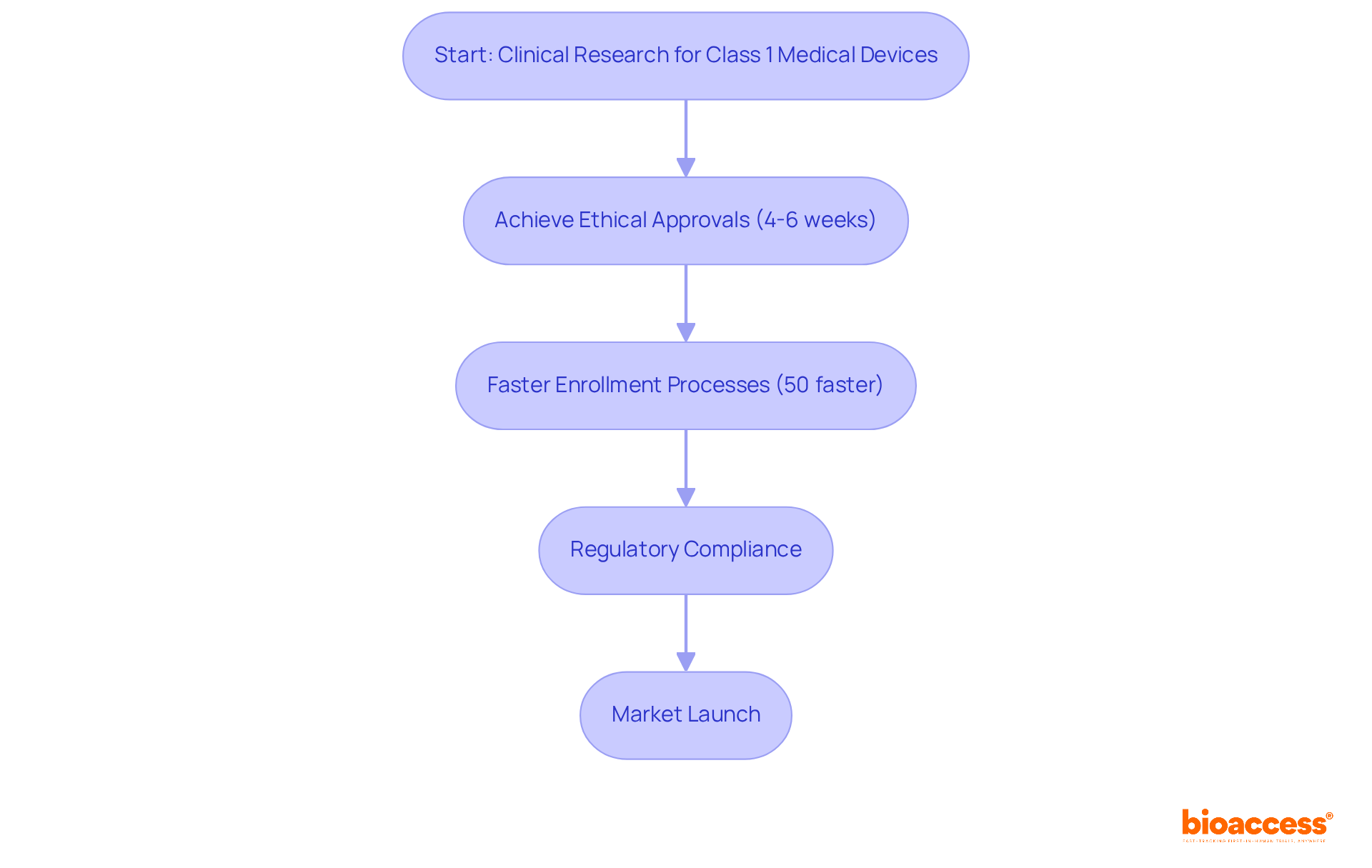
bioaccess® excels in expediting clinical research for products classified as class 1 medical device FDA by leveraging its extensive expertise across Latin America, the Balkans, and Australia. The organization achieves ethical approvals in a remarkable timeframe of just 4-6 weeks, significantly enhancing the speed of enrollment processes, which are 50% faster than traditional markets. This streamlined method is essential for companies aiming to quickly launch low-risk products to the market, ensuring compliance with regulatory standards while enhancing patient access to innovative technologies.
Industry leaders emphasize that timely ethical approvals are crucial for maintaining the integrity of clinical trials, facilitating faster market entry while upholding the rights and safety of participants. Furthermore, Colombia offers R&D tax incentives, including a 100% tax deduction and various financial benefits, making it an even more attractive destination for clinical trials. With a healthcare system ranked among the best globally and a population of over 50 million, Colombia provides a robust environment for patient recruitment.
Successful clinical trials for class 1 medical device FDA products in Latin America showcase the region's potential as a strategic center for Medtech innovation, where bioaccess® plays a crucial role in connecting regulatory requirements and clinical implementation.
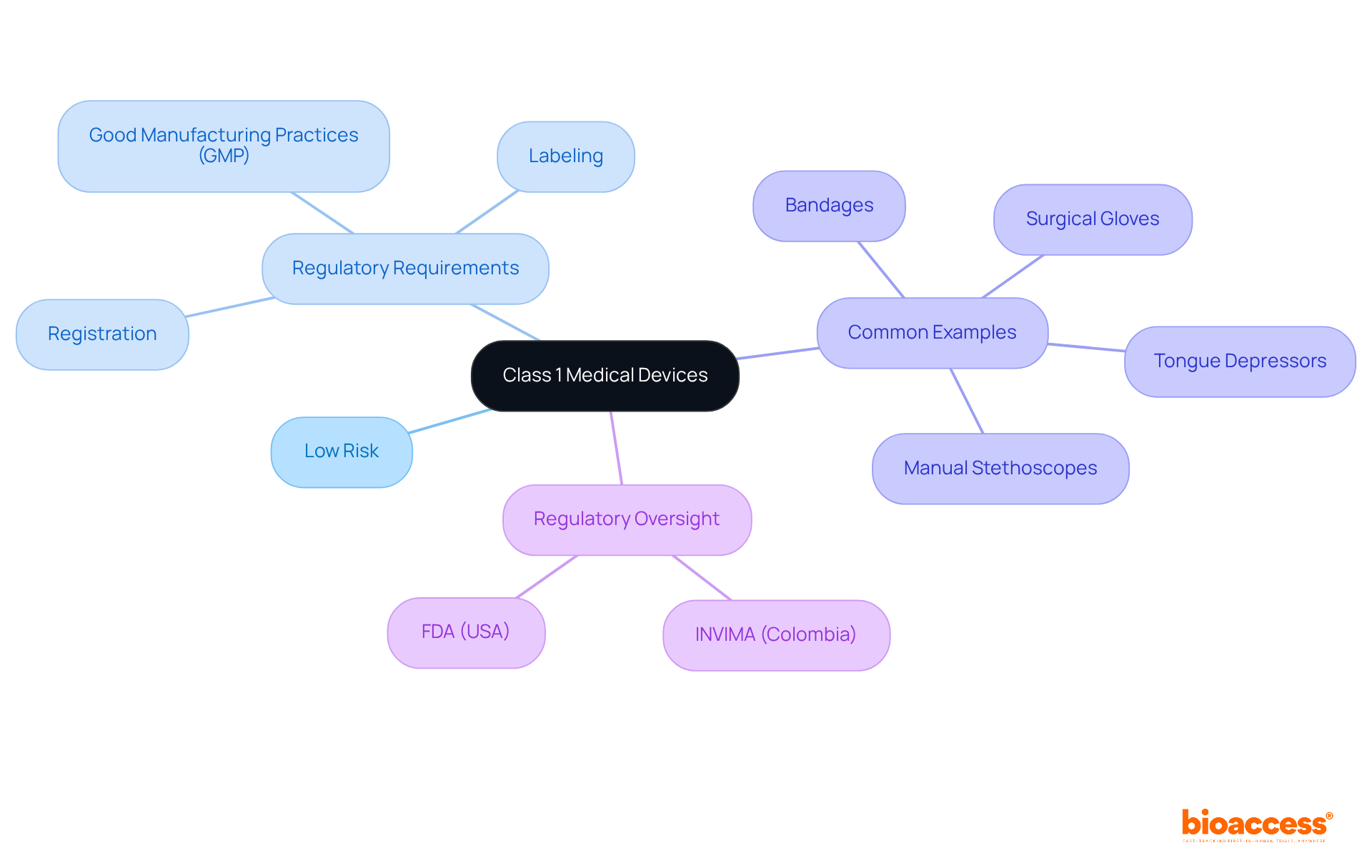
Class 1 medical device FDA items are classified as low-risk products that pose minimal potential harm to patients. These instruments, regulated primarily by general controls, must adhere to essential regulations such as:
Notably, the class 1 medical device FDA products are generally exempt from premarket notification requirements, facilitating a more streamlined route to market entry. Common examples include:
These devices are designed for safety and efficacy without the intent to support or sustain life. In Colombia, regulatory oversight for these instruments is under the jurisdiction of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which ensures adherence to health standards and best practices. Approximately 35% of all regulated medical instruments fall into this category, underscoring their prevalence and significance in healthcare.
Regulatory Requirements for Class 1 Medical Devices
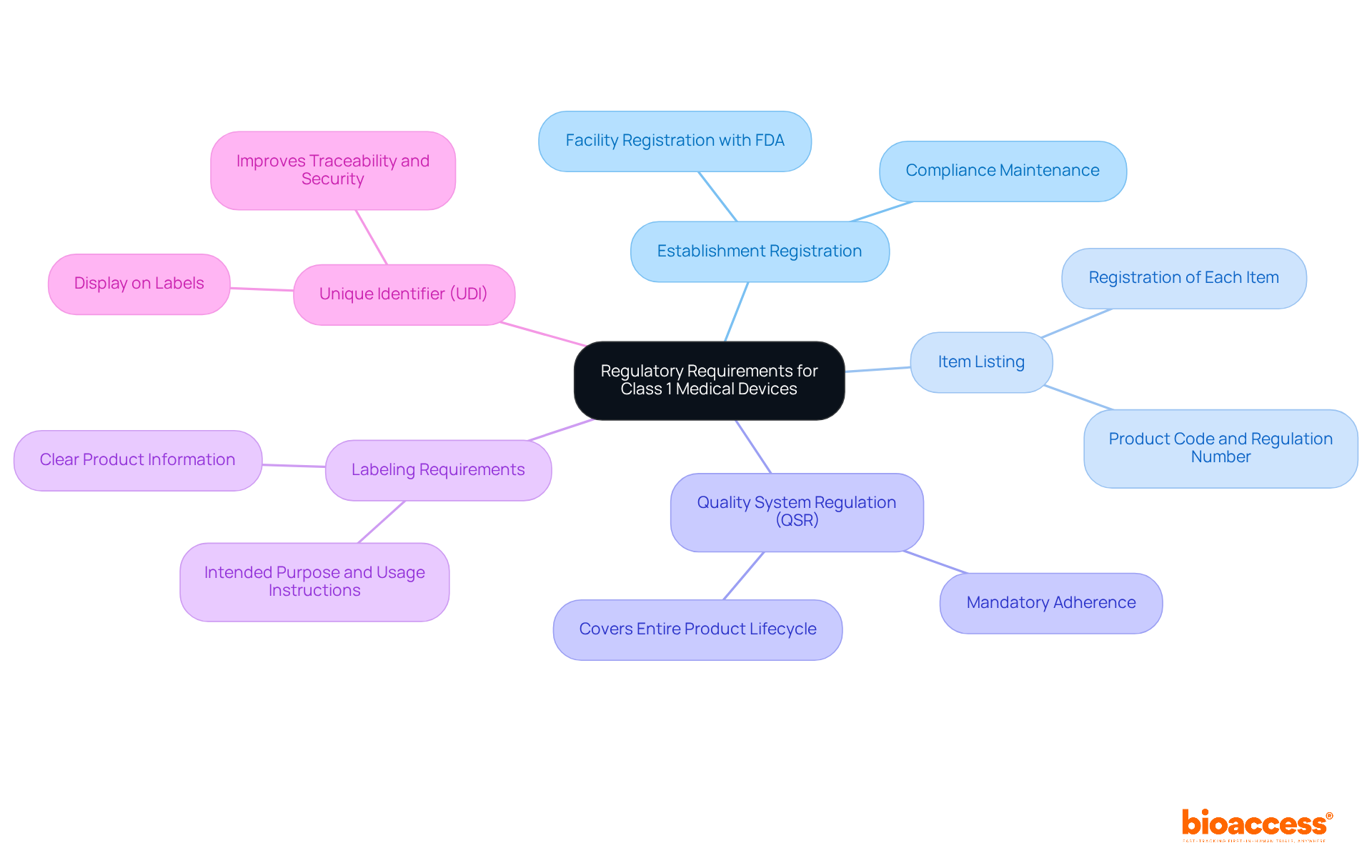
Manufacturers of Class 1 medical devices must adhere to several essential regulatory requirements to ensure safety and efficacy. These include:
These requirements are essential for ensuring that items classified as Class 1 medical device FDA, which are considered low-risk by regulatory authorities, are safe and effective for public use. According to FDA officials, maintaining compliance with regulations for class 1 medical device fda is essential for both protecting public health and facilitating market access. In fact, around 80% of manufacturers have effectively navigated these requirements, demonstrating the industry's commitment to quality and security. Recent FDA guidelines highlight the significance of strong quality management systems and accurate labeling to reduce risks linked to the use of equipment. By following these regulations, manufacturers not only meet legal standards but also enhance the overall safety and effectiveness of medical products in the market. Specialists such as Ana Criado, Director of Regulatory Affairs, underscore the essential importance of regulatory compliance in the creation and distribution of medical equipment. To ensure success, manufacturers should regularly review FDA guidelines and maintain a proactive approach to compliance.
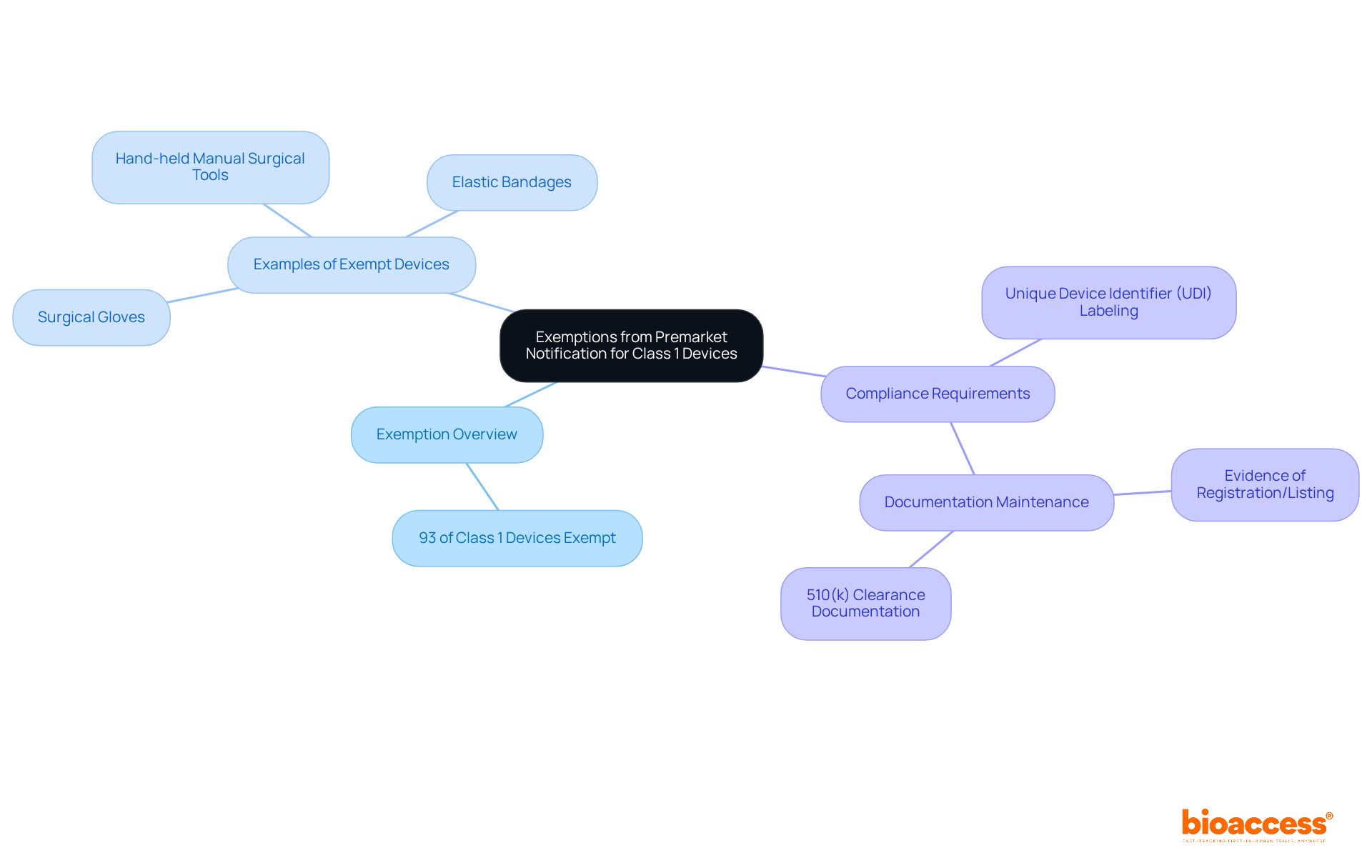
Most instruments categorized as class 1 medical device FDA are exempt from the premarket notification procedure, commonly known as the 510(k) submission. This exemption applies to items that do not serve significant purposes in preventing impairment or that do not pose major health risks. Approximately 93% of Category 1 items, categorized as class 1 medical device FDA, fall under this exemption, allowing manufacturers to navigate the regulatory landscape more effectively.
Nevertheless, compliance with general controls is crucial, and manufacturers of class 1 medical device FDA must maintain comprehensive documentation to demonstrate adherence to FDA regulations. Importantly, all Category I product labels and packages produced on or after September 24, 2023, must feature a Unique Product Identifier (UDI), which is a critical compliance requirement.
The FDA provides an extensive list of exempt products, which serves as a vital resource for manufacturers of class 1 medical device FDA who aim to streamline their approval processes. Examples of items categorized as class 1 medical device FDA that are excluded from 510(k) submissions include:
This showcases the diversity within this category. Furthermore, the FDA's initiatives to reduce regulatory burdens on the medical equipment sector reflect a commitment to fostering innovation. Staying updated on the latest FDA developments and maintaining an administrative file with evidence of registration/listing and 510(k) clearance documentation are essential steps for manufacturers to ensure compliance and mitigate potential regulatory challenges.
General controls for a class 1 medical device FDA encompass several critical requirements aimed at ensuring safety and regulatory compliance. Device registration is paramount; manufacturers of class 1 medical device FDA must register their establishments with the FDA, a crucial step in maintaining oversight and accountability within the industry. Additionally, devices must adhere to stringent labeling regulations, providing essential information that ensures safe usage and informs users about potential risks. Adherence to Quality System Regulation (QSR) is crucial for manufacturers, as it ensures that products are manufactured consistently and fulfill established quality standards. This regulation is essential in preventing defects and ensuring reliability in the class 1 medical device FDA category. Furthermore, manufacturers are required to report any adverse events or device malfunctions to the FDA within a specified timeframe. This reporting is crucial for identifying hazards and facilitating timely corrective actions.
In 2023, the FDA announced a significant rise in recalls, with 31 class 1 medical device FDA recalls emphasizing persistent challenges in protection. This underscores the importance of rigorous compliance with these general controls. Industry leaders emphasize that adherence to these regulations is not only a legal obligation but also a commitment to patient safety. As Amanda K. Sarata observed, the intricacy of medical equipment regulation requires a proactive strategy for compliance, guaranteeing that unsafe or ineffective products do not enter the market. Ongoing oversight and enhancement of quality control protocols are essential to protect public health and improve the efficacy of first-category medical products.

Category 1 medical items encompass a diverse array of products that play crucial roles in healthcare, characterized by their low-risk attributes. Notable examples include:
These examples underscore the extensive range of Category 1 equipment, which is essential to daily healthcare practices, showcasing their vital role in patient care and safety. Significantly, nearly half of all medical instruments fall under Category 1, highlighting their prevalence in healthcare environments. As the industry evolves, trends such as the increasing use of biodegradable materials and energy-efficient production techniques are shaping the future of Category 1 products. Healthcare providers must remain vigilant and informed about these innovations to ensure the continued reliability and effectiveness of these indispensable tools.

Labeling requirements for class 1 medical device FDA products are crucial for guaranteeing user safety and adherence to FDA regulations. The key elements include:
These labeling criteria are not merely regulatory formalities; they serve a crucial function in enhancing patient protection and ensuring effective utilization of the equipment. Approximately 76% of healthcare instruments lack essential labeling information, highlighting the need for manufacturers to prioritize compliance. Regulatory experts emphasize that meticulous review of labeling practices is vital for meeting the requirements for class 1 medical device FDA, as labeling errors remain one of the most cited causes of FDA warning letters. Efficient labeling can significantly influence market access and user comprehension, making it essential for producers of class 1 medical device FDA to stay updated on revisions to FDA labeling guidelines.
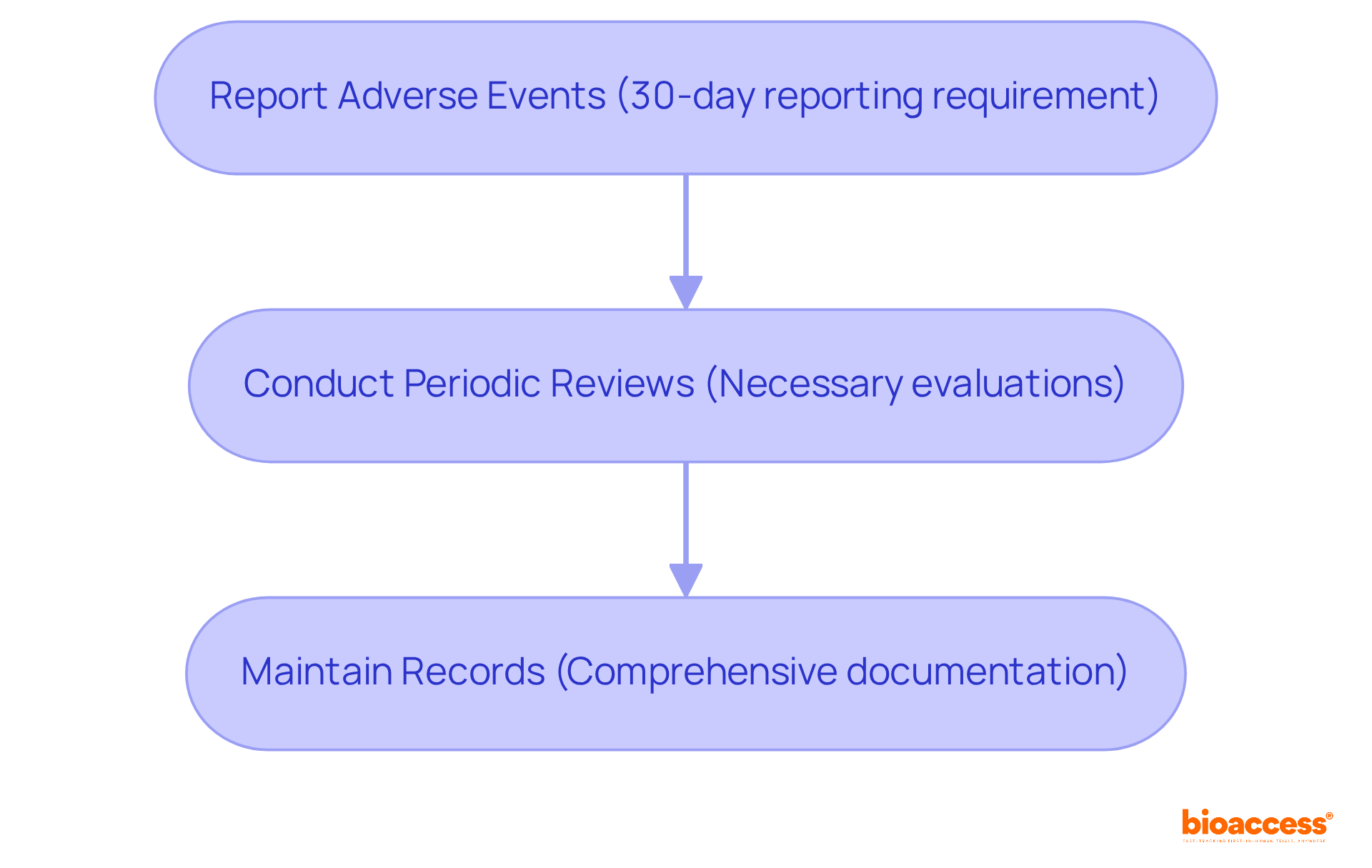
Post-market surveillance for Class 1 medical devices FDA is crucial for monitoring their performance and safety once they are in use. Manufacturers are mandated to report adverse events, conduct periodic reviews, and maintain comprehensive records to ensure compliance and enhance patient safety.
Report Adverse Events: Any serious injuries or malfunctions must be reported to the FDA within 30 days of awareness. In 2020, there were 536,055 reports of medical equipment adverse events, underscoring the critical nature of timely reporting. In Colombia, INVIMA performs a similar role, supervising the marketing and production of health products, ensuring adherence to health standards, and monitoring medical equipment through its Directorate for Medical Equipment and other Technologies.
Conduct Periodic Reviews: Regular evaluations of equipment performance data are necessary to identify potential hazard issues. This proactive approach is essential, as delayed reporting can obstruct early identification of patient welfare concerns. INVIMA's oversight includes monitoring pre- and post-market initiatives, aligning with the requirement for ongoing assessment of product reliability and effectiveness.
Maintain Records: Comprehensive documentation of complaints and adverse events is vital for regulatory assessment, ensuring adherence and facilitating continuous evaluations. INVIMA's classification as a Level 4 health authority by PAHO/WHO highlights its proficiency in overseeing medical equipment, further emphasizing the significance of thorough record-keeping and compliance.
This ongoing oversight is vital for guaranteeing the long-term reliability and efficacy of the class 1 medical device FDA products. Industry leaders stress the necessity for robust monitoring systems to enhance equipment performance and security. As Niranjan Maharajh stated, "Discover how the FDA is utilizing real-world information to proactively oversee equipment reliability and what it signifies for patients, providers, and manufacturers." Additionally, manufacturers should regularly review their reporting processes to ensure compliance and improve patient safety.
Common compliance pitfalls for Class 1 medical devices include:
Avoiding these pitfalls is essential for maintaining compliance and ensuring successful market access. As mentioned by regulatory specialists, a proactive strategy for documentation and compliance can greatly reduce risks linked to class 1 medical device fda products.

The FDA classification process for medical devices encompasses several essential steps that manufacturers must navigate to ensure compliance and successful market entry:
Determine Product Classification: Manufacturers must evaluate whether their product falls into Category I, II, or III based on its risk profile. Category I products, which pose minimal risk, are the simplest to introduce to the market and are frequently exempt from premarket notification obligations. Examples of Class I items include electric toothbrushes, tongue depressors, and bandages.
Consult the FDA Product Classification Database: This vital resource provides information on existing classifications and the specific regulatory requirements linked to each type of equipment. Currently, the FDA has categorized over 1,700 unique medical instruments across three classes, reflecting a diverse range of specialties.
Prepare Necessary Documentation: Depending on the classification, manufacturers may need to compile documentation for premarket notification (510(k)) or premarket approval (PMA). For Category I products, the documentation needs are minimal, facilitating faster market entry. Additionally, it is crucial for manufacturers to implement a Quality Management System (QMS) to ensure compliance with regulatory standards.
Submit to the FDA: The subsequent step involves submitting the required documentation to the FDA for review. The 510(k) review process typically aims for a decision within 90 days; however, complex submissions may extend this timeline.
Receive FDA Clearance or Approval: Following the review of the submission, the FDA will issue a clearance or approval decision. Successful submissions for Class I devices often result in a Substantially Equivalent decision, allowing manufacturers to market their products in the U.S.
Understanding this classification process is crucial for manufacturers to effectively navigate regulatory requirements and ensure compliance, ultimately leading to successful market entry. As noted by industry experts, early engagement with the FDA can significantly enhance the quality of submissions and streamline the approval process.

The exploration of Class 1 medical devices underscores their vital role in healthcare, characterized by low-risk attributes and a streamlined regulatory process. A comprehensive understanding of FDA regulations, from ethical approvals to labeling requirements, is essential for manufacturers seeking to introduce safe and effective products to market. The insights provided illuminate not only the regulatory landscape but also the opportunities for innovation and compliance within this category.
Key arguments presented throughout this article emphasize the importance of:
The discussion surrounding exemptions from premarket notification and common compliance pitfalls further highlights the necessity for manufacturers to remain vigilant and informed about the ever-evolving landscape of medical device regulation. By leveraging resources like bioaccess® for clinical research, companies can enhance their market entry strategies while ensuring patient safety and regulatory compliance.
In summary, the landscape of Class 1 medical devices is ripe with potential for innovation and improvement. As the industry continues to evolve, staying updated on FDA guidelines and maintaining rigorous compliance practices will be paramount for manufacturers. Embracing these insights not only facilitates successful market access but also reinforces the commitment to enhancing patient care and safety. Proactively engaging with regulatory requirements can lead to a future where medical devices not only meet but exceed standards, ultimately benefiting healthcare providers and patients alike.
What is bioaccess® and what role does it play in clinical research for Class 1 medical devices?
bioaccess® specializes in accelerating clinical research for Class 1 medical devices by leveraging its expertise in regions like Latin America, the Balkans, and Australia. It achieves ethical approvals in 4-6 weeks, speeding up enrollment processes by 50% compared to traditional markets.
Why are timely ethical approvals important in clinical trials?
Timely ethical approvals are crucial for maintaining the integrity of clinical trials, facilitating faster market entry, and ensuring the rights and safety of participants.
What advantages does Colombia offer for clinical trials of Class 1 medical devices?
Colombia provides R&D tax incentives, including a 100% tax deduction, a strong healthcare system, and a population of over 50 million, making it an attractive location for patient recruitment in clinical trials.
What are Class 1 medical devices?
Class 1 medical devices are low-risk products that pose minimal potential harm to patients. They are primarily regulated by general controls and are generally exempt from premarket notification requirements.
What are some common examples of Class 1 medical devices?
Common examples include bandages and surgical gloves.
What regulatory requirements must manufacturers of Class 1 medical devices adhere to?
Manufacturers must comply with several requirements, including establishment registration, item listing with the FDA, adherence to Quality System Regulation (QSR), labeling requirements, and displaying a unique identifier (UDI) on labels.
What is the significance of the Quality System Regulation (QSR) for Class 1 medical devices?
QSR ensures that products are manufactured under strict quality standards throughout their lifecycle, from design to post-market surveillance, enhancing safety and efficacy.
How does regulatory compliance impact the market access of Class 1 medical devices?
Maintaining compliance with regulations is essential for protecting public health and facilitating market access, as it ensures that products are safe and effective for public use.
What percentage of manufacturers have successfully navigated the regulatory requirements for Class 1 medical devices?
Approximately 80% of manufacturers have effectively navigated these regulatory requirements, demonstrating the industry's commitment to quality and security.