


The article underscores the essential FDA medical device label requirements critical for compliance, highlighting the crucial elements manufacturers must adhere to for successful market access and consumer safety. It asserts that precise labeling—featuring clear identification, intended use, and comprehensive instructions—is vital to avert costly misbranding issues and secure regulatory approval. This focus on compliance significantly influences both product launches and consumer trust, illustrating the profound impact of adherence to these standards.
Navigating the intricate landscape of FDA medical device labeling requirements is essential for manufacturers seeking to launch safe and effective healthcare products. With the healthcare product marking market anticipated to experience substantial growth, a thorough understanding of the key regulations can not only streamline compliance but also enhance market access and foster consumer trust. However, given that a staggering percentage of devices fail to meet these critical standards, the pressing question remains: how can companies guarantee that their labeling practices are not only compliant but also effective in safeguarding patient safety and maintaining regulatory approval?
bioaccess® excels in assisting Medtech, Biopharma, and Radiopharma firms through the intricacies of FDA product marking requirements. With a profound understanding of the regulatory landscape, bioaccess® streamlines the compliance process, enabling clients to expedite their innovative products to market. Notably, bioaccess® offers accelerated site activation services, achieving FDA/EMA/MDR-ready datasets in under 8 weeks, which is critical for expediting clinical trials.
The healthcare product marking market is expected to expand considerably, approaching around USD 6.16 billion by 2033, increasing from USD 4.16 billion in 2024, with a predicted growth rate of 4.4%. By leveraging its extensive experience in early-phase clinical research, bioaccess® provides customized solutions that not only meet FDA standards but also enhance the likelihood of successful product launches.
Recent updates from the FDA highlight the necessity for precise and compliant identification in accordance with FDA medical device label requirements, particularly in light of increased enforcement actions, making bioaccess®'s expertise invaluable for companies aiming to navigate these evolving requirements effectively.
Effective compliance strategies encompass:
All of which bioaccess® supports to ensure that clients stay at the forefront of the healthcare market.
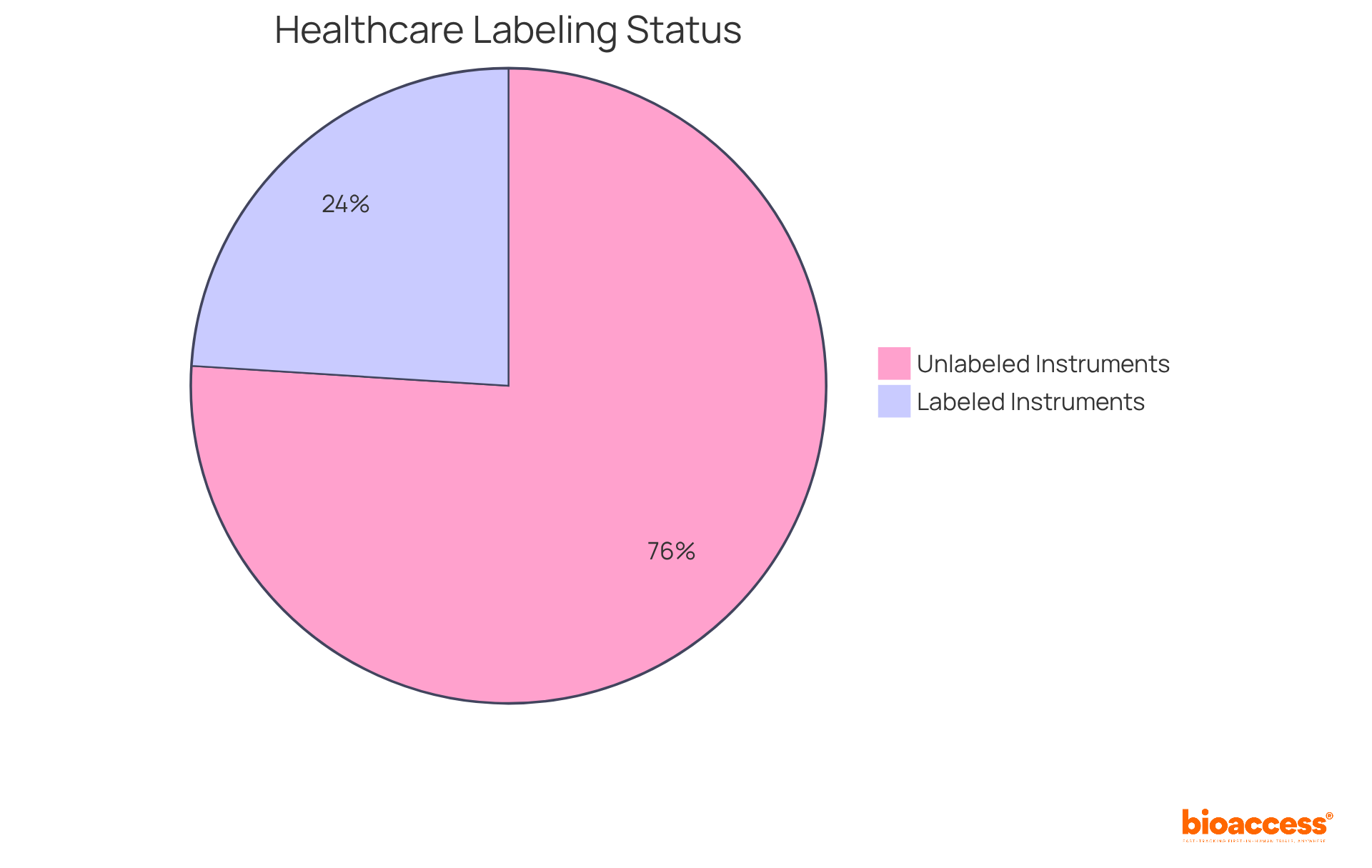
Under 21 CFR 801.1, every healthcare product label must prominently display the name and location of the manufacturer, packer, or distributor. This requirement is crucial for traceability and accountability, allowing consumers and healthcare professionals to identify the origin of the equipment.
Alarmingly, approximately 76% of healthcare instruments lack this essential information, leading to significant misbranding issues. Mislabeling incidents can cost manufacturers an average of $1.3 million annually and may result in production line shutdowns, with 52% of manufacturers reporting weekly interruptions due to mislabeled products.
Such oversights may trigger regulatory actions, including fines or product recalls, underscoring the necessity for manufacturers to adhere strictly to packaging regulations. Ensuring that this information is easily noticeable is not merely a regulatory obligation; it is a vital measure to protect consumer confidence and ensure the safe utilization of healthcare equipment.
Manufacturers should regularly review their labeling practices to ensure compliance with FDA medical device label requirements and avoid the costly repercussions of misbranding.
The intended purpose of a medical instrument must be clearly specified on its label to comply with the FDA medical device label requirements as outlined in 21 CFR 801.4. This definition should include the specific conditions for which the apparatus is intended and the target population it serves. A clear intended use is crucial not only for regulatory approval but also for protecting patient safety by ensuring appropriate application of the equipment.
Vague language can lead to misinterpretation and misuse; thus, manufacturers are urged to provide clear and concise descriptions. In fact, studies indicate that a significant percentage of healthcare instruments suffer from ambiguous intended use descriptions, complicating the regulatory process and delaying market entry.
Successful FDA approvals often depend on clearly defined intended use statements, emphasizing that clarity in labeling is crucial for adhering to FDA medical device label requirements and effective regulation in healthcare products.
21 CFR 801.5 mandates that medical equipment labels must adhere to the FDA medical device label requirements by providing sufficient instructions for use. This critical requirement is part of the FDA medical device label requirements, ensuring users can operate the equipment safely and effectively. Directions should be clear and encompass all necessary information, including:
Insufficient directions can lead to improper use, resulting in adverse events and potential liability for the manufacturer if they fail to comply with FDA medical device label requirements. Therefore, it is essential to invest time in crafting clear and comprehensive instructions, as this is crucial for both compliance and user safety.

According to the FDA medical device label requirements under 21 CFR 801.6, labels for healthcare instruments are strictly prohibited from containing false or misleading statements. This regulation is essential for safeguarding consumers, ensuring they receive accurate and reliable information about the products they use. Misleading claims can have severe repercussions, including regulatory penalties and significant harm to a manufacturer's reputation.
Research indicates that approximately 30% of medical devices have been found to contain misleading claims, raising serious concerns about transparency in the industry. For instance, regulatory bodies have imposed penalties on manufacturers for non-compliance, such as fines exceeding $1 million for false advertising. This reinforces the necessity for rigorous oversight.
Experts in regulatory affairs, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasize the importance of compliance with these regulations. Therefore, it is crucial for manufacturers to meticulously review their labeling practices to meet FDA medical device label requirements, ensuring that all statements are truthful and substantiated by robust evidence.
Maintaining transparency not only builds trust among stakeholders but also improves the overall safety and efficiency of healthcare tools. Additionally, to meet FDA medical device label requirements, labels must provide adequate directions for use and warnings against unsafe usage conditions.

According to 21 CFR 801.15, the FDA medical device label requirements dictate that certain statements on healthcare product labels must be displayed prominently to ensure they are easily observed by consumers. This requirement encompasses:
The prominence of these statements is crucial for user safety, as it helps prevent misuse and ensures that users are aware of important safety information. Manufacturers are urged to utilize:
to enhance the visibility of these key statements.
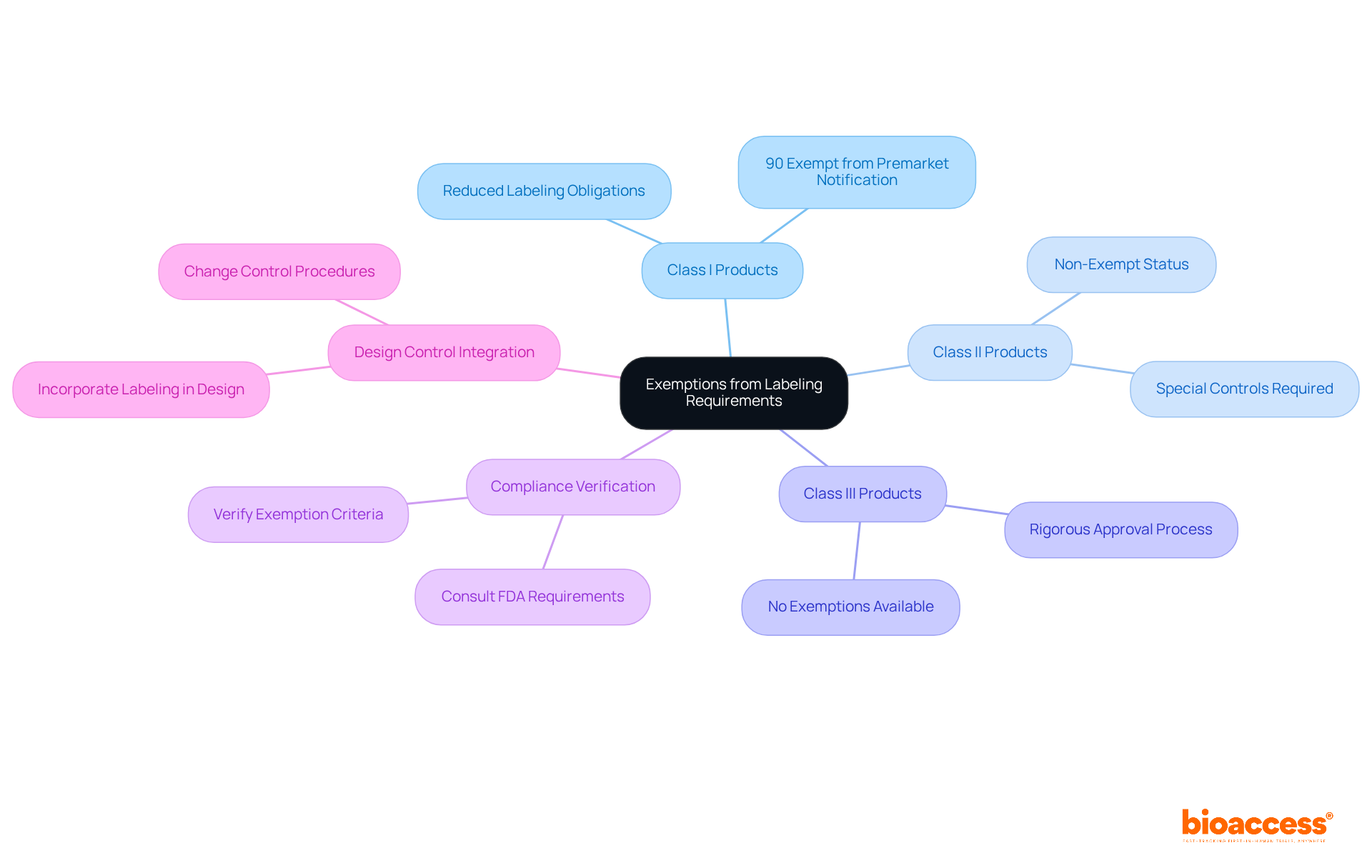
Certain medical instruments may qualify for exemptions from specific marking requirements related to FDA medical device label requirements, which is essential for producers aiming to streamline compliance efforts. For instance, many Class I products enjoy reduced labeling obligations compared to their Class II and III counterparts. Notably, approximately 90% of Class I products are exempt from premarket notification, facilitating a more efficient path to market.
However, it is imperative for manufacturers to meticulously verify the specific criteria for these exemptions to ensure compliance with the FDA medical device label requirements and all other applicable regulations. Consulting the FDA medical device label requirements is vital to determine if a product is eligible for any exemptions, as this knowledge can significantly impact market strategy and regulatory challenges.
Moreover, integrating marking considerations into the design control process is crucial for ensuring compliance and enabling smoother regulatory navigation. Authorities such as Ana Criado, Director of Regulatory Affairs, emphasize the necessity for this integration, while Katherine Ruiz, a specialist in regulatory matters for healthcare products and in vitro diagnostics in Colombia, highlights the importance of understanding both local and international regulations to ensure successful market entry.
The FDA medical device label requirements establish essential criteria that all healthcare product tags must fulfill. These criteria include:
Non-compliance with these marking provisions can lead to misbranding, a prevalent issue noted in FDA enforcement actions and one of the top five causes for Form 483 citations issued to medical equipment companies, particularly related to FDA medical device label requirements.
To mitigate these risks, manufacturers should implement standard operating procedures (SOPs) for labeling review and validation, ensuring that they meet FDA medical device label requirements by including all necessary information and presenting it clearly.
Essential label components, as outlined by FDA medical device label requirements, encompass:
Additionally, labels must be legible and securely attached under all storage and distribution conditions to meet the FDA medical device label requirements. A comprehensive understanding of the FDA medical device label requirements is crucial for effective product marketing and ensuring consumer safety, as high-quality markings can significantly reduce review cycles and enhance market access.
Furthermore, adherence to the FDA medical device label requirements, along with Unique Device Identification (UDI) requirements, is vital for monitoring and recognizing health instruments, underscoring the importance of proper marking preparation.
Ana Criado, with her extensive experience in regulatory matters, underscores the necessity of adhering to the FDA medical device label requirements to ensure both conformity and safety within the medical equipment sector.

Ensuring adherence to FDA medical device label requirements is crucial for maintaining up-to-date information on product packaging. Producers must conduct regular assessments and revisions of their labels to accurately reflect any changes in specifications, usage instructions, or FDA medical device label requirements. A significant percentage of medical devices are found to have outdated information on their labels, which can lead to misuse, adverse events, and potential legal liabilities.
Therefore, creating a systematic review process for identification is essential to ensure that all information remains accurate and compliant with the FDA medical device label requirements. Firms that have effectively managed updates, particularly those adhering to the FDA's five quality controls:
demonstrate the success of proactive regulatory strategies. This ultimately safeguards patient safety and enhances operational efficiency.
FDA compliance is essential for manufacturers of healthcare products aiming for successful market entry. Adhering to identification requirements not only secures regulatory approval but also fosters consumer trust and confidence in the product. Notably, over 30% of healthcare product recalls are associated with marking issues, underscoring the necessity for precision and clarity in this sector. Non-compliance can result in delays, fines, and product recalls, which can significantly undermine a company's market position and reputation. Thus, manufacturers must prioritize compliance as a strategic component of their market access strategy. By leveraging the expertise of organizations like bioaccess®, companies can navigate the complexities of FDA medical device label requirements, ensuring that their labeling practices meet stringent standards and ultimately bolstering consumer trust in medical devices.

Ensuring compliance with FDA medical device labeling requirements is paramount for manufacturers aiming to successfully navigate the regulatory landscape. This article highlights the critical components of effective labeling, emphasizing the importance of accurate identification, clear intended use, comprehensive instructions, and the avoidance of misleading statements. By adhering to these requirements, companies not only mitigate the risk of costly penalties and recalls but also enhance consumer trust and safety.
Key insights discussed include:
The article underscores that a strategic approach to labeling can significantly impact market access and product success, as non-compliance can lead to severe repercussions, including financial losses and damage to reputation.
Ultimately, prioritizing FDA compliance in medical device labeling is not just a regulatory obligation; it is a vital investment in the safety and efficacy of healthcare products. Manufacturers are encouraged to adopt rigorous compliance strategies and leverage expert resources to navigate these complex requirements effectively. By doing so, they can ensure successful market entry and contribute to a safer healthcare environment for all.
What services does bioaccess® provide to assist with FDA medical device labeling requirements?
bioaccess® assists Medtech, Biopharma, and Radiopharma firms by streamlining the compliance process with FDA product marking requirements, offering accelerated site activation services, and achieving FDA/EMA/MDR-ready datasets in under 8 weeks.
What is the projected growth of the healthcare product marking market?
The healthcare product marking market is expected to grow from approximately USD 4.16 billion in 2024 to around USD 6.16 billion by 2033, with a predicted growth rate of 4.4%.
Why is precise labeling important according to recent FDA updates?
Precise labeling is crucial due to increased enforcement actions by the FDA. Companies must navigate evolving requirements effectively to avoid regulatory issues.
What are some effective compliance strategies supported by bioaccess®?
Effective compliance strategies include comprehensive documentation, following specific labeling formats like Pressure Sensitive Labels and Glue Applied Labels, ensuring adherence to FDA medical device label requirements, and proactive involvement with regulatory changes.
What essential information must be displayed on healthcare product labels under 21 CFR 801.1?
Labels must prominently display the name and location of the manufacturer, packer, or distributor, which is vital for traceability and accountability.
What are the consequences of mislabeling healthcare instruments?
Mislabeling can lead to significant financial losses, averaging $1.3 million annually for manufacturers, production line shutdowns, and regulatory actions such as fines or product recalls.
What does 21 CFR 801.4 require regarding the intended use of medical instruments?
21 CFR 801.4 requires that the intended purpose of a medical instrument be clearly specified on its label, including specific conditions for use and the target population.
Why is clarity in intended use descriptions important for medical devices?
Clarity in intended use descriptions is crucial for regulatory approval and patient safety, as vague language can lead to misinterpretation and misuse of the equipment.