


The article titled "10 Key Insights for Clinical Trials of Medical Devices" presents essential factors that significantly impact the success and efficiency of clinical trials within the medical device sector. It underscores the necessity of:
These elements are crucial as they contribute to expedited approvals and enhanced outcomes throughout the development process. By understanding these insights, stakeholders can navigate the complexities of clinical trials more effectively, ensuring that innovations reach the market in a timely manner. The collaboration among various entities in the Medtech landscape is vital, as it addresses key challenges and fosters advancements in clinical research.
The landscape of clinical trials for medical devices is rapidly evolving, driven by the urgent need for speed and efficiency in a sector where nearly 90% of research projects struggle to meet enrollment targets. As innovators seek to navigate the complexities of regulatory compliance and patient recruitment, understanding the unique challenges and best practices has never been more crucial.
What strategies can stakeholders adopt to enhance their trial outcomes and ensure timely market access? This article delves into ten key insights that illuminate the path forward in the clinical trial process for medical devices, offering valuable guidance for Medtech professionals aiming to thrive in this competitive environment.
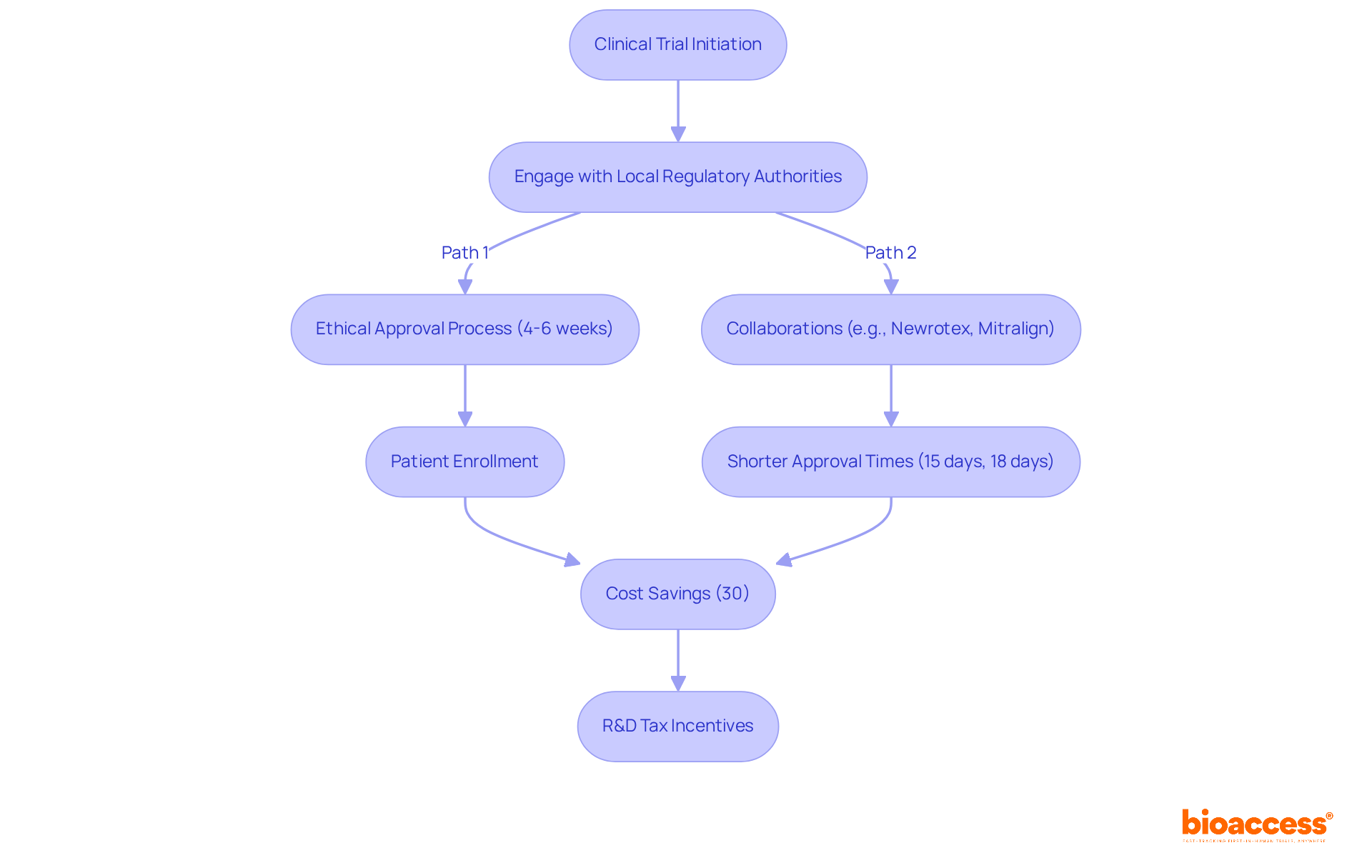
bioaccess® excels in accelerating the clinical trial of medical devices by leveraging the regulatory efficiency of Latin America, obtaining ethical approvals in an impressive 4-6 weeks. This efficiency is further enhanced by access to diverse patient populations in Colombia, where the total IRB/EC and MoH (INVIMA) review takes only 90-120 days, and approximately 95% of the population is covered by universal healthcare.
Notably, the partnership with Newrotex in Panama resulted in a rapid 15-day ethical endorsement for their nerve regeneration technology study, underscoring the area's growing significance in research. Additionally, the collaboration with Mitralign in Colombia yielded ethical approval in just 18 days, facilitating swift patient enrollment.
These success narratives underscore bioaccess®'s prominence in enabling prompt and effective clinical trials of medical devices for Medtech innovators, addressing the critical demand for speed in an industry where nearly 90% of research projects fail to achieve their enrollment objectives.
Industry experts emphasize that engaging with local regulatory authorities early can significantly enhance the likelihood of obtaining timely approvals. To leverage these advantages, research directors should consider partnering with bioaccess® to navigate the complexities of study execution effectively.
Colombia offers competitive benefits, including significant cost savings of over 30% compared to North America and Western Europe, along with robust R&D tax incentives that can provide substantial financial advantages for innovation projects.
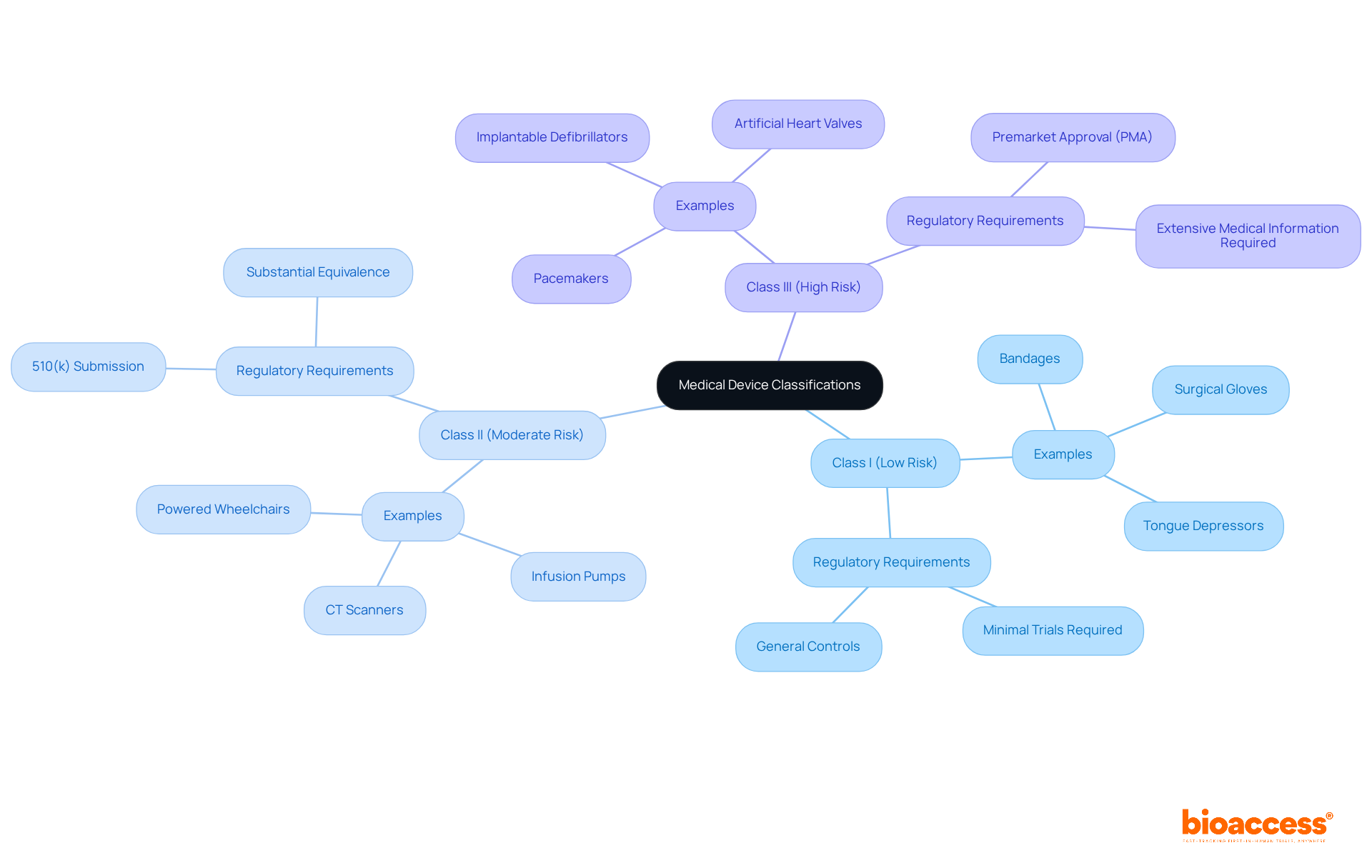
Medical instruments are classified into three main categories:
Each classification dictates the degree of regulatory supervision and the type of research studies required for a clinical trial of medical devices. For instance, Class I products, such as bandages and surgical gloves, typically encounter minimal regulatory examination and frequently do not necessitate extensive trials prior to marketing. In contrast, Class II items, such as CT scanners and infusion pumps, require a 510(k) submission to show substantial equivalence to existing products, which involves moderate medical information gathering.
Class III products, such as pacemakers and implantable defibrillators, are subject to the most rigorous regulatory requirements, including the Premarket Approval (PMA) process. This procedure requires extensive medical information to confirm safety and effectiveness, making it crucial for producers to comprehend these classifications for efficient study planning in a clinical trial of medical devices.
The classification process also has implications for quality systems and cybersecurity readiness, which are essential for ensuring compliance and safety throughout the product lifecycle. Moreover, comprehending the phases of the distribution funnel is vital for producers, as it influences research planning and market entry strategies.
Real-world examples illustrate the implications of these classifications. For example, Medtronic's creation of a new cardiac instrument demanded thorough trials to satisfy Class III criteria, highlighting the significant risks associated with guaranteeing patient safety. Likewise, Abbott Laboratories successfully maneuvered through the regulatory environment for its Class II diagnostic products by following the 510(k) pathway, which facilitated their market entry.
The FDA plays an essential role in establishing suitable regulatory measures for each category of equipment, ensuring that producers align their medical strategies with these classifications. Furthermore, postmarket monitoring is a necessity for all categories, crucial for continuous safety and effectiveness.
Comprehending medical device categories is essential for the success of a clinical trial of medical devices, as these categories determine not only the regulatory route but also the data prerequisites and study design. Manufacturers must align their medical strategies with these classifications to facilitate smoother reviews and ensure compliance with regulatory expectations. With bioaccess's extensive study management services—including feasibility assessments, compliance evaluations, site selection, project oversight, and reporting—manufacturers can navigate these complexities more effectively. Additionally, bioaccess allows for quicker patient enrollment, achieving 50% faster enrollment rates and $25K savings per patient with FDA-ready data, further improving the efficiency of research studies.
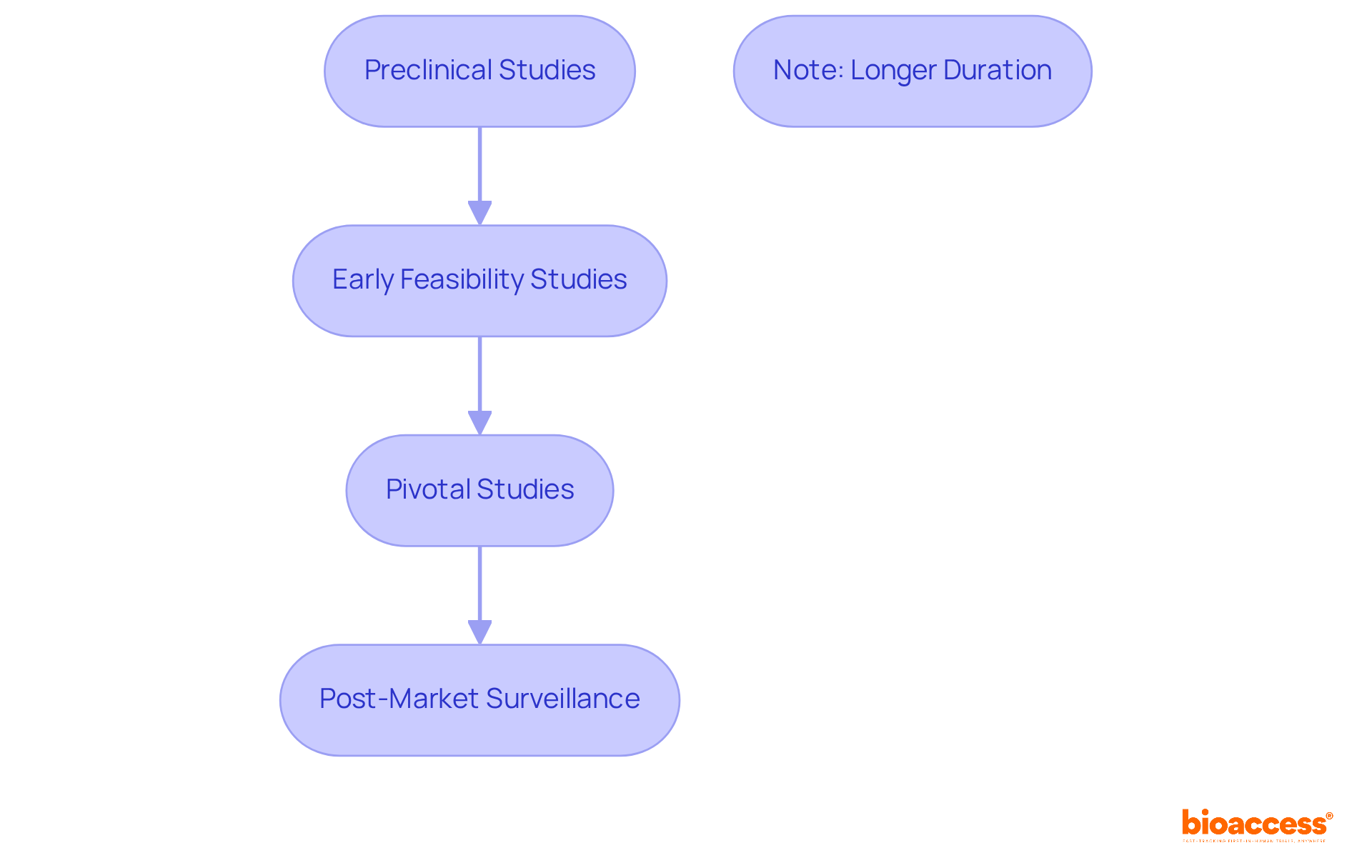
The clinical evaluation process for medical instruments encompasses several critical phases.
Each phase is meticulously organized to gather essential information that informs subsequent steps in the development process, ensuring that products are both safe and effective prior to market release.
Notably, the median duration for Phase 1 studies is approximately 1.6 years, whereas pivotal assessments can take considerably longer, underscoring the complexity and thoroughness required in these evaluations.
Insights from research experts further emphasize the importance of robust preclinical studies and critical evaluations in determining a product's safety profile and effectiveness, ultimately facilitating informed decision-making during the regulatory assessment process.
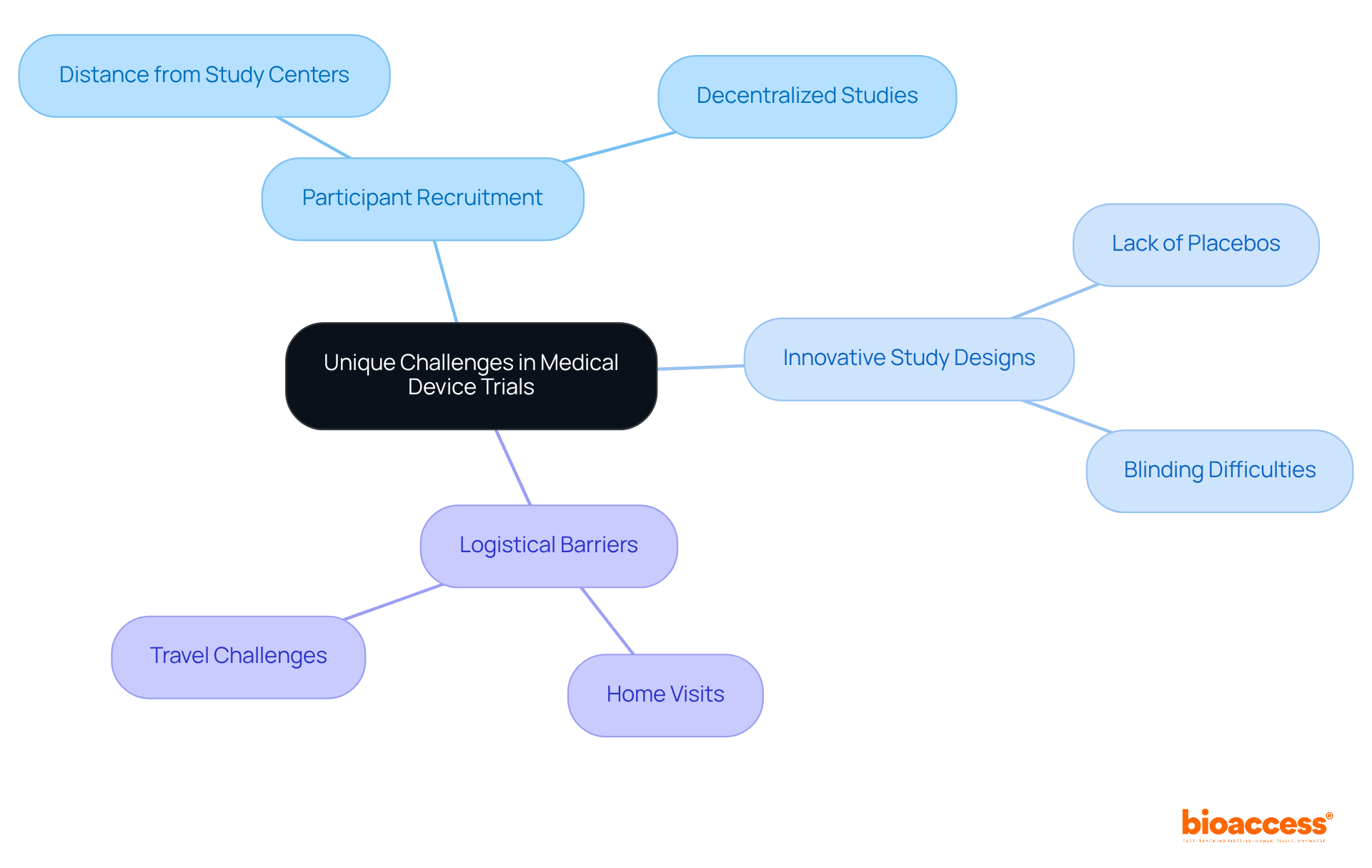
Clinical trials of medical devices present unique challenges that distinguish them from pharmaceutical studies. A significant obstacle is the necessity for practical equipment use during experiments, complicating participant recruitment and data gathering. Unlike pharmaceutical studies, medical equipment research rarely employs placebos, necessitating innovative study designs that effectively evaluate equipment performance without conventional control methods.
For instance, achieving blinding can be particularly difficult, especially with intrusive equipment that possesses distinct physical characteristics. Moreover, logistical barriers often impede recruitment, with approximately 70% of potential participants residing more than two hours from study centers.
To address these challenges, strategies such as decentralized studies and home visits can enhance recruitment efforts, particularly among populations facing additional hurdles. Understanding these unique features is crucial for the effective management of experiments, particularly in the context of the clinical trial of medical devices, and ensuring the successful advancement of medical instruments.
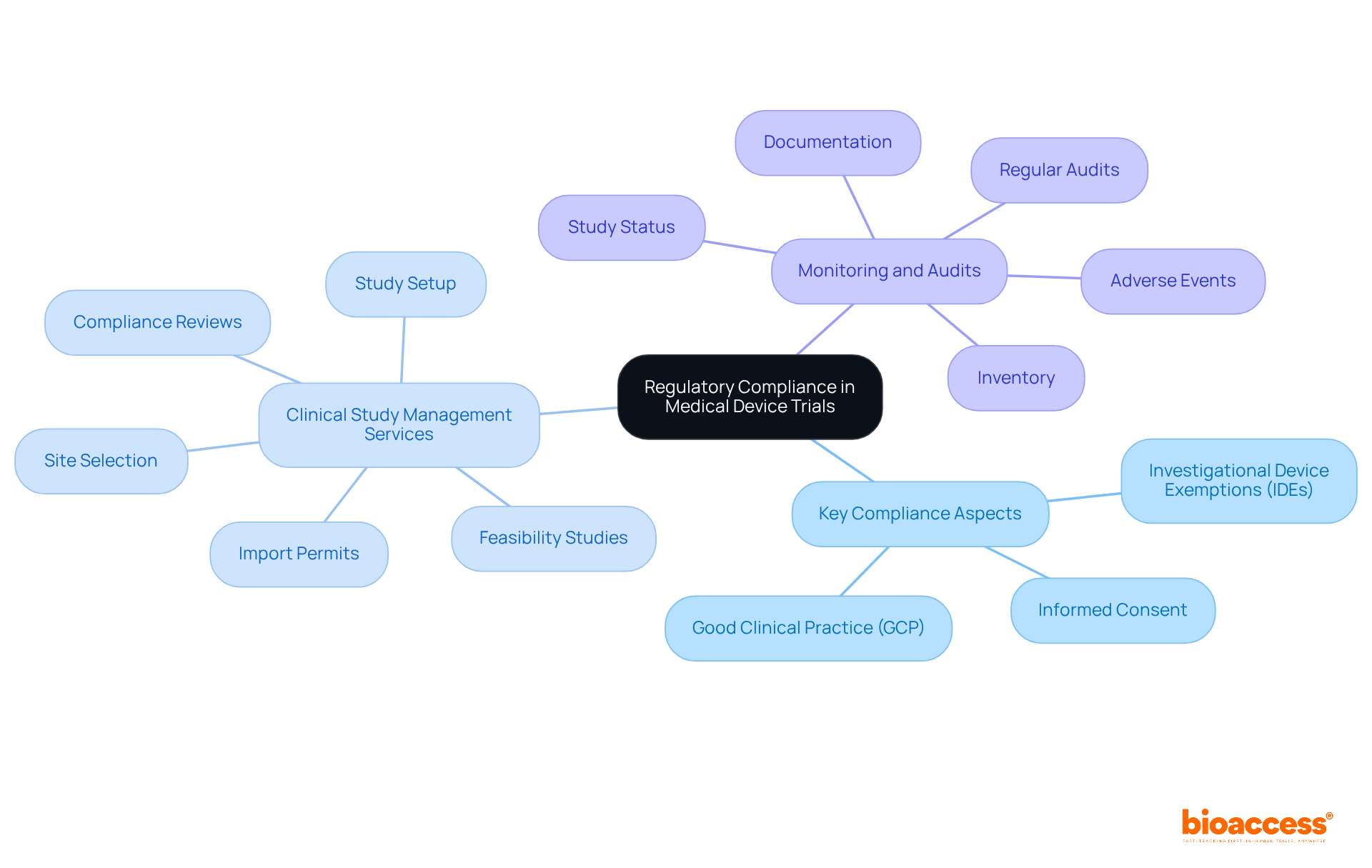
Navigating regulatory compliance in medical device studies is crucial for success in the clinical research landscape. Understanding the specific requirements established by regulatory bodies such as the FDA and EMA is essential. Key compliance aspects include:
At bioaccess, we excel in providing extensive clinical study management services, encompassing:
Our skilled project management ensures that all facets of the study are meticulously monitored and reported, including:
Regular audits and thorough documentation are indispensable for maintaining compliance and facilitating seamless regulatory reviews. This is particularly vital in the complex landscape of Latin America, where local expertise plays a pivotal role in achieving successful market access. Collaboration with experienced partners like bioaccess can significantly enhance your clinical research outcomes.
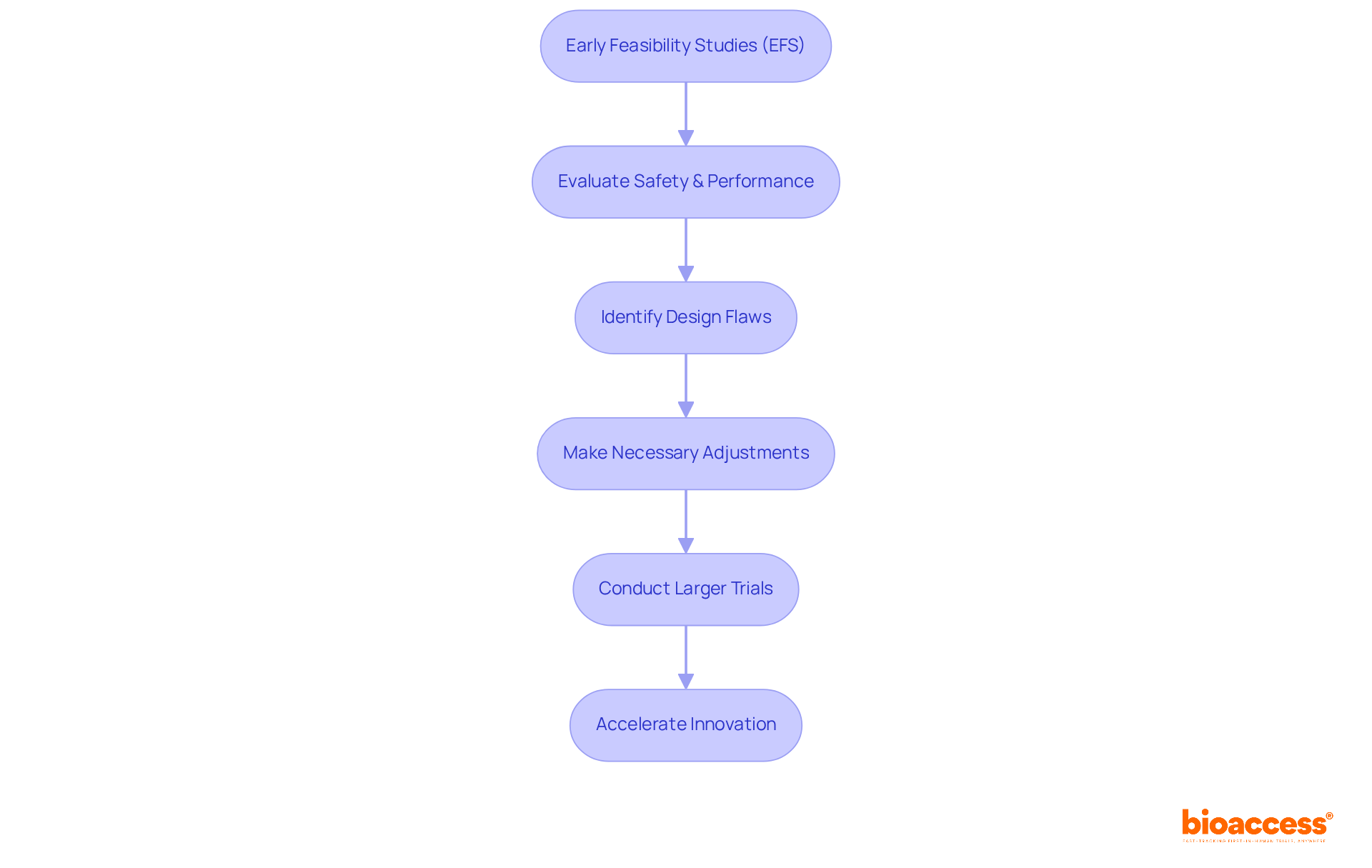
Early Feasibility Studies (EFS) play a pivotal role in the clinical trial of medical devices development landscape, helping researchers evaluate initial safety and performance within a limited participant group. By identifying potential design flaws early, EFS facilitate necessary adjustments before progressing to larger critical evaluations. This proactive strategy mitigates the risk of costly failures later in the development process, establishing EFS as an indispensable asset for innovators conducting clinical trials of medical devices.
Recent discoveries underscore the significance of EFS in medical studies. For instance, ReGelTec's Early Feasibility Study in Barranquilla, Colombia, successfully treated eleven patients suffering from chronic low back pain using HYDRAFIL™ hydrogel technology, illustrating the tangible benefits of EFS. This research not only confirms the effectiveness of new tools but also highlights bioaccess's expertise in conducting clinical trials of medical devices, achieving over a 50% reduction in recruitment time and 95% retention rates.
Typically, EFS involve a small number of participants, generally ranging from 10 to 30, which allows for focused data collection on usability, preliminary safety, and potential efficacy. This limited scope is essential for gathering early insights that can inform larger-scale research initiatives.
Experts emphasize that engaging healthcare professionals and patients during the clinical trial of medical devices is vital for incorporating valuable user feedback into the product design. As industry leaders note, EFS serve as a crucial link between conceptual development and the clinical trial of medical devices, ultimately reducing development risks and accelerating innovation cycles within the Medtech sector. The Accelerate EFS Toolkit by MDIC further enhances this process by providing best practices and resources for effective EFS management, ensuring that recommendations are grounded in extensive knowledge and practical experience. Additionally, bioaccess addresses logistical challenges by streamlining recruitment processes and enhancing patient consent procedures, thereby facilitating smoother EFS execution.
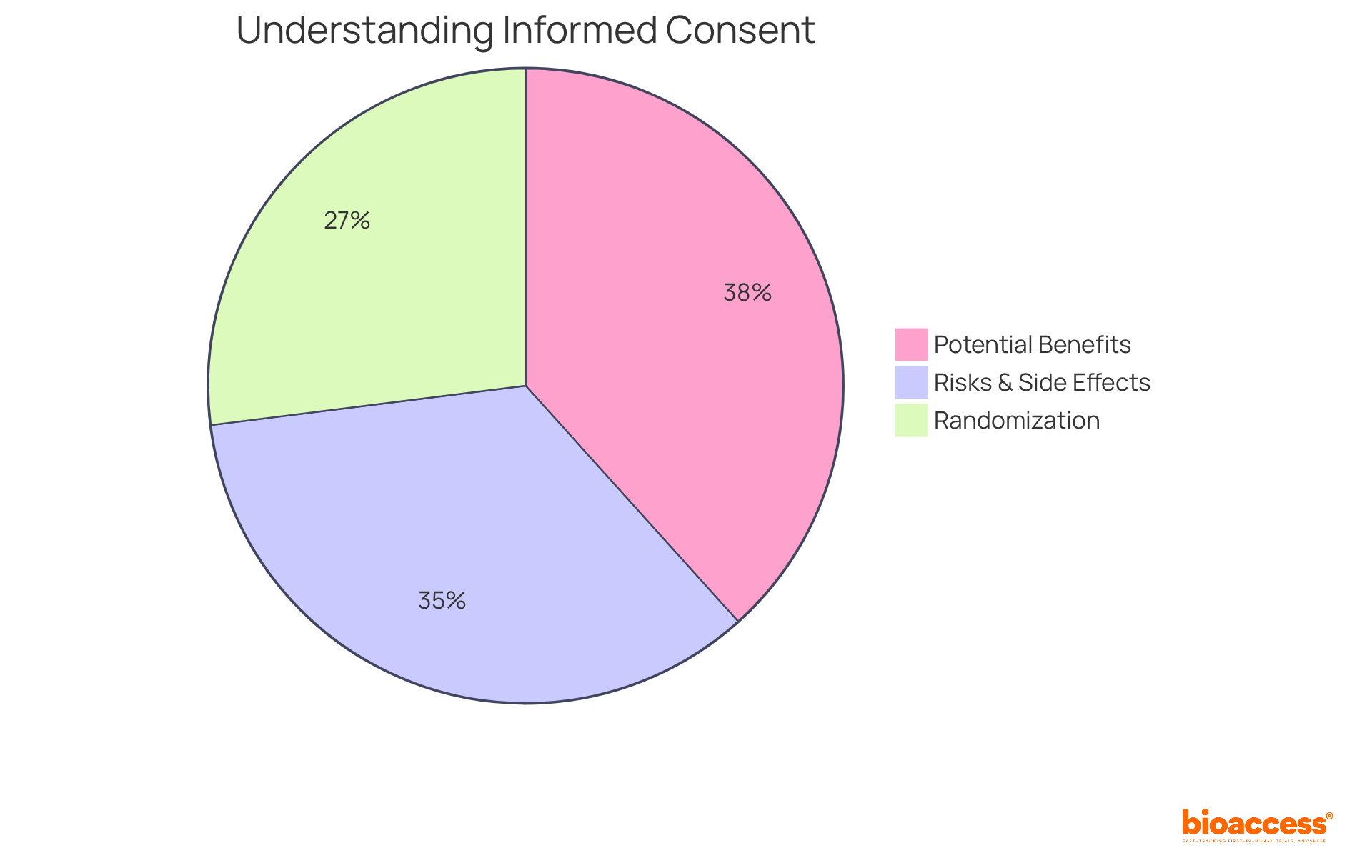
Ethical factors in the clinical trial of medical devices are paramount, particularly in securing informed consent, ensuring participant safety, and maintaining confidentiality. Researchers have a duty to provide comprehensive information about the study's purpose, potential risks, and benefits to participants. Recent data reveals that:
This indicates a more balanced perspective on participants' understanding of informed consent components. Nonetheless, there exists a significant gap in grasping complex concepts such as placebo and randomization, with only 52.1% understanding the latter. This highlights the urgent need for enhanced communication strategies in the informed consent process.
Institutional Review Boards (IRBs) play a vital role in reviewing study protocols for the clinical trial of medical devices to ensure that ethical standards are upheld, thereby protecting the rights and welfare of participants. The rise in serious events reported in 2023, especially within the Complication of Procedure/Treatment/Test category, underscores the necessity for rigorous oversight. Bioethicists assert that informed consent transcends mere formality; it is a critical component in a clinical trial of medical devices that fosters participant welfare and safeguards their rights. As specialists emphasize, "Acquiring informed consent from participants in medical research is vital as it enhances their well-being and protects their rights." Enhancing the quality of the informed consent procedure is essential for cultivating trust and ensuring that participants are fully informed about their involvement in research studies. Furthermore, concerns regarding the exploitation of healthy individuals in phase I studies must be addressed to provide a comprehensive view of the ethical framework in medical research.
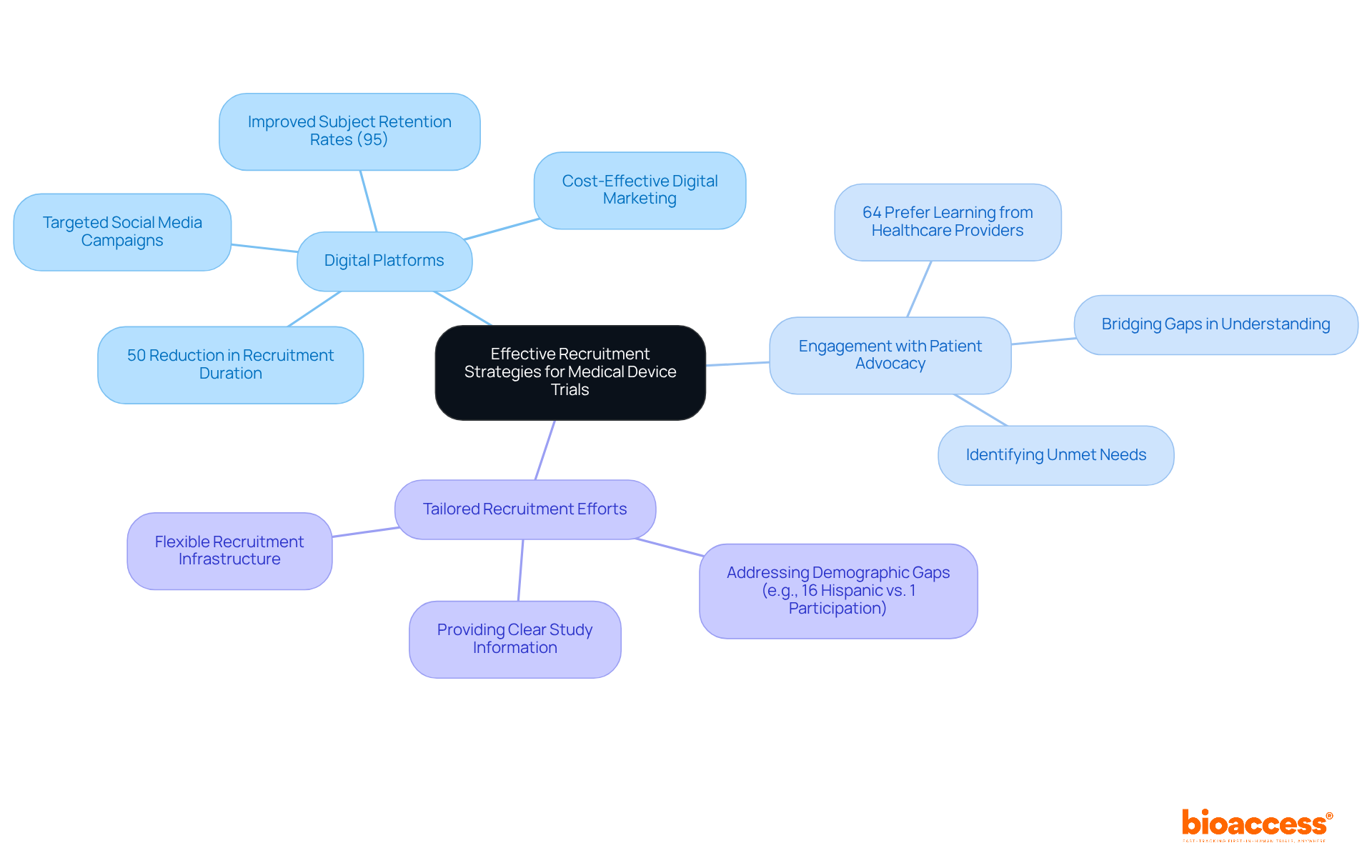
To enhance participation rates in medical device studies, effective recruitment strategies are essential. Leveraging digital platforms has emerged as a transformative approach, enabling researchers to connect with diverse populations more efficiently. For instance, the collaboration between GlobalCare Clinical Trials and bioaccess™ has led to a remarkable reduction in participant recruitment duration by over 50%, alongside an improvement in subject retention rates exceeding 95% in Colombia. This underscores the importance of innovative methods in overcoming recruitment challenges, especially considering that nearly 90% of medical studies face delays due to these issues.
Engaging with patient advocacy groups can significantly bolster outreach efforts, as these organizations possess a deep understanding of the unmet needs within their communities, effectively bridging the gap between researchers and potential participants. It is crucial to tailor recruitment efforts to the specific demographics of the target population. For example, while 16% of the U.S. population is Hispanic, they represent only 1% of clinical study participants, highlighting a critical gap that demands attention. Providing clear and comprehensive information about the study procedures can alleviate anxieties and misunderstandings, fostering confidence and encouraging participation.
Recent trends indicate that digital marketing strategies, including targeted social media campaigns, are increasingly effective. These approaches facilitate precise targeting of potential participants, rendering recruitment efforts more efficient and cost-effective. Experts in the field emphasize that understanding patient needs and cultivating relevant connections within the health community are vital components of a successful recruitment process. Furthermore, the CISCRP survey reveals that 64% of patients prefer to learn about a study from their healthcare provider rather than an external source, underscoring the importance of community outreach. By implementing these strategies, along with the comprehensive management services for research studies offered by bioaccess™, research participation rates can rise, ultimately enhancing the quality of medical investigations.
Efficient information management in the clinical trial of medical devices is paramount, and it heavily relies on the implementation of electronic information capture (EDC) systems, which are currently utilized in over 75% of clinical studies. These systems streamline the information-gathering process, enhance precision, and ensure compliance with regulatory standards. By automating data entry and providing real-time access to study details, EDC systems can reduce operational costs by up to 30% and significantly expedite study timelines.
Incorporating robust information collection protocols is essential, along with regular validation and cleaning processes. Establishing transparent governance policies ensures that information integrity is maintained throughout the study. Furthermore, educating personnel on these procedures not only enhances adherence but also fosters a culture of information literacy, a quality increasingly recognized as vital in today’s data-driven landscape.
Recent advancements in data management, such as cloud storage and AI-driven analytics, further bolster the effectiveness of medical studies. These innovations facilitate improved tracking of patient progress and enable quicker identification of issues, ultimately leading to more reliable outcomes. As noted by industry specialists, the adoption of EDC systems is not merely a passing trend; it represents a crucial evolution in medical research, especially for the clinical trial of medical devices, empowering organizations to remain competitive and responsive to the dynamic healthcare environment.
Global trends in the clinical trial of medical devices are increasingly characterized by the adoption of digital health technologies, the rise of decentralized studies, and a growing emphasis on patient-centric approaches. Innovations such as artificial intelligence and real-world evidence are revolutionizing study designs and data collection methods.
Firms like bioaccess® are at the forefront of these transformations, offering extensive trial management services that encompass:
Their expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies is essential for stakeholders striving to maintain a competitive edge in the clinical trial of medical devices.
Moreover, collaborations with organizations such as GlobalCare Clinical Trials, which have achieved over a 50% reduction in recruitment duration and a 95% retention rate, underscore the significant impact of the clinical trial of medical devices on local economies, fostering job creation and enhancing healthcare.
Staying informed about these trends is crucial for stakeholders to remain agile in the ever-evolving landscape of clinical research.
In conclusion, the clinical trials of medical devices stand as a complex yet vital segment of the healthcare landscape. Understanding the nuances—from regulatory compliance to effective recruitment strategies—is essential for success. The insights presented underscore the importance of leveraging regional advantages, particularly those in Latin America, to expedite the clinical trial process while ensuring adherence to ethical standards and regulatory requirements.
Throughout the discussions, the critical role of medical device classifications, the phased approach to clinical trials, and the unique challenges faced compared to drug trials have been emphasized. The necessity of early feasibility studies has emerged as a pivotal strategy to mitigate risks and enhance the overall development process. Furthermore, effective recruitment strategies, especially those utilizing digital platforms and community engagement, are paramount to overcoming the common barriers that hinder participant enrollment.
As the landscape of medical device clinical trials continues to evolve, stakeholders must stay informed about emerging trends and best practices. Embracing innovative approaches and forming strategic partnerships can significantly enhance the efficiency and effectiveness of clinical trials. Ultimately, these efforts will lead to improved patient outcomes and accelerated market access for new medical technologies.
What is bioaccess® and how does it accelerate clinical trials for medical devices?
bioaccess® is a company that accelerates the clinical trial process for medical devices by leveraging the regulatory efficiency of Latin America, achieving ethical approvals in 4-6 weeks. They also have access to diverse patient populations, particularly in Colombia, where the total IRB/EC and MoH review takes 90-120 days.
What are the advantages of conducting clinical trials in Colombia?
Colombia offers significant advantages for clinical trials, including cost savings of over 30% compared to North America and Western Europe, access to universal healthcare for approximately 95% of the population, and robust R&D tax incentives that provide financial benefits for innovation projects.
How quickly can ethical approvals be obtained in Latin America?
Ethical approvals can be obtained in as little as 15 days, as demonstrated by bioaccess®'s partnership with Newrotex in Panama for a nerve regeneration technology study. In another instance, a collaboration with Mitralign in Colombia achieved ethical approval in just 18 days.
What are the classifications of medical devices and their significance in clinical trials?
Medical devices are classified into three categories: Class I (low risk), Class II (moderate risk), and Class III (high risk). Each classification dictates the level of regulatory supervision and the type of research studies required for clinical trials, influencing study planning and regulatory compliance.
What are the phases involved in medical device clinical trials?
The clinical evaluation process includes several phases: Preclinical studies (laboratory and animal testing for safety and functionality), Early Feasibility Studies (initial clinical safety and performance evaluation with a limited number of participants), Pivotal studies (larger cohort studies to confirm efficacy for regulatory approval), and Post-market surveillance (monitoring long-term safety and effectiveness after market release).
How does bioaccess® facilitate faster patient enrollment in clinical trials?
bioaccess® achieves 50% faster patient enrollment rates and can save $25,000 per patient by providing FDA-ready data, which enhances the efficiency of research studies.
What is the importance of engaging with local regulatory authorities in clinical trials?
Engaging with local regulatory authorities early in the process can significantly enhance the likelihood of obtaining timely approvals, which is crucial for the success of clinical trials in the medical device industry.
Why is understanding medical device classifications critical for manufacturers?
Understanding medical device classifications is essential for manufacturers because it determines the regulatory route, data requirements, and study design necessary for successful clinical trials, ensuring compliance with regulatory expectations.