


This article provides crucial insights into the updated FDA annual reporting guidance for clinical research directors, focusing on the significant shift from IND annual reports to Development Safety Update Reports (DSURs). Understanding this transition is vital for clinical research operations, as it underscores the importance of compliance with new standards. Key components of compliance include:
Both of which are essential for improving patient safety and streamlining the regulatory process.
In the evolving Medtech landscape, adapting to these new guidelines is not just a regulatory requirement; it’s a strategic necessity. By embracing these changes, clinical research directors can ensure that their operations align with the latest safety protocols, ultimately fostering a more efficient and effective research environment.
The importance of collaboration in navigating these updates cannot be overstated. As the industry adapts, stakeholders must work together to address the challenges posed by these new reporting requirements. The next steps involve not only understanding the implications of DSURs but also actively engaging in discussions about best practices and compliance strategies.
The recent updates to the FDA's annual reporting guidelines mark a pivotal moment for clinical research directors, as adherence to these new standards is essential for success. This shift presents organizations with a vital opportunity to boost their operational efficiency and enhance patient safety by implementing comprehensive strategies that align with these evolving requirements.
However, as the landscape changes, how can sponsors effectively navigate the complexities of these guidelines while ensuring timely and accurate reporting? This article explores ten key insights that illuminate the path forward, equipping clinical research leaders with the knowledge necessary to excel in a rapidly changing regulatory environment.
bioaccess® stands at the forefront of assisting Medtech, Biopharma, and Radiopharma companies in navigating the updated annual report FDA guidance for compliance. Our comprehensive strategy encompasses:
This approach enables clients to achieve compliance efficiently, significantly reducing delays in meeting new requirements.
As the FDA intensifies its focus on stringent documentation standards, the implications for research timelines and market entry become increasingly critical. In 2024 alone, the FDA approved over 5,564 marketing submissions, highlighting the urgent need for robust compliance strategies to expedite product approvals. Regulatory specialists emphasize that organizations prioritizing transparency and quality in their practices can enhance their credibility and improve patient outcomes.
By cultivating a culture of compliance and leveraging bioaccess's end-to-end services—especially in Latin America—companies can align with FDA expectations and ensure their annual report follows FDA guidance to strategically position themselves in a competitive landscape. This alignment not only accelerates their path to market but also fosters collaboration that is essential for success in the evolving clinical research environment.
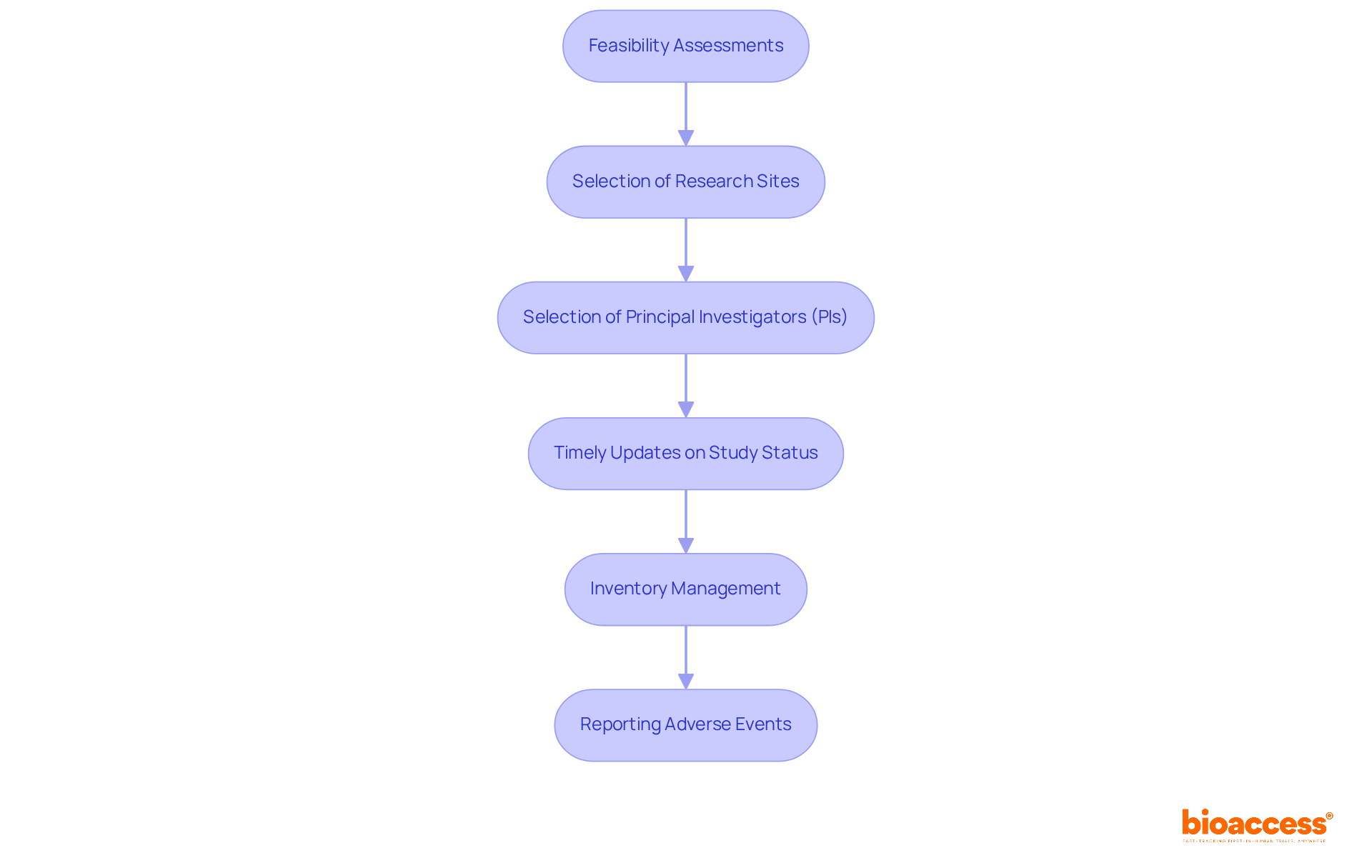
The FDA's updated yearly documentation requirements for Investigational New Drug (IND) applications demand that sponsors provide comprehensive updates on the current status of their research studies. These updates must encompass data on safety, adverse event reports, and any modifications to the study protocol. This shift towards thorough documentation aims to enhance patient well-being and ensure data accuracy throughout the clinical trial process.
In 2025, it is projected that around 60% of sponsors will comply with these new reporting requirements, reflecting a growing commitment to regulatory adherence. Essential components of the IND annual reports now include:
Organizations that have adopted extensive research study management services, such as those offered by bioaccess—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—have successfully navigated compliance challenges. A notable case study illustrated that comprehensive documentation significantly enhanced safety signal identification through effective data integration.
As research directors, understanding these requirements is crucial for ensuring compliance and avoiding potential regulatory repercussions, such as increased scrutiny from regulatory bodies or delays in approval processes. Furthermore, sponsors must submit the Development Safety Update Report (DSUR) no later than 60 calendar days after the data lock point, underscoring the importance of timely submission. Insights from industry leaders, like Robert M. Califf, emphasize that the proposed annual report FDA guidance aligns with international standards, further highlighting the significance of these updated requirements. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, offers valuable guidance in navigating these complexities.

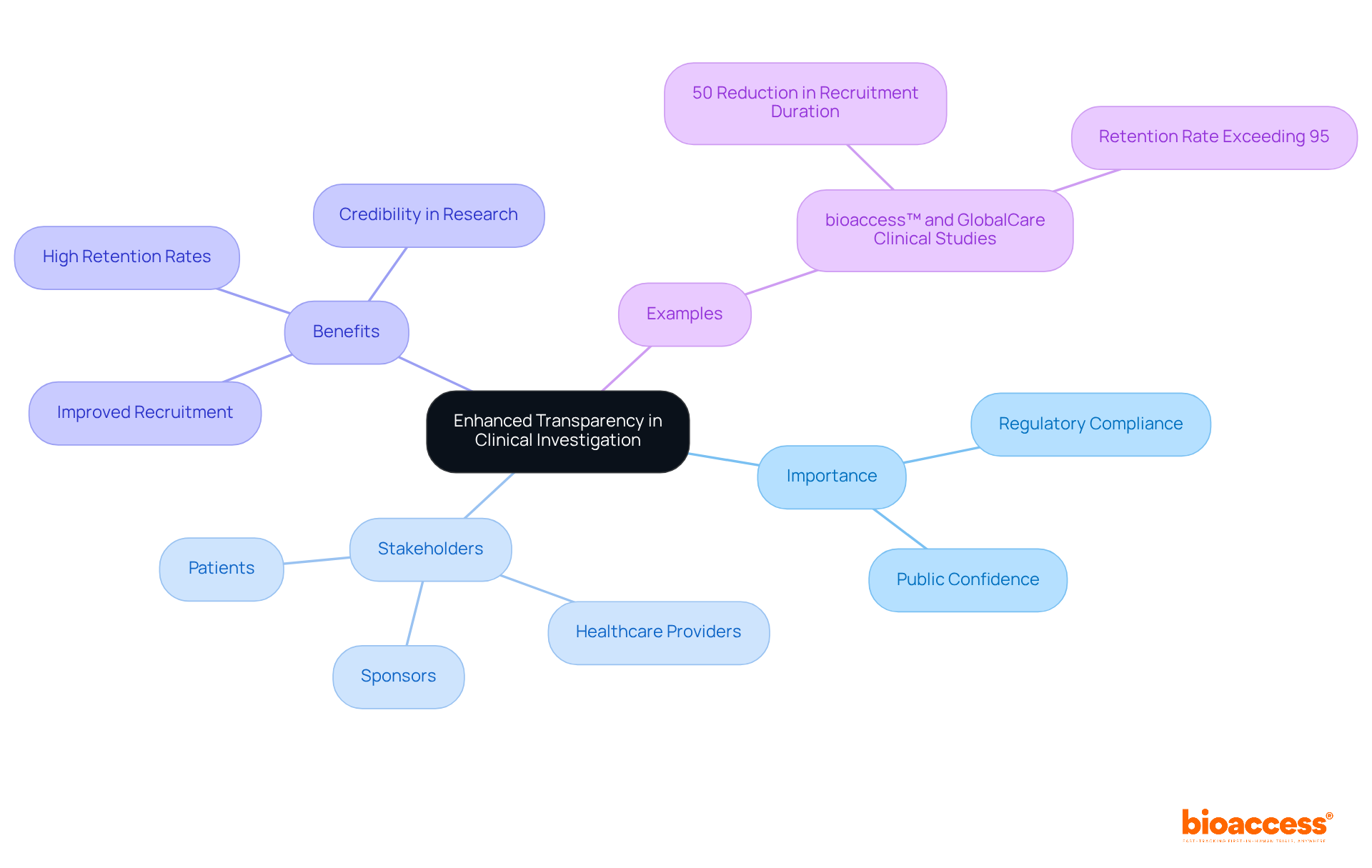
The recent FDA guidelines underscore the critical importance of transparency in research reporting, mandating that sponsors provide comprehensive information about study outcomes and safety data. This pivotal change is designed to bolster public confidence in medical research, ensuring that stakeholders—including patients and healthcare providers—have access to vital information. By adopting these transparency practices, sponsors not only comply with regulatory requirements but also cultivate a culture of openness that enhances patient recruitment and engagement.
For instance, companies like bioaccess™, in collaboration with GlobalCare Clinical Studies, have achieved over a 50% reduction in subject recruitment duration and a retention rate exceeding 95%. Such outcomes illustrate that prioritizing transparent reporting can profoundly impact the credibility of medical research.
Furthermore, media coverage by Clinical Leader highlights the growing focus on research studies in Latin America and Colombia, showcasing the region's potential for innovative partnerships, such as IDx Technologies' collaboration with bioaccess™ to identify ophthalmology centers for AI-driven disease detection.
As healthcare experts emphasize, openness is essential for maintaining public trust in research studies, ultimately leading to improved patient outcomes and a more informed medical community.
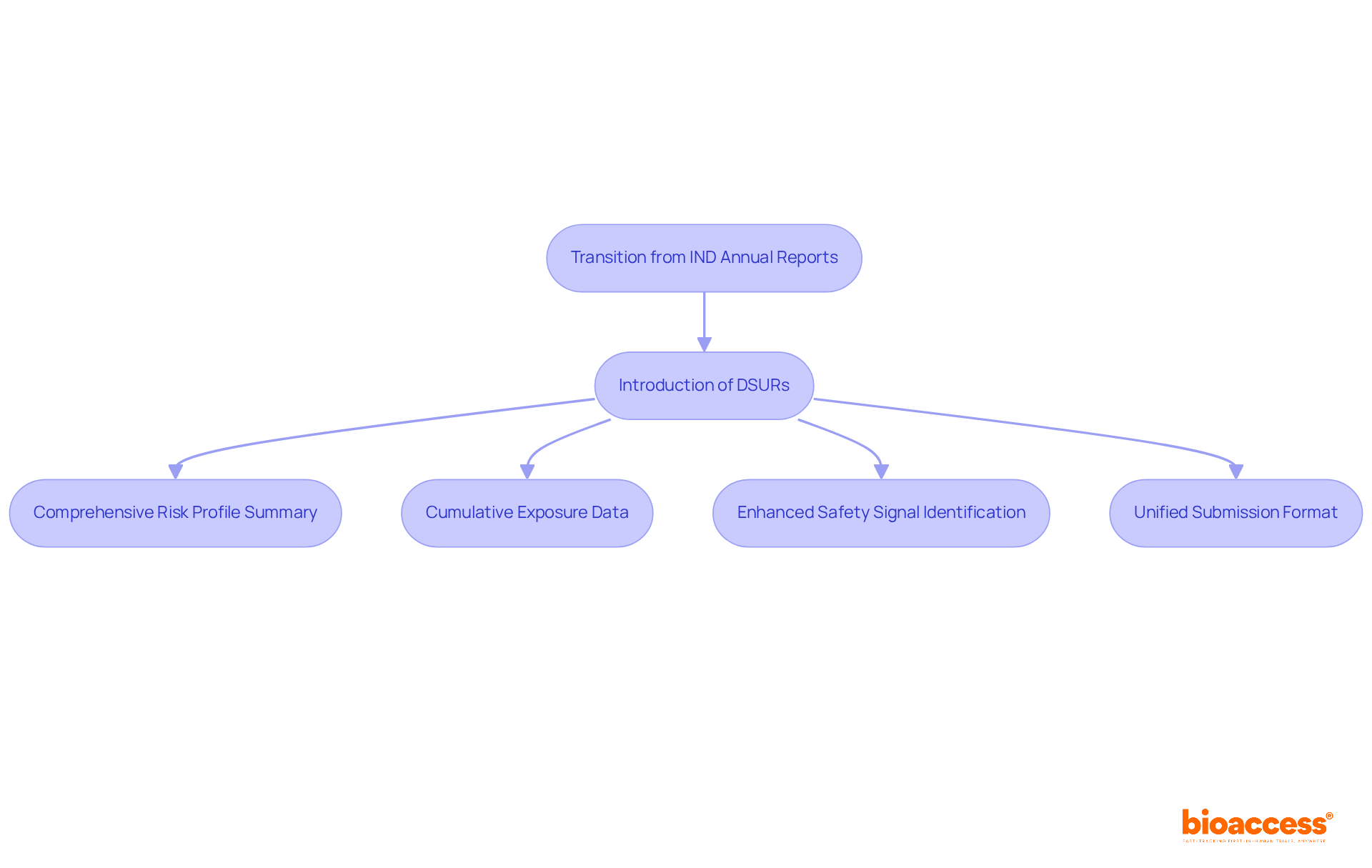
The FDA's transition from IND annual accounts to Development Safety Update Reports (DSURs) marks a significant evolution in data presentation within clinical research. DSURs are crafted to deliver a comprehensive summary of the risk profile of investigational medications, integrating data from all ongoing trials. This shift is not merely procedural; it aims to streamline documentation methods while substantially improving the quality of information available to regulators.
As part of this new framework, sponsors must provide cumulative exposure data and detailed descriptions of investigations. This requirement enhances the FDA's capacity to identify safety signals earlier in the study process. Regulatory affairs specialists stress that grasping the DSUR format and content requirements is essential for sponsors to ensure compliance and facilitate efficient regulatory interactions.
The proposed DSUR requirements are anticipated to alleviate the burden on sponsors by allowing a unified submission format for multiple regulatory authorities. This change not only simplifies the process but also promotes global drug development, underscoring the importance of collaboration in navigating the complexities of clinical research.
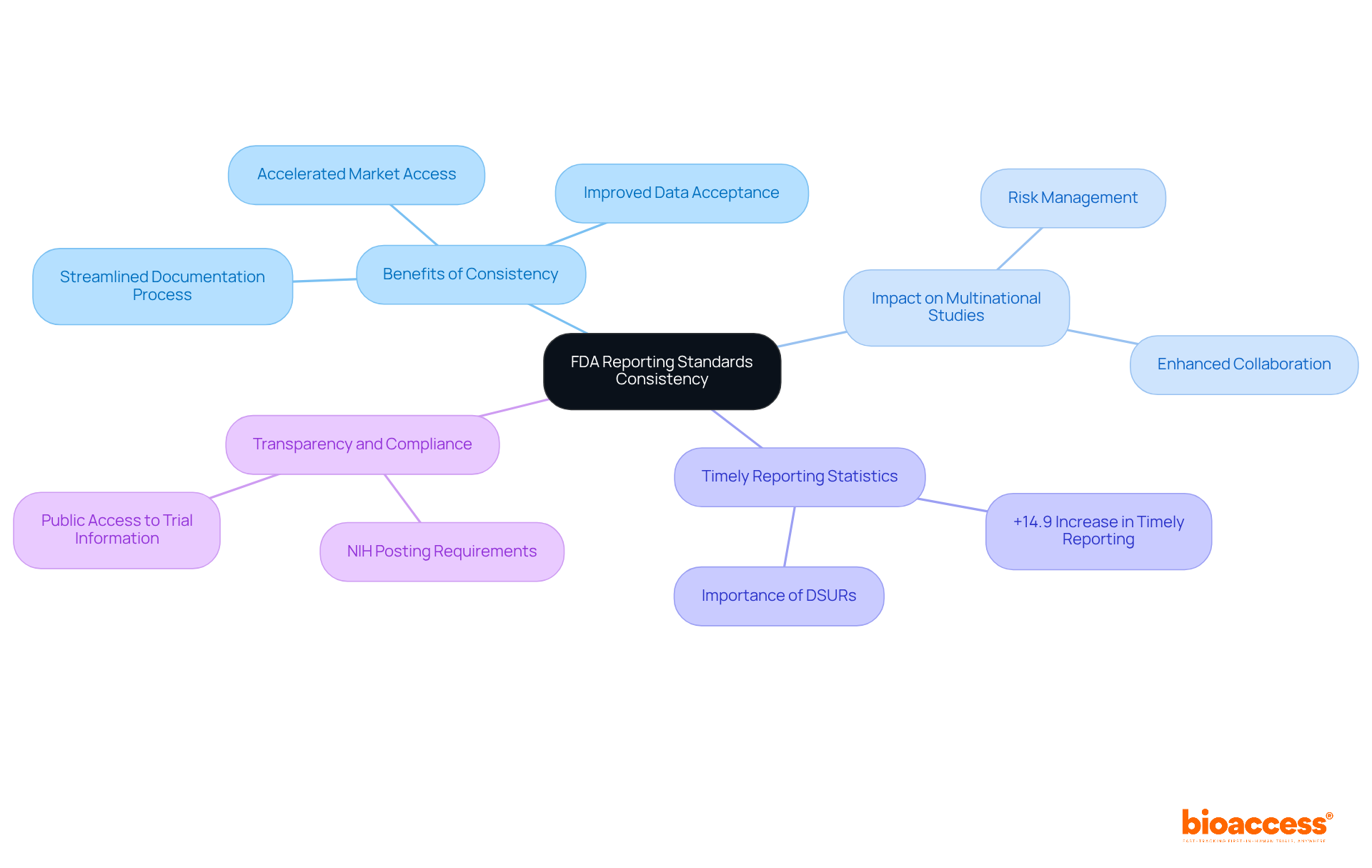
The FDA's revised guidelines are designed to establish consistency in documentation standards, particularly for Development Safety Update Reports (DSURs). This alignment is crucial for sponsors engaged in multinational studies, as it streamlines the documentation process and minimizes the risk of non-compliance. By adhering to international standards, sponsors can improve the acceptance of their data by regulatory authorities worldwide, facilitating smoother approvals and enhancing market access for Medtech products. Regulatory specialists emphasize that such consistency not only streamlines the operational aspects of clinical studies but also significantly accelerates the pace at which innovative products reach the market, ultimately improving patient access to new therapies.
Recent statistics reveal a remarkable +14.9% increase in timely reporting within 12 months, underscoring the positive impact of these guidelines. The DSUR is vital for providing risk evaluations to address potential patient hazards, highlighting its importance in the context of multinational studies. Furthermore, the requirement for the NIH to post all submitted information on ClinicalTrials.gov within 30 days enhances transparency and compliance, further supporting the objectives of the new guidelines.
At bioaccess, our comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are tailored to help sponsors navigate these guidelines effectively. This not only contributes to job creation and economic growth but also leads to improved healthcare outcomes in local communities. As we move forward, collaboration is key to overcoming the challenges in clinical research and ensuring that innovative therapies reach those who need them most.
Comprehensive Development Safety Update Reports (DSURs) play a crucial role in enhancing the protection profiles of investigational drugs. These reports must include detailed analyses of adverse events, cumulative risk data, and emerging hazard signals. By offering a thorough summary of security information, sponsors can effectively communicate ongoing risk evaluations to regulatory bodies and stakeholders. This proactive approach not only ensures compliance with regulatory requirements but also bolsters the integrity of clinical research.
A systematic review of risk data analysis methods illustrates that the implementation of DSURs significantly improves the identification of potential hazards. Prior to DSUR implementation, many adverse events went unreported or were inadequately analyzed, leading to monitoring gaps. After implementing DSURs, studies showed a notable increase in the detection of serious adverse events, with some risks identified that had not been previously reported by sponsors.
Furthermore, establishing a cross-functional team for DSUR preparation is essential for comprehensive data collection and analysis. This collaborative approach allows for a more thorough assessment of risk information, ultimately leading to better-informed decisions regarding the continuation of research studies.
In addition to the importance of DSURs, the extensive management services provided by bioaccess enhance the overall security and effectiveness of research. These services include:
By meticulously overseeing all aspects of the trial process, bioaccess improves risk monitoring and adherence to regulations.
Data analysts emphasize that the organized format of DSURs facilitates clearer information presentation, making it easier to identify trends and emerging signals. This structured approach not only aids in regulatory compliance but also fosters confidence among stakeholders by demonstrating a commitment to patient welfare and thorough oversight during the development process. Moreover, the impact of medtech clinical studies extends beyond safety; they also contribute significantly to job creation, economic growth, healthcare improvement, and international collaboration, ultimately benefiting local economies.
Effective strategic planning is crucial for sponsors aiming to successfully navigate the new annual report FDA guidance. This process begins with a thorough evaluation of existing documentation methods to pinpoint compliance gaps. Data indicates that many organizations struggle with significant shortcomings in their documentation, leading to considerable risks.
To address these challenges, sponsors should develop a robust compliance strategy that aligns with the annual report FDA guidance, encompassing:
By proactively tackling these areas, sponsors can greatly reduce the risk of non-compliance and facilitate more efficient research operations. Compliance officers stress the necessity of adapting to these changes, highlighting that a well-structured approach not only fulfills regulatory requirements but also boosts overall operational efficiency.
The proposed FDA guidance regarding the annual report is set to significantly impact sponsors, particularly in terms of compliance requirements and reporting expectations. To align with these new standards, sponsors must adjust their strategies proactively, which may involve revising research protocols and enhancing data management practices. Regulatory experts stress that maintaining compliance is essential, as the evolving landscape necessitates a thorough understanding of these guidelines' implications.
For instance, companies have successfully navigated these changes by implementing robust safety surveillance plans and regularly reviewing serious adverse events (SAEs). By staying informed and agile, sponsors can effectively position themselves to meet the challenges posed by the updated guidance, ensuring they maintain a competitive edge in the market. Recent data indicates that research protocol amendments have surged due to updates in the annual report FDA guidance, underscoring the importance for sponsors to remain alert and adaptable to regulatory changes.
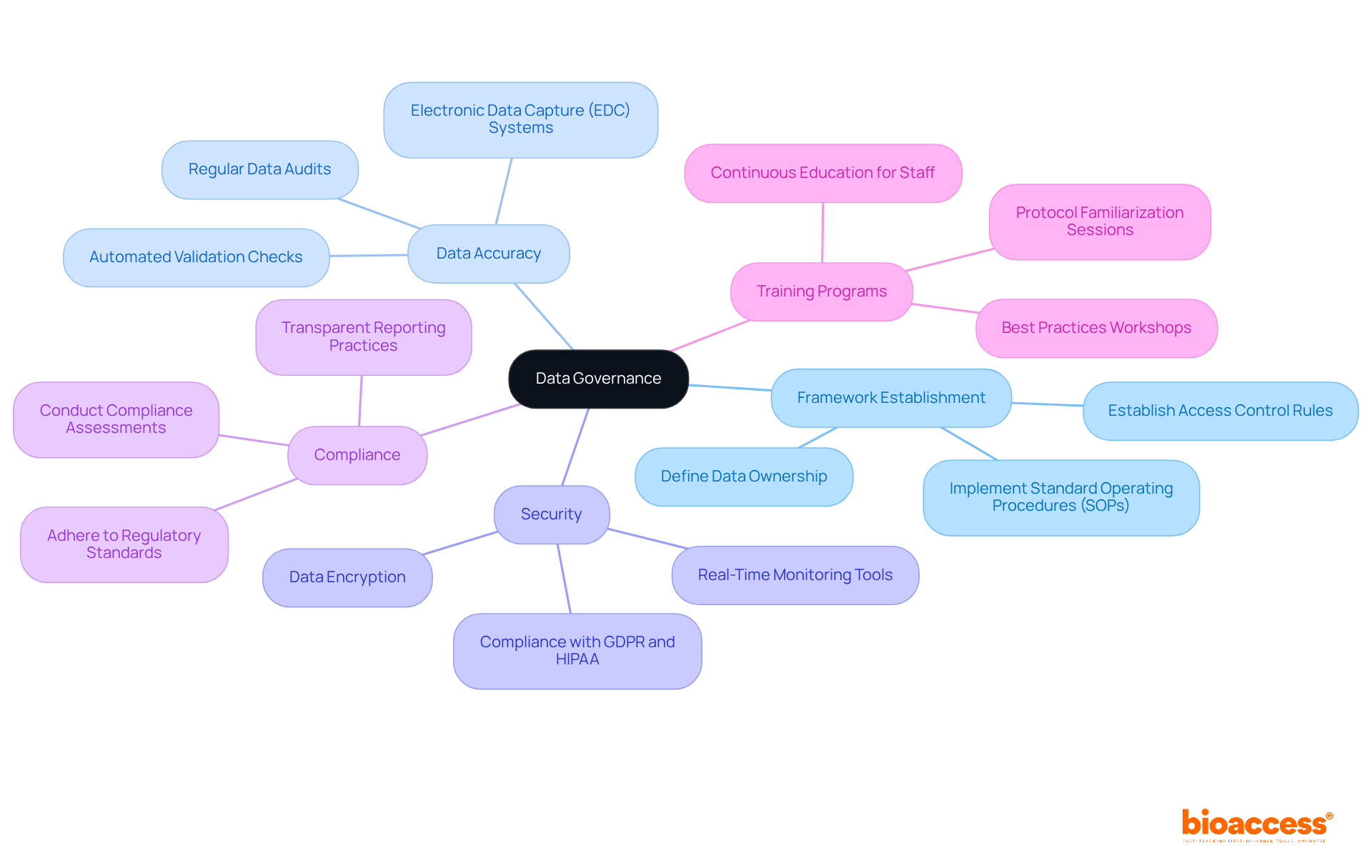
Data governance stands as a cornerstone for adapting to the new FDA submission standards. Sponsors are tasked with establishing comprehensive data governance frameworks to guarantee that all clinical trial data is accurate, secure, and compliant with regulatory requirements. This involves setting clear protocols for data collection, storage, and presentation, alongside implementing robust training programs for all team members on best practices in data management. By prioritizing data governance, sponsors can significantly enhance the reliability of their disclosures and mitigate the risk of compliance issues.
Effective data governance not only streamlines processes but also fosters a culture of accountability, ultimately leading to improved compliance rates in medical research. Moreover, extensive trial management services—including:
play a crucial role in ensuring that trials are conducted efficiently and effectively. These services not only elevate the quality of clinical research but also contribute to local economies through job creation and healthcare improvements.
The recent updates to annual report FDA guidance by the FDA signify a crucial shift from IND annual reports to Development Safety Update Reports (DSURs). This transition not only enhances transparency requirements but also mandates comprehensive data reporting, both of which are vital for improving patient well-being and streamlining the reporting process. DSURs are designed to provide regulators with timely and relevant information, fostering a more robust oversight framework.
By adapting to these changes, sponsors can ensure compliance while significantly enhancing the quality of their research trials. Companies that have embraced these new standards have reported notable improvements in patient safety outcomes, showcasing the tangible benefits of aligning with the FDA's updated requirements. As clinical research leaders acknowledge the importance of these updates, they can better equip their teams to navigate the evolving regulatory landscape effectively.

The updated FDA annual reporting guidance marks a crucial turning point for clinical research directors, underscoring the need for comprehensive compliance strategies. By shifting from IND annual reports to Development Safety Update Reports (DSURs), the FDA seeks to enhance transparency and data accuracy, ultimately bolstering patient safety and regulatory oversight. This transition not only aligns with international standards but also equips organizations to navigate the complexities of clinical trials in an increasingly competitive landscape.
Key insights from the article underscore the significance of robust compliance measures, such as:
Organizations that proactively adapt to these changes can expect improved operational efficiency and a stronger market reputation. Moreover, the focus on transparency cultivates public confidence in medical research, which is vital for patient engagement and recruitment.
As the clinical research environment evolves, embracing these new FDA guidelines transcends mere regulatory compliance; it becomes a strategic imperative. By prioritizing compliance and transparency, sponsors can enhance their research integrity, expedite product approvals, and ultimately contribute to better healthcare outcomes. The call to action is unmistakable: organizations must invest in comprehensive planning and execution to meet the challenges posed by the updated FDA guidance and continue advancing the field of clinical research.
What is bioaccess® and how does it assist companies with FDA compliance?
bioaccess® helps Medtech, Biopharma, and Radiopharma companies navigate the updated FDA annual report guidelines for compliance. Their services include feasibility assessments, selection of research sites and principal investigators, timely updates on study status, inventory management, and reporting of adverse events.
Why is compliance with the new FDA annual reporting guidelines important?
Compliance is crucial as the FDA is focusing on stringent documentation standards, which can significantly impact research timelines and market entry. Adhering to these guidelines helps expedite product approvals and enhances organizational credibility.
What are the key components of the updated FDA annual reporting requirements for IND applications?
The key components include cumulative enrollment figures, demographic distributions of study participants, and a summary of findings from relevant studies.
What is the significance of the Development Safety Update Report (DSUR)?
The DSUR must be submitted no later than 60 calendar days after the data lock point, emphasizing the importance of timely reporting in compliance with FDA requirements.
How does transparency in clinical investigation reporting benefit sponsors?
Enhanced transparency helps build public confidence in medical research, improves patient recruitment and engagement, and ensures stakeholders have access to vital information about study outcomes and safety data.
What impact has bioaccess™ had on subject recruitment and retention in clinical studies?
bioaccess™, in collaboration with GlobalCare Clinical Studies, has achieved over a 50% reduction in subject recruitment duration and a retention rate exceeding 95%, demonstrating the benefits of prioritizing transparent reporting.
How do the new FDA guidelines align with international standards?
The proposed annual report FDA guidance is designed to align with international standards, reflecting a growing commitment to regulatory adherence among sponsors and enhancing the overall quality of clinical research.