


This article delineates the essential insights required for achieving success in Phase 2 clinical studies within the Medtech sector. It underscores the significance of:
Furthermore, it emphasizes the necessity for collaboration among stakeholders, all of which are vital for ensuring timely and effective clinical trial outcomes that can facilitate the successful advancement of medical innovations.
The landscape of clinical study phase 2 represents a pivotal moment for Medtech innovations, where the efficacy and safety of treatments are rigorously evaluated to ascertain their potential for success. As Medtech firms navigate this intricate phase, it becomes essential to grasp the nuances of:
Notably, approximately 30% of drugs fail to progress beyond this stage, prompting an important question: what key insights can drive these trials toward success and ensure that promising therapies reach the market?
bioaccess® effectively leverages its extensive network and regulatory expertise to expedite the clinical study phase 2 trials, facilitating the swift transition of Medtech innovations from concept to market. By harnessing the regulatory agility of Latin America and the diverse patient populations in the Balkans, bioaccess® secures ethical approvals in an impressive 4-6 weeks. This rapid progression is crucial for Medtech firms striving to maintain a competitive edge, as it allows for the quicker collection of vital efficacy and safety data during the clinical study phase 2 compared to traditional markets. Additionally, the total IRB/EC and MoH review process in Colombia takes only 90-120 days, underscoring the efficiency of the regulatory framework in the region.
Moreover, bioaccess® employs advanced project management techniques, including meticulous planning and real-time data tracking, to ensure that every facet of the study is executed with precision. This structured methodology not only accelerates the process but also guarantees adherence to all regulatory standards, reinforcing bioaccess®'s status as a frontrunner in facilitating Medtech innovations. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked, "bioaccess® provided significant benefits during our initial human study in Colombia, illustrating the successful collaboration between local expertise and innovative medical practices." With an average enrollment rate that is 50% faster than conventional methods, bioaccess® is revolutionizing the research landscape within the Medtech sector. Furthermore, Colombia offers financial incentives, such as a 100% tax deduction for investments in science and technology, positioning it as an attractive destination for Medtech firms considering conducting a clinical study phase 2.
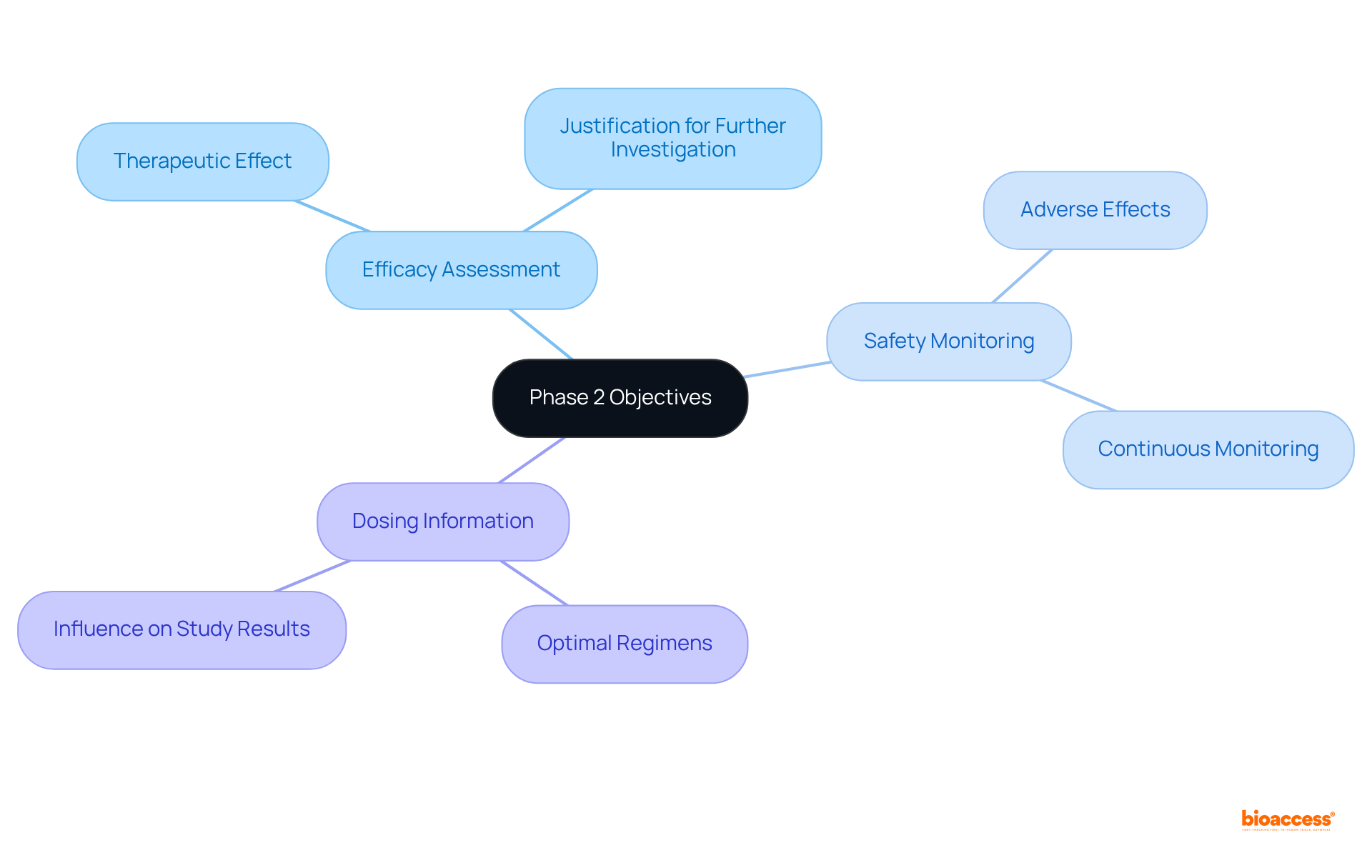
Clinical study phase 2 is pivotal for evaluating the effectiveness and safety of new therapies, typically involving 100 to 300 individuals who have the targeted condition. The primary objectives of this phase encompass:
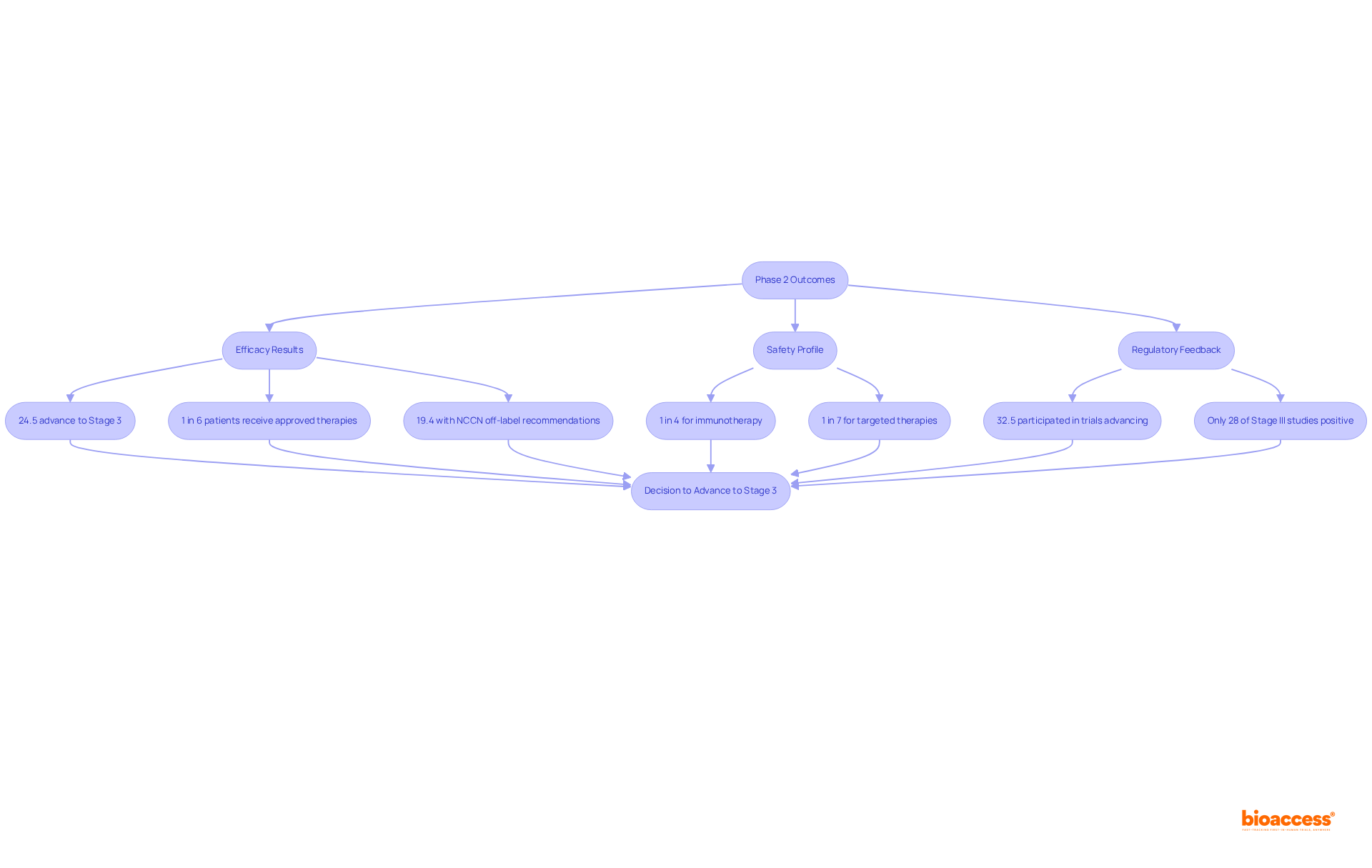
These objectives are not only essential for progressing to clinical study phase 2 evaluations but also play a crucial role in the overall success of medical development. With approximately 33% of medications in clinical study phase 2 assessments advancing to Stage 3, it is noteworthy that over 30% of drugs entering clinical study phase 2 evaluations do not progress, while more than 58% fail in Stage III assessments. The knowledge acquired during this stage is vital for making informed decisions regarding the treatment's future. Furthermore, successful Stage 2 studies often focus on efficient dosing methods and comprehensive safety oversight, underscoring the importance of these factors in the research environment. Stage 2 studies typically last several months to two years, further emphasizing the commitment required in this critical stage.
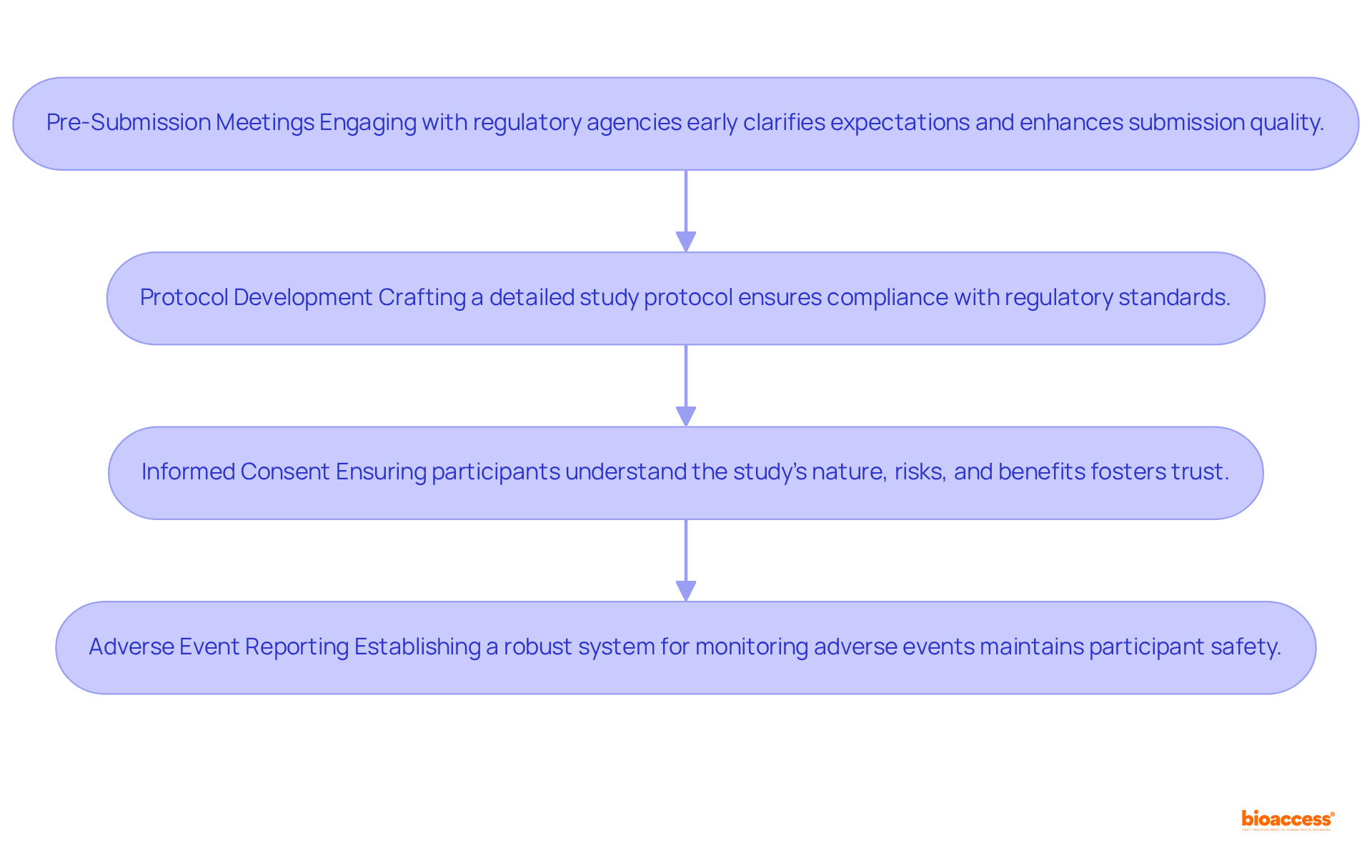
Navigating the regulatory environment for clinical study phase 2 necessitates a comprehensive understanding of the guidelines established by regulatory agencies such as the FDA and EMA. Key considerations include:
By adhering to these regulatory requirements, Medtech companies can mitigate risks and significantly enhance the likelihood of positive outcomes. Engaging regulatory experts early in the process can further clarify complex requirements and improve the chances of successful IND submissions. Notably, bioaccess® achieves regulatory approvals in just 6-8 weeks, significantly faster than the typical 6-12 months in the US/EU, underscoring the potential for expedited processes when navigating these requirements.
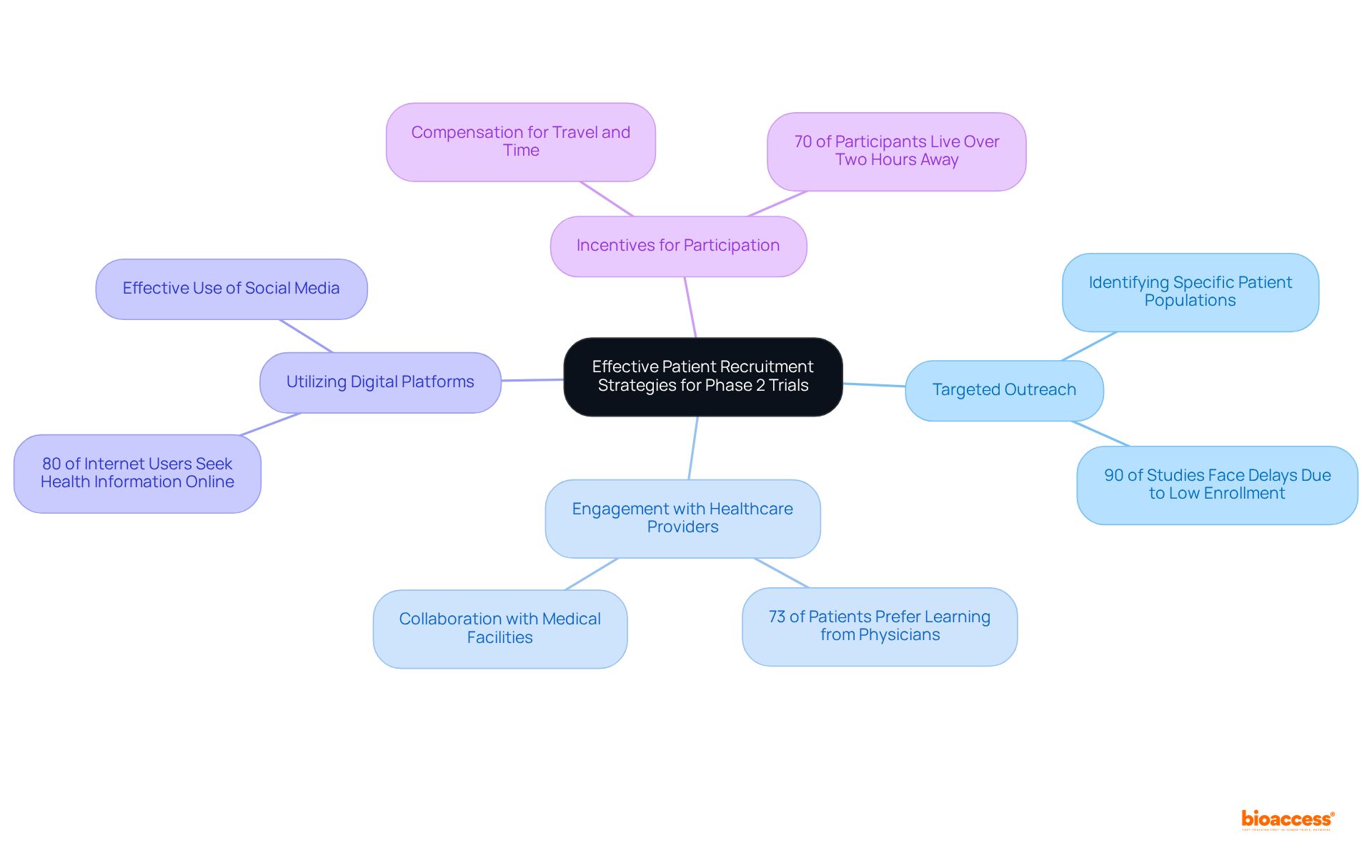
Efficient patient recruitment techniques are essential for the success of the clinical study phase 2. Key approaches include:
By applying these approaches, Medtech firms can enhance enrollment rates and ensure that studies are completed on time, ultimately accelerating medical advancements. Furthermore, bioaccess® achieves enrollment 50% faster than traditional markets, demonstrating the effectiveness of these strategies.
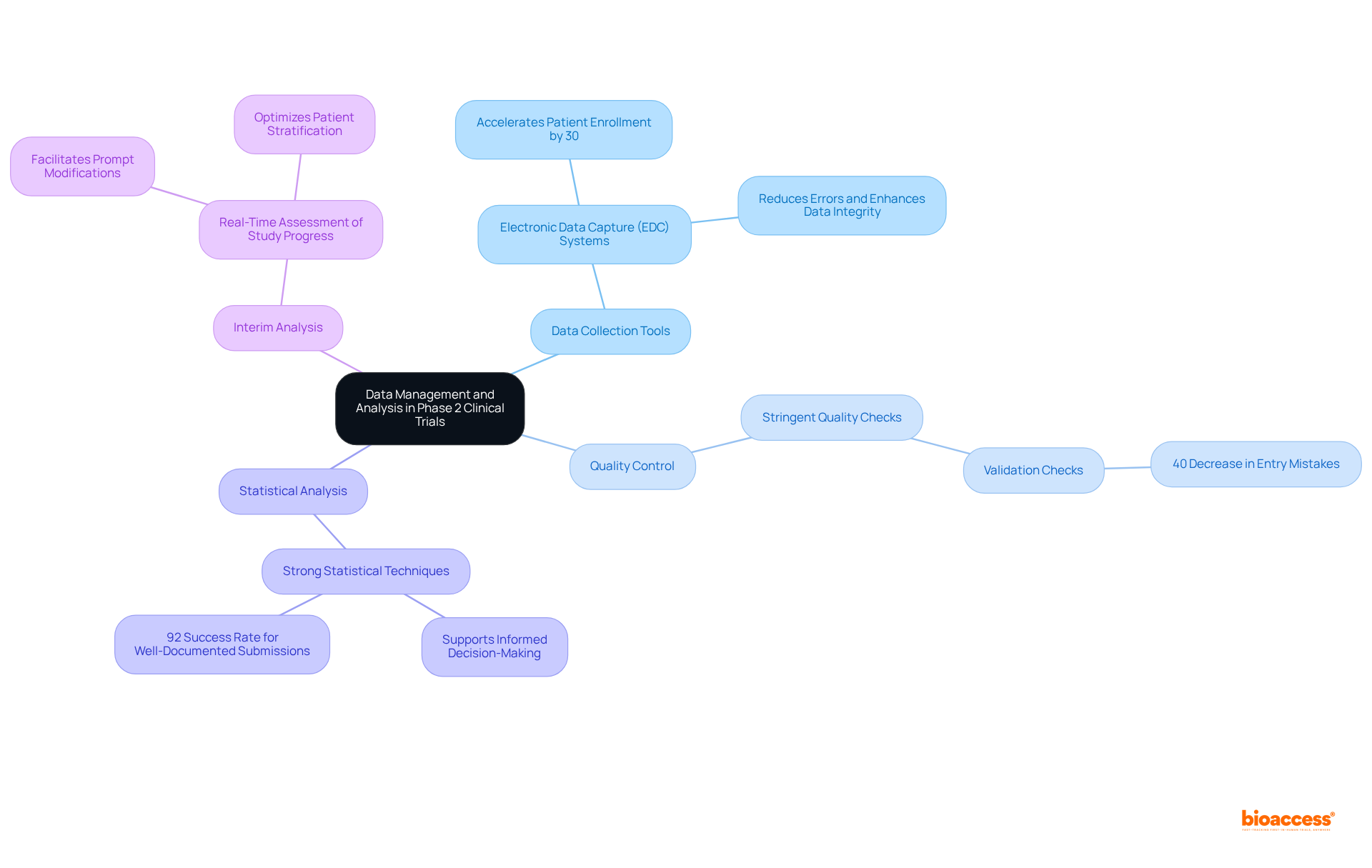
In clinical study phase 2, data management and analysis are crucial, as best practices can significantly influence outcomes. Key strategies include:
Data Collection Tools: Leveraging electronic data capture (EDC) systems streamlines data collection, reduces errors, and enhances data integrity. The adoption of EDC has been shown to accelerate patient enrollment by 30%, as evidenced in various studies, demonstrating its effectiveness in improving trial efficiency.
Quality Control: Implementing stringent quality checks is essential for ensuring accuracy and completeness. This encompasses validation checks that recognize and address discrepancies during information entry, which can result in a 40% decrease in entry mistakes, as demonstrated in the 'Oncology Trial Optimization' case study.
Statistical Analysis: Utilizing strong statistical techniques is essential for examining experimental results and extracting valuable insights. Effective statistical analysis not only supports informed decision-making but also enhances the likelihood of regulatory approval, which has a success rate of 92% for well-documented submissions.
Interim Analysis: Performing interim evaluations permits real-time assessment of study progress, facilitating prompt modifications to protocols based on emerging information trends. This proactive approach can reveal safety trends and optimize patient stratification, ensuring that the most promising subpopulations are targeted.
By prioritizing these information management practices, Medtech companies can significantly enhance the reliability of their research outcomes, ultimately aiding successful regulatory submissions and promoting medical innovations. As Veda Bawo emphasizes, "You can have all of the fancy tools, but if your data quality is not good, you're nowhere.
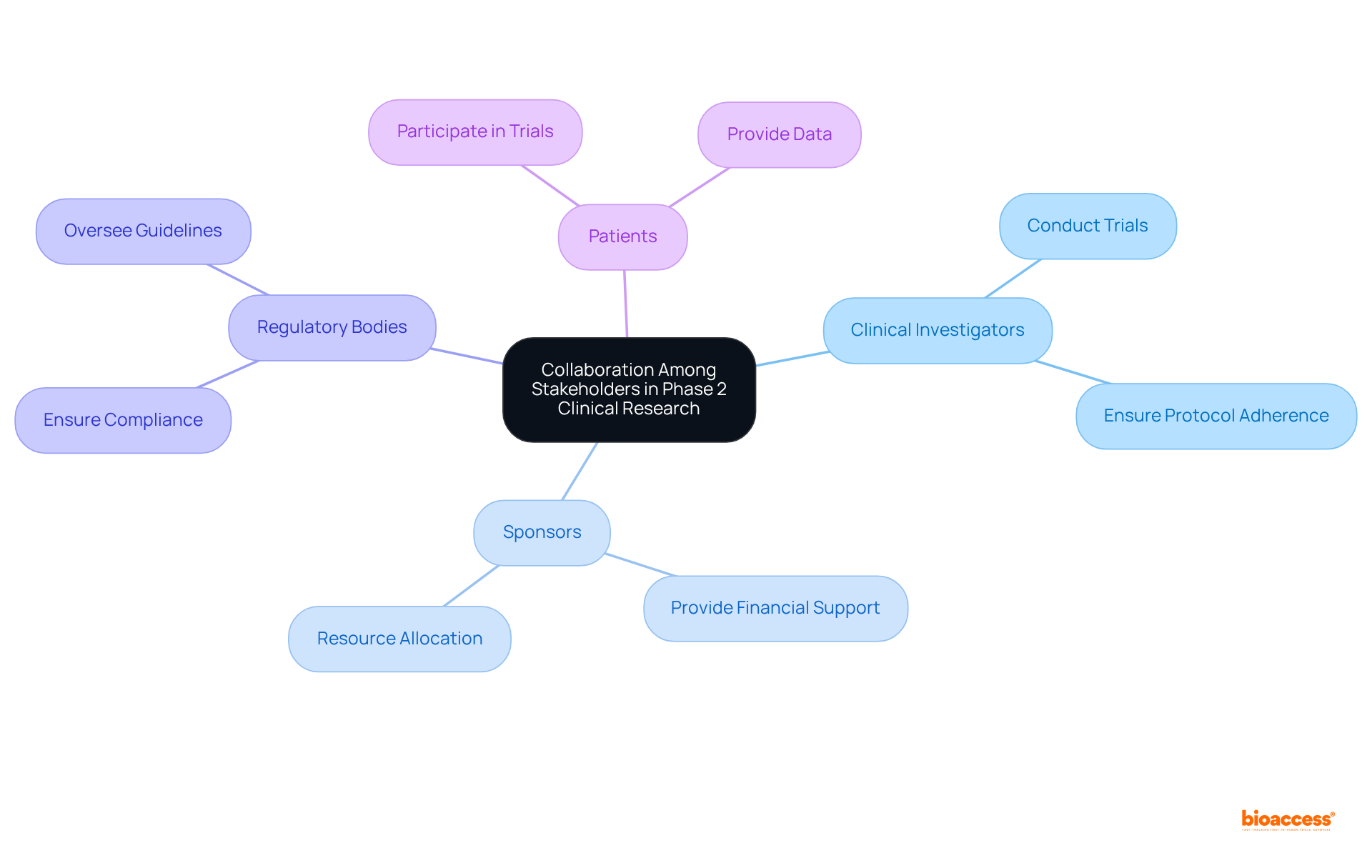
Cooperation among stakeholders is essential for the success of clinical study phase 2. Key stakeholders include:
Effective communication and collaboration among these stakeholders can lead to enhanced results. This synergy fosters a shared understanding of goals and expectations, ultimately driving the success of clinical research initiatives.
The results of the clinical study phase 2 are pivotal in determining whether a treatment progresses to Stage 3 development. Key considerations include the following:
Successful clinical study phase 2 experiments not only lay the groundwork for larger-scale studies but also validate the treatment's efficacy and safety, highlighting their essential role in the drug development process.
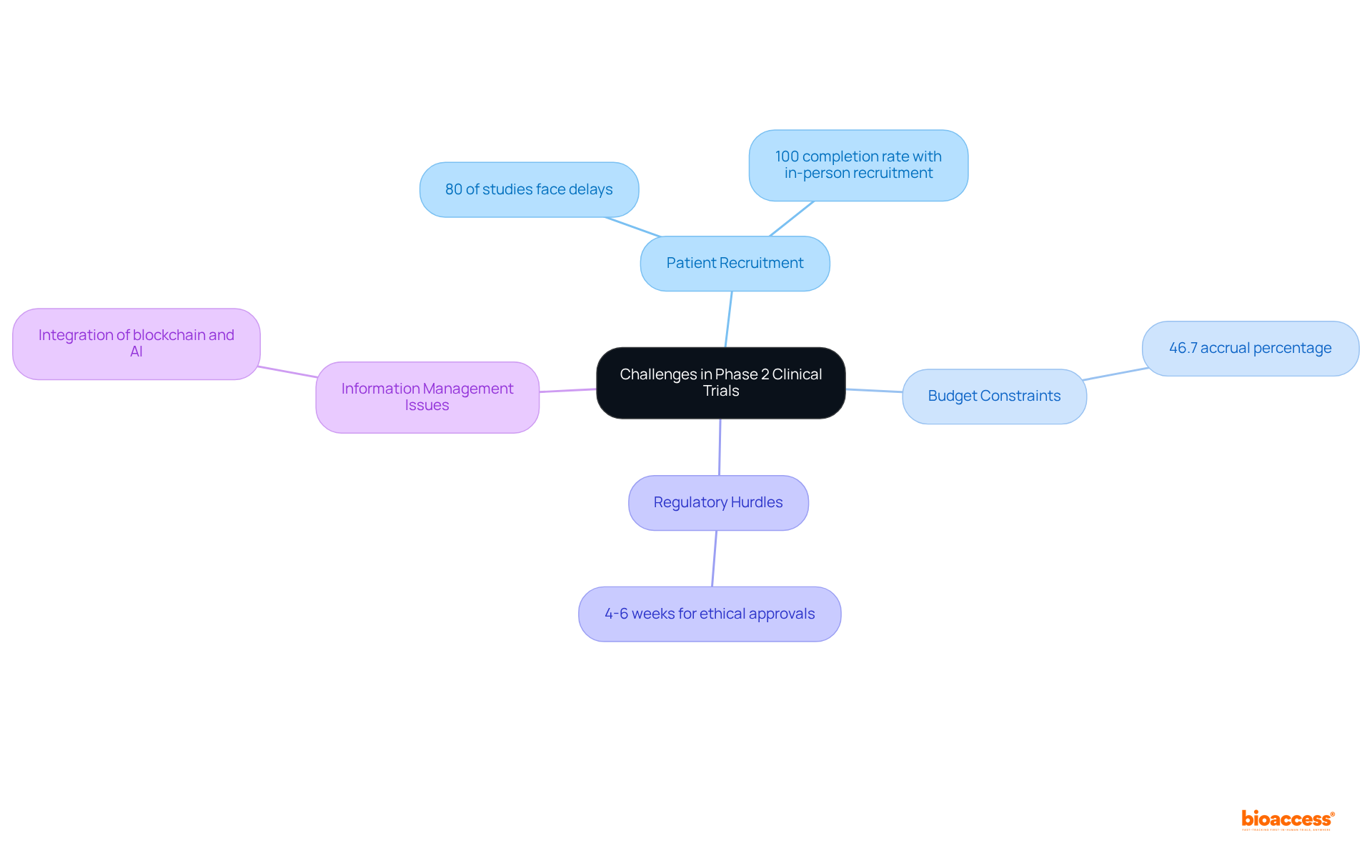
Conducting Phase 2 clinical trials presents several common challenges, including:
Patient Recruitment: Difficulty in finding eligible participants can delay trials. Approximately 80% of clinical studies face delays due to recruitment problems, and only 55% of studies achieve their initially defined recruitment goals. Innovative strategies, such as leveraging digital platforms and community engagement, can enhance recruitment efforts. For instance, in-person recruitment has demonstrated a 100% completion rate among screened participants, showcasing its effectiveness.
Budget Constraints: Limited funding can significantly impact study design and execution. Recruitment budgets for Phase 1 and clinical study phase 2 have increased considerably, necessitating careful allocation of resources to meet enrollment objectives. The average accrual percentage in randomized clinical studies (RCTs) is only 46.7%, underscoring the need for effective budget management to bolster recruitment strategies.
Regulatory Hurdles: Navigating complex regulatory requirements can impede research progress. With over 15 years of experience, organizations like bioaccess® leverage regional regulatory speed to facilitate ethical approvals in just 4-6 weeks, effectively mitigating these challenges.
Information Management Issues: Ensuring information integrity and precision is vital for successful testing. The integration of advanced technologies, such as blockchain and AI, can enhance data management processes, providing secure and efficient solutions for participant monitoring and data collection.
Addressing these challenges requires proactive planning, effective communication, and collaboration among stakeholders to ensure successful results.
Ethical factors in the clinical study phase 2 research are crucial for ensuring participant safety and upholding study integrity. Key ethical principles include:
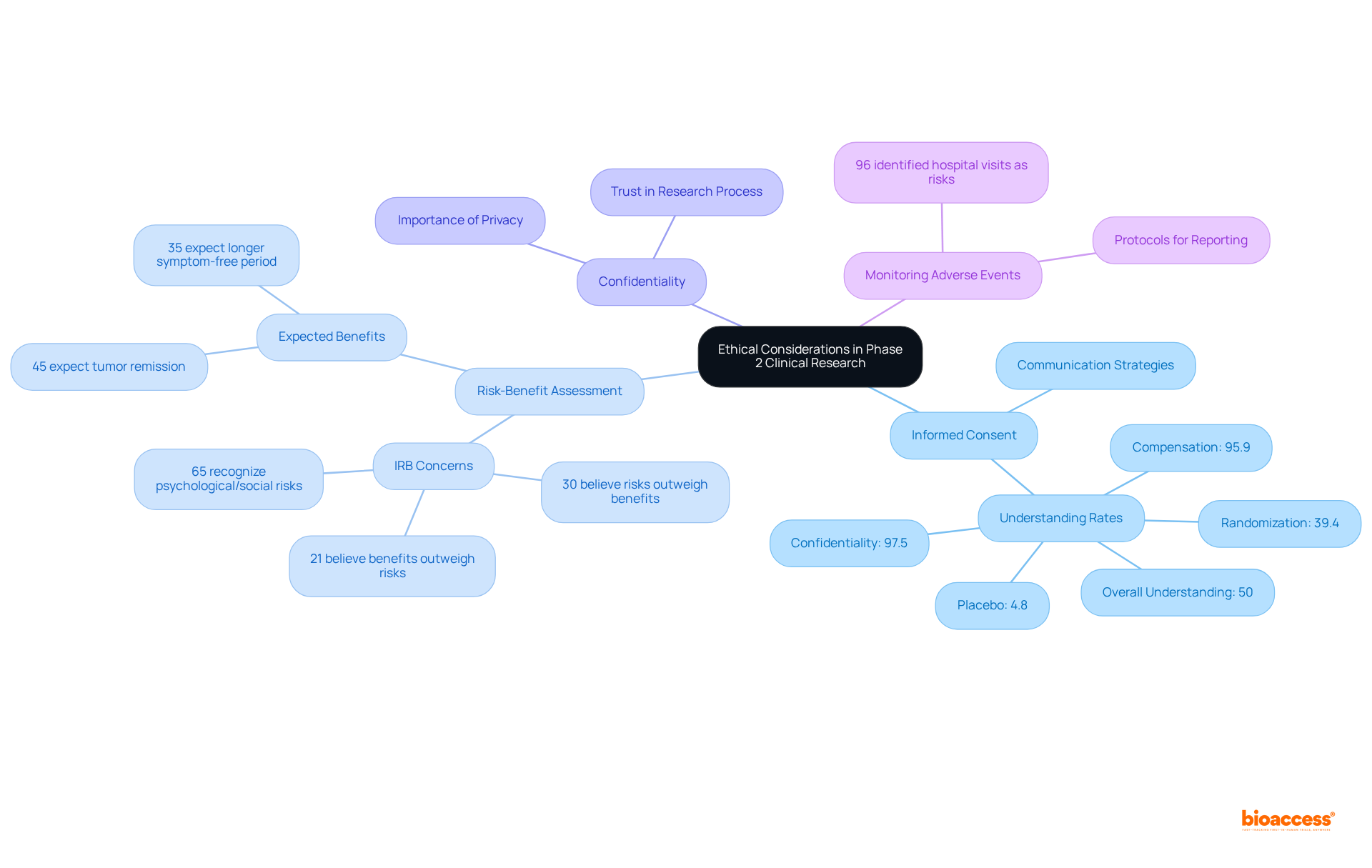
Informed Consent: It is essential that participants are fully informed about the trial's nature, associated risks, and potential benefits prior to enrollment. Despite high rates of understanding regarding confidentiality (97.5%) and compensation (95.9%), comprehension of critical concepts like placebo (4.8%) and randomization (39.4%) remains low. Significantly, only 50% of participants in clinical studies grasped all elements of informed consent, highlighting an urgent requirement for enhanced communication strategies.
Risk-Benefit Assessment: Researchers must rigorously evaluate whether the potential benefits of the treatment outweigh the risks involved. A significant portion of Institutional Review Board (IRB) members (30%) expressed concerns that risks outweighed benefits, while 21% believed benefits outweighed risks. This contrasting perspective underscores the importance of thorough assessments in maintaining ethical standards. Moreover, 65% of respondents recognized psychological and social risks linked to participation in the study, further complicating the risk-benefit landscape.
Confidentiality: Safeguarding participants' privacy and ensuring information confidentiality is essential. This principle not only safeguards individual rights but also fosters trust in the research process.
Monitoring Adverse Events: Establishing robust protocols for monitoring and reporting adverse events is crucial for safeguarding participant welfare. With 96% of respondents identifying hospital visits and extra tests as risks, effective monitoring can mitigate potential harms and enhance participant safety.
Following these ethical guidelines promotes trust and openness in medical research, ultimately boosting the reliability and success of clinical study phase 2.
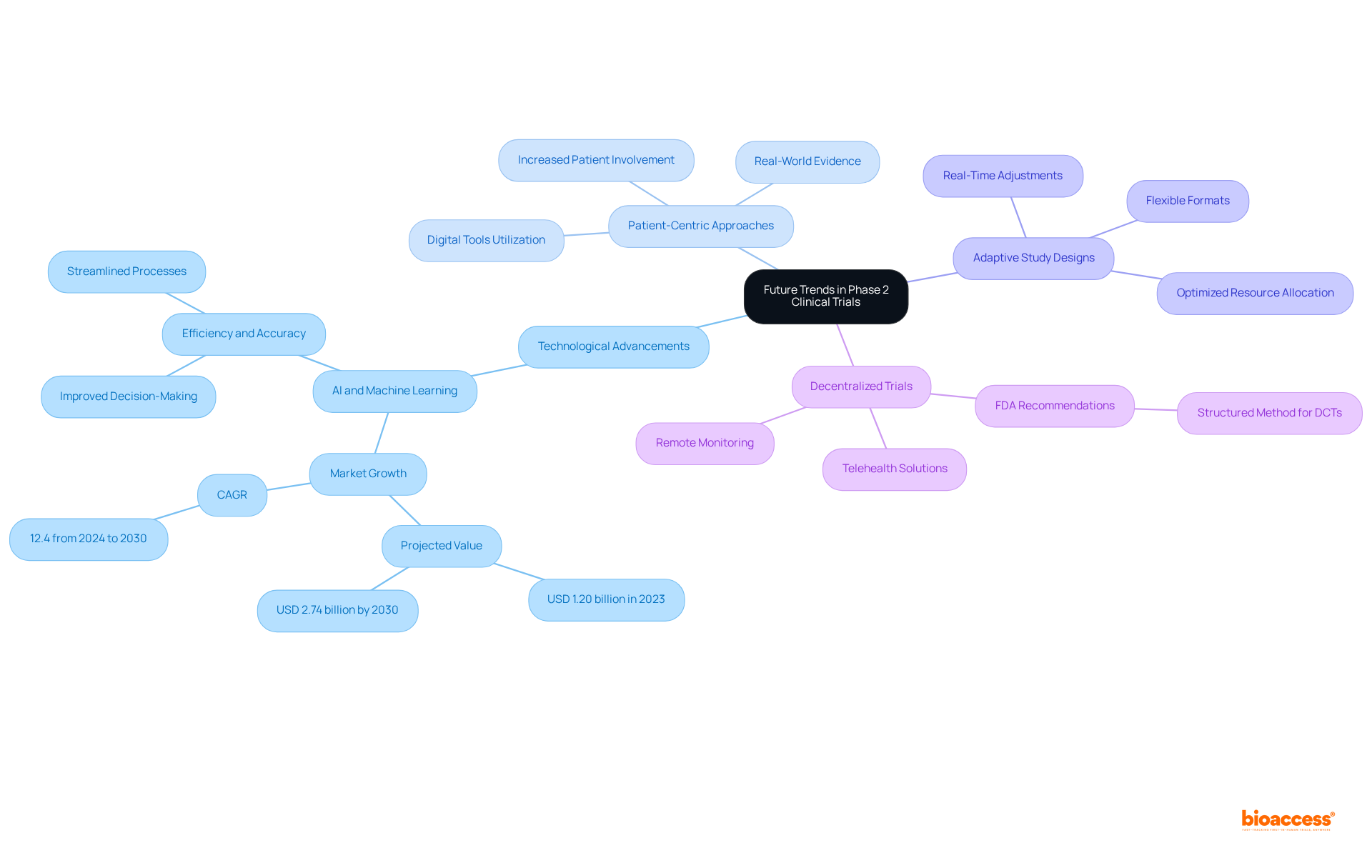
The future of Phase 2 clinical trials is poised to be shaped by several pivotal trends:
Technological Advancements: The incorporation of AI and machine learning into trial design and data analysis is set to significantly enhance efficiency and accuracy. The worldwide AI in clinical studies market is anticipated to expand from USD 1.20 billion in 2023 to USD 2.74 billion by 2030, increasing at a CAGR of 12.4%. These technologies can streamline processes, manage vast data sets, and improve decision-making, ultimately leading to faster and more reliable outcomes.
Patient-Centric Approaches: An increasing focus on patient involvement and experience will guide study designs to better match participants' needs. This shift is evident in the growing utilization of digital tools and real-world evidence to establish more inclusive and accessible studies, which can result in higher enrollment rates and enhanced participant satisfaction. Emphasizing patient engagement in clinical research is critical to improving access, increasing quality, and shortening timelines.
Adaptive Study Designs: The adoption of flexible study formats that allow modifications based on interim results is gaining traction. This approach not only enhances the likelihood of success but also allows for real-time adjustments that can optimize resource allocation and patient outcomes.
Decentralized Trials: The rise of remote monitoring and telehealth solutions is facilitating broader patient participation and streamlining data collection. The FDA released recommendations on the design and execution of decentralized studies (DCTs) in 2023, highlighting the significance of a structured method. Decentralized studies allow researchers to connect with varied populations, thus increasing the representativeness of study samples and enhancing the overall validity of results.
These trends signify a transformative shift towards more efficient, patient-focused, and technologically advanced clinical trials, particularly in the clinical study phase 2, ultimately enhancing the drug development process and addressing the evolving needs of the healthcare landscape.
The significance of Phase 2 clinical trials in the Medtech sector is paramount, serving as a critical juncture for evaluating the efficacy and safety of new therapies. This phase not only determines the viability of treatments but also establishes the groundwork for subsequent development stages. With organizations like bioaccess® at the forefront of expediting these trials, the journey from innovation to market is increasingly streamlined and efficient.
Throughout this discussion, key insights emerge, including:
The collaborative nature of successful Phase 2 trials is emphasized, highlighting the roles of various stakeholders in ensuring that studies meet their objectives while upholding ethical standards. Furthermore, the exploration of emerging trends, such as technological advancements and patient-centric approaches, illustrates the evolving landscape of clinical research.
As the Medtech industry grapples with challenges in patient recruitment and regulatory navigation, the insights provided are invaluable for stakeholders aiming to enhance their clinical study processes. Embracing innovative strategies and fostering collaboration among all parties involved will be essential in overcoming hurdles and driving successful outcomes in Phase 2 trials. The future of clinical research hinges on the ability to adapt and evolve, ensuring that patient safety and treatment efficacy remain at the forefront of medical advancements.
What is bioaccess® and what role does it play in Medtech innovations?
bioaccess® is an organization that leverages its extensive network and regulatory expertise to expedite phase 2 clinical studies for Medtech innovations, facilitating their transition from concept to market.
How quickly can bioaccess® secure ethical approvals for clinical studies?
bioaccess® can secure ethical approvals in an impressive 4-6 weeks, utilizing the regulatory agility of Latin America and diverse patient populations in the Balkans.
What is the average enrollment rate for studies facilitated by bioaccess® compared to conventional methods?
The average enrollment rate for studies facilitated by bioaccess® is 50% faster than conventional methods.
What are the primary objectives of clinical study phase 2?
The primary objectives of clinical study phase 2 are efficacy assessment, safety monitoring, and establishing optimal dosing information.
Why is efficacy assessment important in phase 2 studies?
Efficacy assessment is crucial for determining whether the treatment achieves the desired therapeutic effect, which is necessary for justifying further investigation.
What is the significance of safety monitoring during phase 2 clinical trials?
Continuous safety monitoring ensures patient safety and informs necessary adjustments to the treatment protocol, helping to identify any adverse effects or complications.
What are the key regulatory requirements for conducting phase 2 clinical trials?
Key regulatory requirements include pre-submission meetings with regulatory agencies, detailed protocol development, informed consent from participants, and robust adverse event reporting systems.
How does bioaccess® compare to typical timelines for regulatory approvals in the US/EU?
bioaccess® achieves regulatory approvals in just 6-8 weeks, significantly faster than the typical 6-12 months required in the US/EU.
What financial incentives does Colombia offer to Medtech firms conducting clinical studies?
Colombia offers a 100% tax deduction for investments in science and technology, making it an attractive destination for Medtech firms.
What is the success rate of medications progressing from clinical study phase 2 to phase 3?
Approximately 33% of medications in clinical study phase 2 assessments advance to phase 3. However, over 30% of drugs entering phase 2 evaluations do not progress, and more than 58% fail in phase 3 assessments.