


The article presents crucial insights into the forthcoming EU Clinical Trials Regulation (CTR) and its significant implications for researchers. Set to take effect on January 31, 2025, the CTR aims to streamline the application process for clinical trials across EU member states. This new regulation enhances transparency and expedites the initiation of studies, thereby offering researchers opportunities for more efficient trial management and fostering improved cross-border collaboration.
The landscape of clinical trials is undergoing a significant transformation as the European Union prepares to implement its new Clinical Trials Regulation (CTR) by January 31, 2025. This pivotal change aims to streamline processes, enhance transparency, and foster collaboration among researchers across member states, ultimately promoting faster and more efficient studies.
However, as these regulations unfold, researchers face the pressing challenge of adapting to new compliance requirements while ensuring the integrity and diversity of their trials. What strategies can researchers employ to navigate these changes and seize the opportunities presented by the evolving regulatory environment?
bioaccess® excels in accelerating clinical studies in Latin America, where ethical approvals can be obtained in as little as 4-6 weeks. This rapid turnaround is facilitated by a streamlined regulatory framework, often resulting in patient enrollment occurring 50% faster than in traditional markets.
For instance:
By leveraging local knowledge and navigating the complexities of regional regulations, bioaccess empowers Medtech, Biopharma, and Radiopharma innovators to significantly expedite their research timelines. This efficiency is particularly crucial for first-in-human studies, where timely approvals can profoundly impact the advancement of medical innovations.
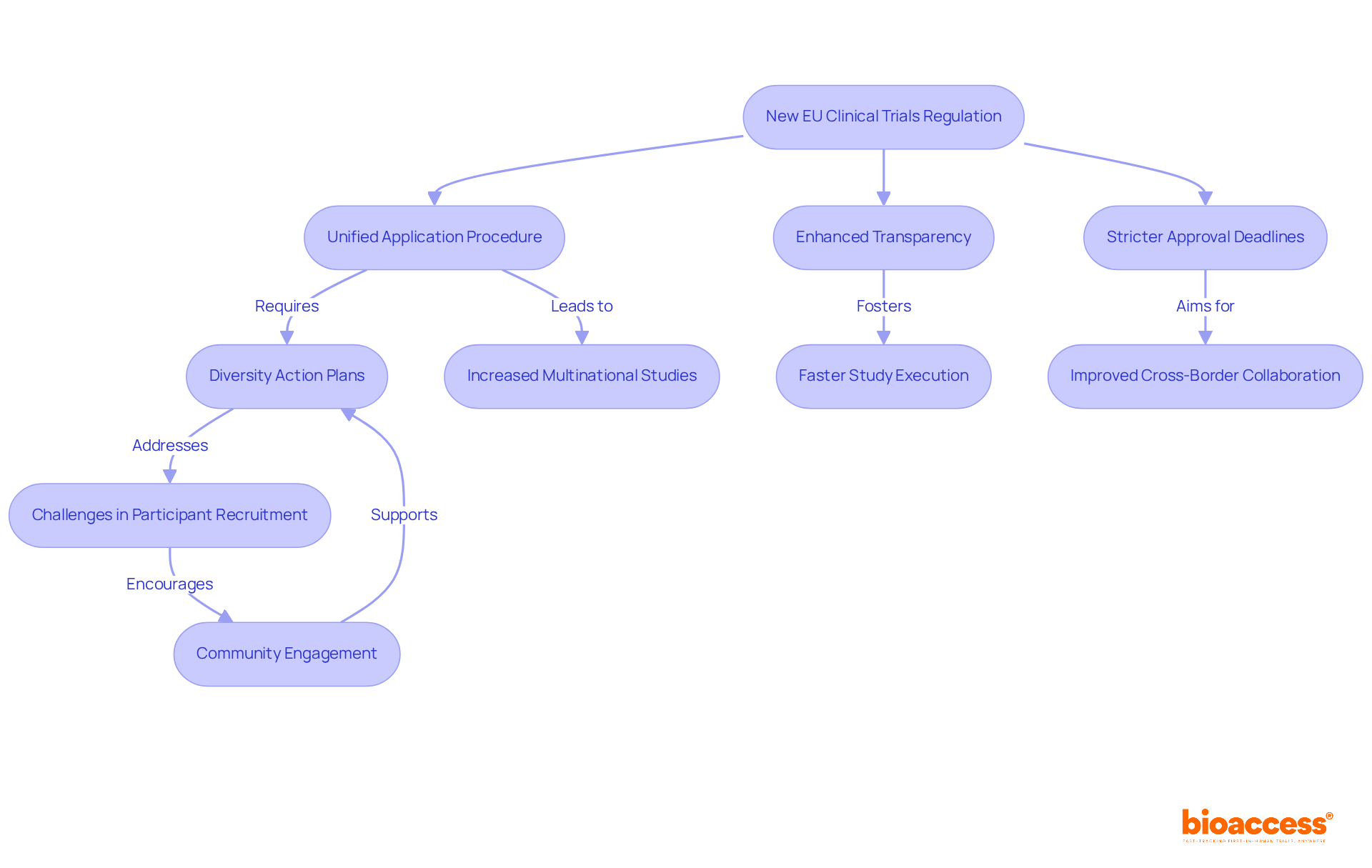
The clinical trial regulation EU, which is the EU Clinical Studies Regulation (CTR), effective from January 31, 2025, marks a pivotal shift in the research landscape across EU member states. This clinical trial regulation EU introduces a unified application procedure for multi-country studies, which is anticipated to streamline submissions and reduce administrative burdens. Enhanced transparency standards will foster greater accountability, while more stringent approval deadlines under clinical trial regulation EU aim to expedite the initiation of research studies. Researchers must adapt to the clinical trial regulation EU, as these regulations offer opportunities for faster study execution and improved cross-border collaboration.
Dr. Stacie Bell underscores the necessity for a proactive approach from researchers, particularly in crafting diversity action plans to bolster participant recruitment. The clinical trial regulation EU aims to create a more favorable environment for research studies, targeting an increase of 500 multinational studies over the next five years. This ambitious objective reflects a commitment to revitalizing the EU's research testing framework, which has seen a decline in its global share over the past decade.
As researchers navigate the clinical trial regulation EU, they must also account for the implications of the Clinical Studies Information System (CTIS), which became mandatory for initial study applications as of January 31, 2023. The integration of CTIS is poised to enhance data sharing and transparency, ultimately benefiting the research community. By embracing the clinical trial regulation EU changes, researchers can position themselves to leverage streamlined processes for more efficient research management and execution.
Currently, an average of 900 medical studies receive approval annually, and the implementation of the CTR is expected to significantly increase this number. However, researchers must also confront the challenges of recruiting diverse participants. Coker highlights that trust issues complicate engagement, suggesting that by focusing on practical strategies—such as community involvement and tailored recruitment initiatives—researchers can enhance diversity in medical studies and improve overall outcomes. Furthermore, utilizing comprehensive research management services, such as those offered by bioaccess, can facilitate feasibility assessments, site selection, compliance evaluations, study setup, and project oversight. This approach addresses patient recruitment challenges and can achieve faster participant enrollment—up to 50% quicker—while saving $25K per patient with FDA-ready information.
The Clinical Trials Information System (CTIS) serves as a centralized platform for submitting and managing clinical study applications in compliance with clinical trial regulation EU. This system streamlines the application process, enabling sponsors to submit a single application for multiple member states. Furthermore, CTIS enhances data transparency by making study information publicly accessible, fostering trust among stakeholders. Researchers must familiarize themselves with CTIS functionalities to refine their study management strategies and ensure compliance with the clinical trial regulation EU.
In addition, with bioaccess®'s innovative approach, regulatory approval can be achieved in just 6-8 weeks, a significant improvement compared to the typical 6-12 months required in the US/EU. This acceleration facilitates the enrollment of treatment-naive cardiology or neurology cohorts at a pace 50% faster than that of Western sites, effectively addressing common patient recruitment challenges faced by medtech and biopharma startups. By leveraging bioaccess®'s extensive management services for studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—researchers can navigate the complexities of early-phase research more efficiently.
The harmonization of clinical trial regulation EU is designed to reduce administrative burdens and enhance the efficiency of trial approvals. This initiative presents several advantages, including quicker participant recruitment and a streamlined regulatory process, which can lead to faster access to innovative treatments. bioaccess® plays a pivotal role in this landscape by providing expedited participant recruitment and site activation services, having activated over 50 pre-qualified sites in under eight weeks. This capability is essential for addressing the challenges posed by participant recruitment delays, which cost the pharmaceutical industry billions and hinder access to new therapies, emphasizing the need for effective navigation through these regulatory complexities.
Moreover, the General Data Protection Regulation (GDPR) has implemented stricter protocols, increasing the administrative burdens for researchers and causing project delays. As Paloma Moraga Alapont pointed out, "Inadequate enrollment of participants can precipitate delays, escalated costs, and compromise scientific integrity."
To fully harness the benefits of harmonization, researchers must adopt proactive strategies. Engaging specialized consulting services, such as those offered by bioaccess®, can provide crucial assistance in navigating the complexities of clinical trial regulation EU. This includes:
Additionally, fostering collaboration among stakeholders—including researchers, policymakers, and patient advocacy groups—can significantly enhance the overall efficiency of study processes and improve patient involvement. As the EU strives to re-establish itself as a competitive center for medical research, addressing these challenges will be vital for promoting innovative health solutions.

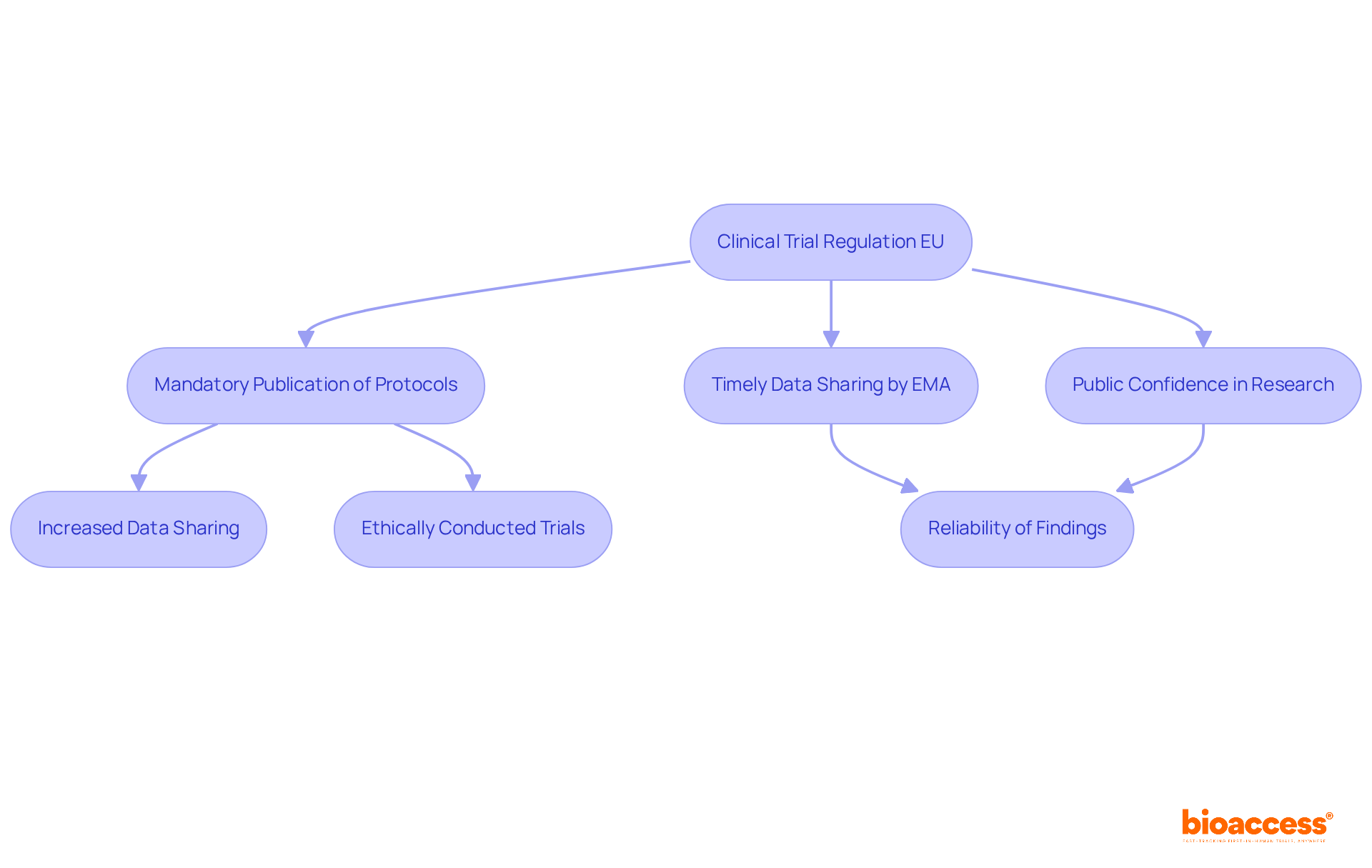
The recent clinical trial regulation EU underscores the vital importance of openness in clinical trials by mandating the publication of trial protocols and outcomes in the Clinical Trials Information System (CTIS). This initiative aims to bolster public confidence in medical research, ensuring that findings are both accessible and ethically conducted. By enforcing transparency, the EU endeavors to cultivate a collaborative environment where researchers can share data more effectively, ultimately enhancing the integrity of medical research.
For instance, studies indicate that trials with published protocols are significantly more likely to report their results, with approximately 36% of trials failing to publish their main findings despite having protocols available. This disparity underscores the necessity for strict adherence to transparency practices.
Furthermore, the European Medicines Agency (EMA) has committed to releasing trial data within established timelines, highlighting the importance of timely information sharing. Such measures not only improve the quality of medical research but also instill public confidence in the regulatory process, as stakeholders can rely on the comprehensiveness and dependability of shared data.
In summary, these guidelines represent a crucial step toward a more transparent and reliable clinical research environment in accordance with clinical trial regulation EU.
To effectively navigate compliance with clinical trial regulation EU, sponsors must implement several strategic actions. Comprehensive training programs for staff on the new guidelines are essential. These programs should focus on the nuances of the clinical trial regulation EU and the common compliance challenges faced by sponsors, such as the need for timely reporting and adherence to ethical standards. Studies show that only 49.5% of experiments reported outcomes, emphasizing the essential need for enhanced training and awareness among clinical research experts.
Utilizing the Clinical Trials Information System (CTIS) for all submissions is another vital step in accordance with clinical trial regulation EU. This platform streamlines the submission process and enhances transparency, allowing sponsors to maintain compliance more effectively. Establishing clear communication channels with regulatory bodies is equally important for adhering to clinical trial regulation EU, as it fosters collaboration and ensures that any regulatory updates are promptly addressed.
Moreover, developing a robust compliance framework is crucial in accordance with clinical trial regulation EU. This framework should incorporate regular audits and updates to research protocols to align with evolving regulatory requirements. Best practices indicate that sponsors who prioritize training and compliance not only enhance their operational efficiency but also improve their overall success rates. As John F. Kennedy aptly stated, 'Leadership and learning are indispensable to each other,' emphasizing the significance of ongoing education in the research landscape.
Furthermore, utilizing extensive research management services, like those provided by bioaccess, can greatly assist in feasibility studies, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. With professionals such as Katherine Ruiz, who focuses on regulatory matters for medical devices and in vitro diagnostics in Colombia, sponsors can maneuver through the intricacies of research studies with increased confidence and success.
The ethics review process stands as a cornerstone of clinical study integrity, ensuring that research is conducted ethically while safeguarding participant rights. Under the clinical trial regulation EU, ethics committees are mandated to assess study protocols in alignment with local laws and ethical standards. Engaging these committees early in the study design phase is imperative for addressing potential ethical issues and ensuring compliance with regulatory standards.
Ethics encompasses all facets of a medical study, extending beyond informed consent procedures, thereby highlighting the comprehensive role of ethics committees. A uniform model across EU Member States based on clinical trial regulation EU would foster standardized procedures and similar protection standards, thereby enhancing the overall efficacy of the ethics review process.
Moreover, implementing best practices for ethics committees is essential to guarantee adequate protection and cultivate community trust. Successful collaboration with ethics committees can facilitate smoother approval processes, as evidenced by the introduction of a single submission procedure that alleviates administrative burdens and bolsters communication.
By fostering a cooperative relationship with ethics committees, researchers can navigate the complexities of ethical oversight more effectively, ultimately contributing to the success of their medical studies.
With the clinical trial regulation EU poised to fully take effect, it is imperative that ongoing clinical studies transition to meet the new requirements by January 31, 2025. This transition poses significant challenges, including:
A comprehensive transition plan is essential for researchers to navigate these complexities effectively. For instance, the expedited transition application process aims to reduce documentation requirements and shorten review timelines; however, sponsors should be prepared for potential delays, as EU member states may not consistently adhere to these timelines.
Furthermore, the introduction of the clinical trial regulation EU is expected to simplify administrative processes, but it necessitates careful updates to research protocols to ensure compliance. As highlighted by industry specialists, adapting to these new guidelines is crucial for preserving the integrity and continuity of clinical studies. Researchers must prioritize participant convenience and ensure that their study designs align with the evolving regulatory landscape to enhance participant engagement and retention.
Effective participant recruitment strategies are critical under the clinical trial regulation EU, which underscore the necessity for timely trial initiation. bioaccess® offers expedited recruitment and site activation services, having successfully activated over 50 pre-qualified locations in under eight weeks. Researchers must leverage digital platforms and social media to connect with potential participants, engage advocacy groups, and utilize registries to identify eligible candidates. Given the hesitance of prospective patients and the financial constraints faced by Medtech and Biopharma startups, it is essential to provide transparent information about the advantages of studies and address concerns to enhance recruitment initiatives and ensure diverse participant representation. By utilizing bioaccess®'s FDA/EMA/MDR-compliant datasets and centralized monitoring, medical studies can effectively navigate common recruitment challenges, streamlining the process for Medtech and Biopharma startups.
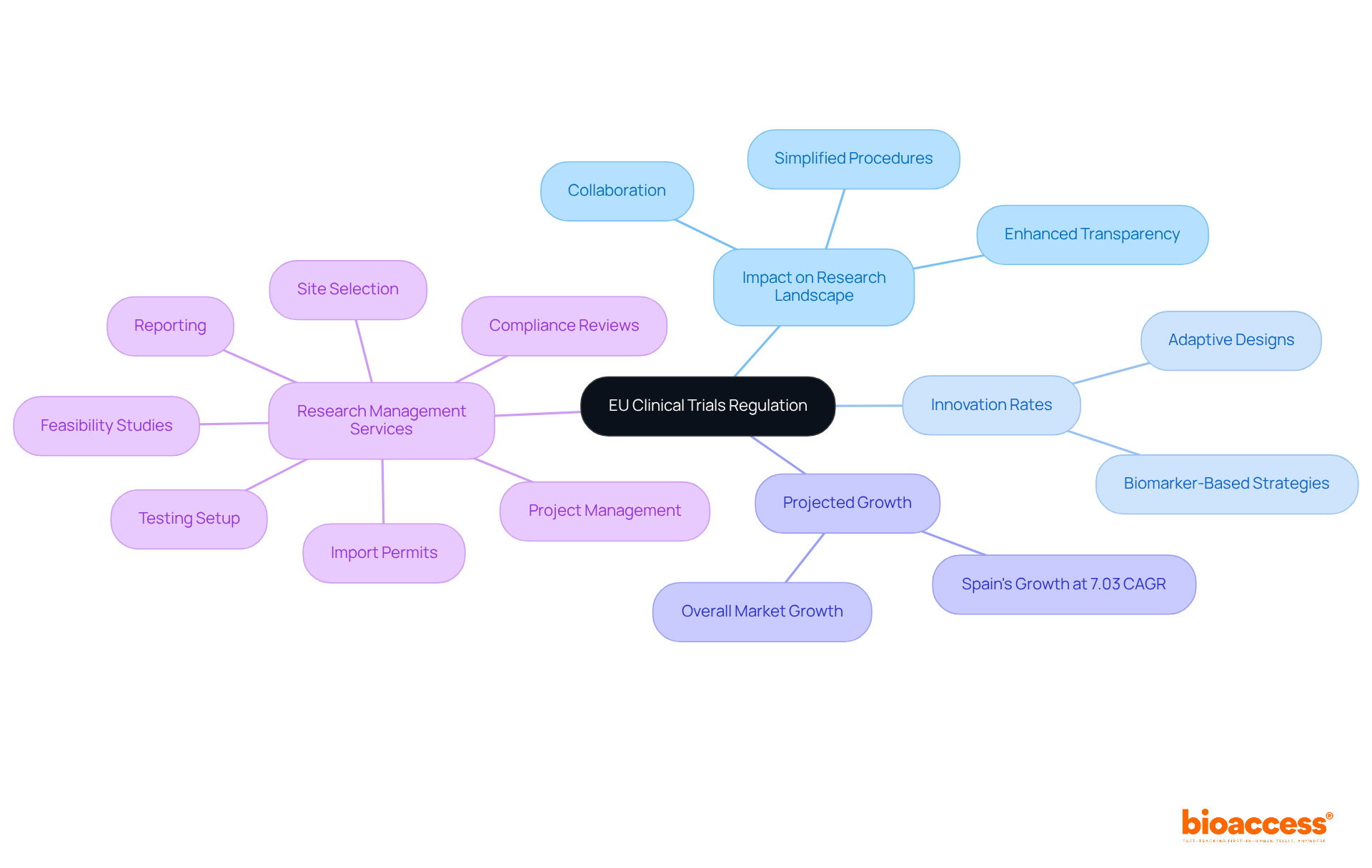
The clinical trial regulation EU is set to significantly transform the research landscape in Europe. By simplifying procedures and enhancing transparency, this policy aims to establish Europe as a leading hub for clinical studies in accordance with the clinical trial regulation EU. Since its implementation, there has been a notable increase in innovation rates, with numerous sponsors adopting adaptive designs and biomarker-based enrollment strategies that facilitate more efficient proof-of-concept validation. For instance, the launch of a centralized online platform under the Clinical Trials Information System (CTIS) has streamlined the submission process for multinational studies, reducing administrative burdens by as much as 50%.
Moreover, the regulation fosters collaboration among stakeholders, nurturing an environment where innovative trial methodologies can flourish. Nations such as Spain are projected to expand at a 7.03% CAGR until 2030, driven by economical enrollment procedures and robust supporter advocacy networks. This growth mirrors the broader trend of enhanced patient access to new therapies, a direct outcome of the regulatory changes.
In this evolving landscape, comprehensive research study management services, such as those offered by bioaccess, play a crucial role. These services encompass:
By ensuring that experiments are conducted effectively and in accordance with guidelines, bioaccess contributes significantly to the overall success of research initiatives.
Looking ahead to 2025 and beyond, the environment for research trials in Europe is expected to progress further, with ongoing adjustments to the regulatory framework. As the European medicines regulatory network continues to monitor and refine these regulations, researchers must remain vigilant and adaptable to seize the opportunities that arise from this dynamic environment. The long-term impacts of the clinical trial regulation EU are likely to encompass not only increased efficiency and transparency but also a sustained boost in innovation across the clinical research sector.
The clinical trial regulation EU heralds a transformative approach to research within Europe, introducing significant changes that promise to enhance the efficiency, transparency, and ethical standards of clinical studies. As these new regulations take effect in 2025, researchers must adapt to a unified application process, streamlined approvals, and heightened transparency requirements that collectively aim to rejuvenate the EU's position in the global research landscape.
Key insights from the regulation underscore the necessity of proactive strategies in participant recruitment and compliance management. The integration of the Clinical Trials Information System (CTIS) is poised to revolutionize trial management by simplifying submissions and promoting data sharing, while the emphasis on diversity in participant demographics reflects an ongoing commitment to ethical research practices. Moreover, engaging with ethics committees and utilizing specialized consulting services can significantly enhance the efficiency of study processes.
As the landscape of clinical research evolves, embracing these changes is crucial for researchers striving to maintain competitiveness. The long-term implications of the EU clinical trial regulation are profound, with the potential to not only accelerate the pace of innovation but also restore public trust in medical research. By prioritizing transparency and collaboration, the research community can effectively navigate these new regulations, ultimately leading to improved patient outcomes and a more robust framework for future studies.
What is bioaccess and how does it impact clinical trials in Latin America?
Bioaccess is a service that accelerates clinical studies in Latin America, enabling ethical approvals to be obtained in as little as 4-6 weeks. This rapid process is supported by a streamlined regulatory framework, allowing for patient enrollment to occur 50% faster than in traditional markets.
Can you provide examples of quick ethical approvals obtained by companies in Latin America?
Yes, for instance, Mitralign obtained ethical approval in only 18 days in Colombia, and Axoft secured testing permission in 60 days in Panama.
What are the key changes introduced by the EU Clinical Trials Regulation (CTR)?
The CTR introduces a unified application procedure for multi-country studies, aims to streamline submissions, reduce administrative burdens, enhance transparency, and enforce more stringent approval deadlines to expedite research initiation.
When will the EU Clinical Trials Regulation take effect?
The EU Clinical Trials Regulation will take effect on January 31, 2025.
What is the Clinical Trials Information System (CTIS) and its significance?
The CTIS is a centralized platform for submitting and managing clinical study applications in compliance with the EU CTR. It streamlines the application process and enhances data transparency by making study information publicly accessible.
How can researchers benefit from the changes in the EU clinical trial landscape?
Researchers can benefit from faster study execution, improved cross-border collaboration, and an increase in the number of multinational studies, with a target of 500 new studies over the next five years.
What challenges do researchers face in participant recruitment under the new regulations?
Researchers face challenges related to recruiting diverse participants, as trust issues can complicate engagement. Strategies such as community involvement and tailored recruitment initiatives are suggested to enhance diversity.
How does bioaccess help address patient recruitment challenges?
Bioaccess provides comprehensive research management services, including feasibility assessments, site selection, compliance evaluations, and project oversight, which can lead to faster participant enrollment—up to 50% quicker—and save costs.
What is the typical timeline for regulatory approval with bioaccess compared to the US/EU?
Bioaccess can achieve regulatory approval in just 6-8 weeks, significantly faster than the typical 6-12 months required in the US/EU.