


The article titled "10 Key Insights on Clinical Trials for Medical Devices" presents crucial elements that significantly enhance the efficiency and effectiveness of clinical trials within the medical device sector. It underscores the importance of:
This optimization is vital for accelerating the market entry of innovative medical devices, addressing key challenges within the Medtech landscape. As such, the insights provided serve as a valuable resource for stakeholders aiming to navigate the complexities of clinical research.
The landscape of clinical trials for medical devices is rapidly evolving, particularly in regions like Latin America. Here, regulatory efficiencies and diverse patient populations create unique opportunities for Medtech innovators. As the demand for faster evaluations intensifies, understanding the intricacies of these trials becomes essential for stakeholders aiming to navigate the complexities of bringing new technologies to market.
However, with challenges such as participant recruitment, regulatory compliance, and ethical considerations looming large, how can companies ensure their clinical trials not only meet but exceed expectations? This article delves into ten key insights that illuminate the path forward in the realm of medical device trials, offering valuable strategies and perspectives for success.
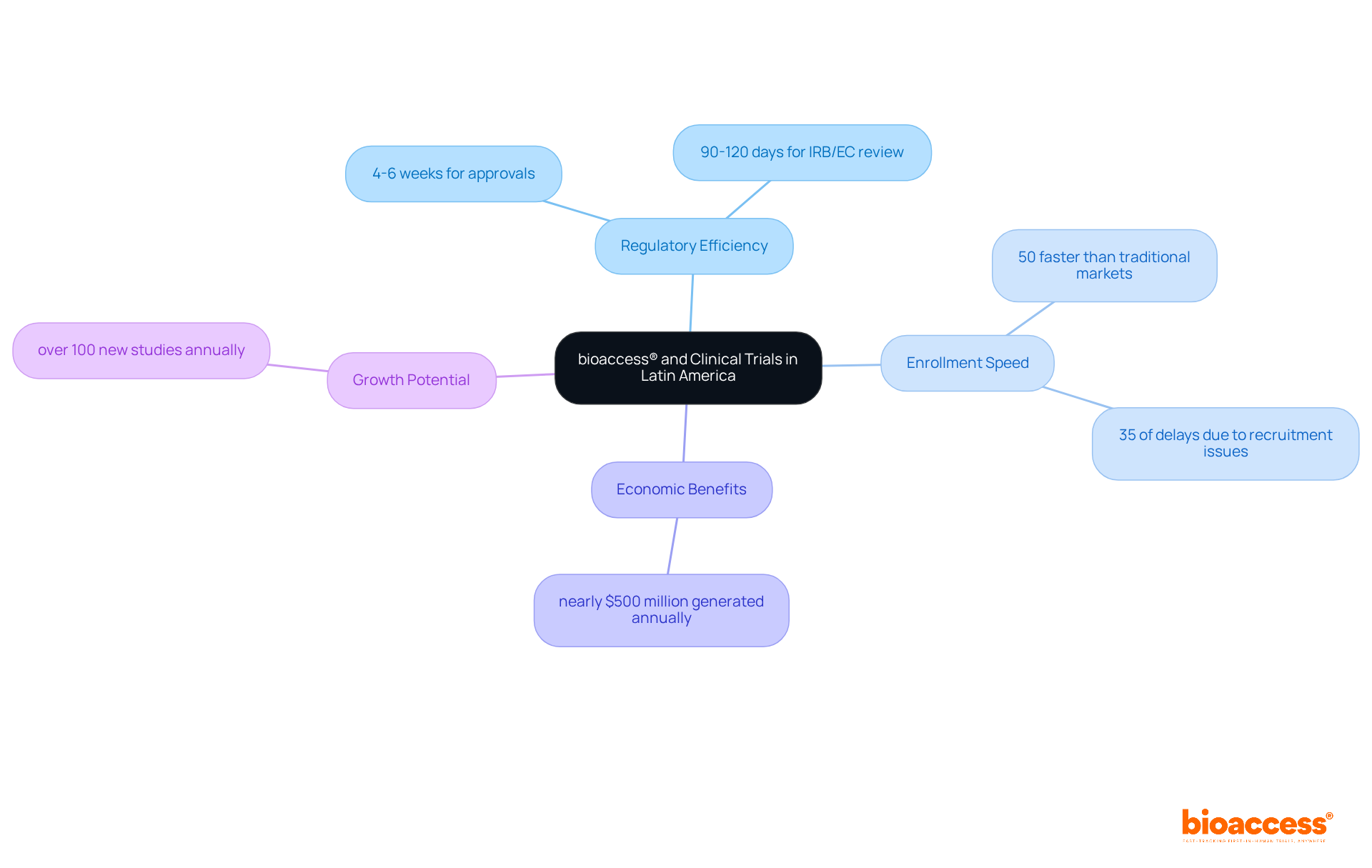
bioaccess® excels in optimizing clinical trials for medical devices by leveraging the regulatory efficiency of Latin America and its diverse patient populations. With ethical approvals secured in just 4-6 weeks and enrollment procedures that are 50% faster than traditional markets, bioaccess® stands out as a pioneer in accelerating the research process. This efficiency is vital for Medtech innovators conducting clinical trials for medical devices, aiming to bring their products to market swiftly and effectively. Medtech executives emphasize that regulatory pace is essential for maintaining a competitive edge, especially as R&D investment in medtech increases by 10 percent annually, highlighting the growing demand for quicker evaluations.
By capitalizing on this rapid regulatory environment, bioaccess® has successfully facilitated numerous research studies, showcasing its ability to adapt to the unique needs of the region. Colombia, recognized for its robust healthcare system ranked 22nd by the WHO and regarded as one of the best in Latin America, has emerged as a hub for research studies, attracting significant foreign investment and generating nearly $500 million in economic benefits each year. Furthermore, investments in science, technology, and innovation projects in Colombia are bolstered by R&D tax incentives, including a 100% tax deduction and a 25% tax discount. The country offers cost savings of over 30% compared to North America and Western Europe, with its total IRB/EC and MoH (INVIMA) review process taking only 90-120 days.
Experts agree that Colombia could see more than 100 new research studies annually, further underscoring the region's potential for growth. Additionally, with 35 percent of study delays attributed to inadequate participant recruitment, bioaccess®'s expedited enrollment processes are crucial for overcoming these challenges. Collectively, these factors position bioaccess® as an indispensable partner for Medtech firms striving to navigate the complexities of clinical trials for medical devices in Latin America.
Navigating the regulatory landscape is crucial for the success of clinical trials for medical devices related to health technologies. Each country presents distinct regulations that must be adhered to, including pre-market submissions, clinical trial applications, and post-market surveillance. For instance, the 510(k) process in the U.S. offers a faster pathway to market for Class II products, typically taking about 90 days, compared to the 180 days required for Pre-Market Approval (PMA) for Class III items. This disparity underscores the importance of selecting the appropriate regulatory pathway based on product classification.
In Latin America, regulatory frameworks are undergoing significant evolution, with many countries streamlining their processes to attract innovative medical technologies. Engaging with local regulatory authorities early in the process can greatly enhance the likelihood of obtaining timely approvals and minimizing potential delays. Statistics indicate that compliance issues remain a challenge in clinical trials for medical devices, highlighting the need for thorough preparation and a deep understanding of local regulations.
Expert insights suggest that a proactive approach to regulatory involvement not only aids in compliance but also optimizes the planning of testing schedules. By capitalizing on the unique regulatory advantages presented by regions such as Latin America, Medtech and Biopharma companies can expedite their clinical trials for medical devices and more effectively bring their innovations to market.

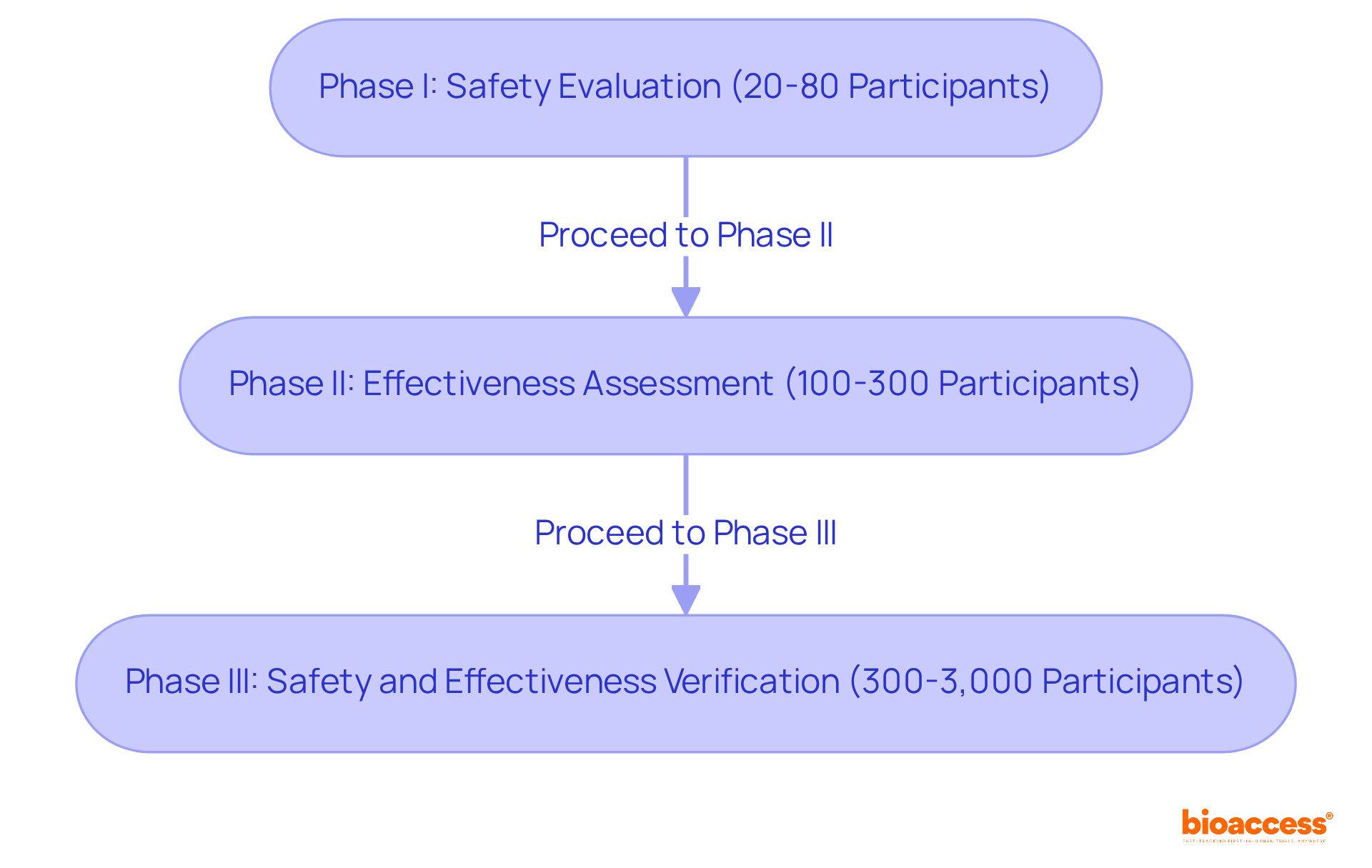
Clinical trials for medical devices are organized into distinct phases, each playing a crucial role in the development process.
Phase I studies, which are clinical trials for medical devices, typically lasting several weeks, focus on evaluating safety and determining the appropriate dosage, often involving 20 to 80 participants. These initial tests are vital for identifying any adverse effects and ensuring the intervention is manageable. As one expert noted, 'At the core of every apparatus assessment is a person who believes in our commitment.'
Phase II studies, which are a type of clinical trials for medical devices, generally encompass 100 to 300 participants and aim to assess the effectiveness of the device while monitoring side effects over a treatment duration of several weeks. This phase is essential for determining whether the device effectively addresses the targeted condition. Successful cases include trials for innovative instruments that have significantly improved patient outcomes, such as real-time glucose monitors that have revolutionized diabetes management.
Phase III studies are critical in clinical trials for medical devices, involving larger groups of 300 to 3,000 participants and typically lasting several months to years. They compare the new device against standard treatments to verify its overall safety and effectiveness. The outcomes of these clinical trials for medical devices are crucial for regulatory approval, as they provide extensive data on the device's performance across diverse populations. As highlighted in the Australian Clinical Research Handbook, 'Clinical trials for medical devices are vital for the advancement and assessment of medical instruments, supplying evidence-based information that aids decision-making and guarantees patient safety.'
Understanding these phases is essential for stakeholders to effectively strategize their research and allocate resources, ensuring each step contributes to the overarching goal of enhancing patient care through innovative medical technologies.
Informed consent is a fundamental aspect of clinical trials for medical devices, as it ensures that participants are fully aware of the study's nature, potential risks, and their rights. Clear and comprehensive communication is essential; consent forms must be straightforward, enabling participants to grasp complex information with ease. This clarity not only safeguards participants but also significantly enhances the credibility of the study, ultimately favorably influencing enrollment rates.
Ethical standards in informed consent processes are paramount, as they foster trust and transparency—crucial elements for participant engagement. To uphold these standards, researchers should prioritize the development of accessible consent materials and encourage open dialogue, allowing participants to ask questions and express concerns.
Furthermore, integrating compliance reviews and effective project management into the informed consent process can further reinforce ethical standards, ensuring that all regulatory requirements are met. Expert opinions underscore that a robust informed consent procedure in clinical trials for medical devices can lead to increased enrollment rates and improved participant retention, thereby reinforcing the ethical foundation of medical research.

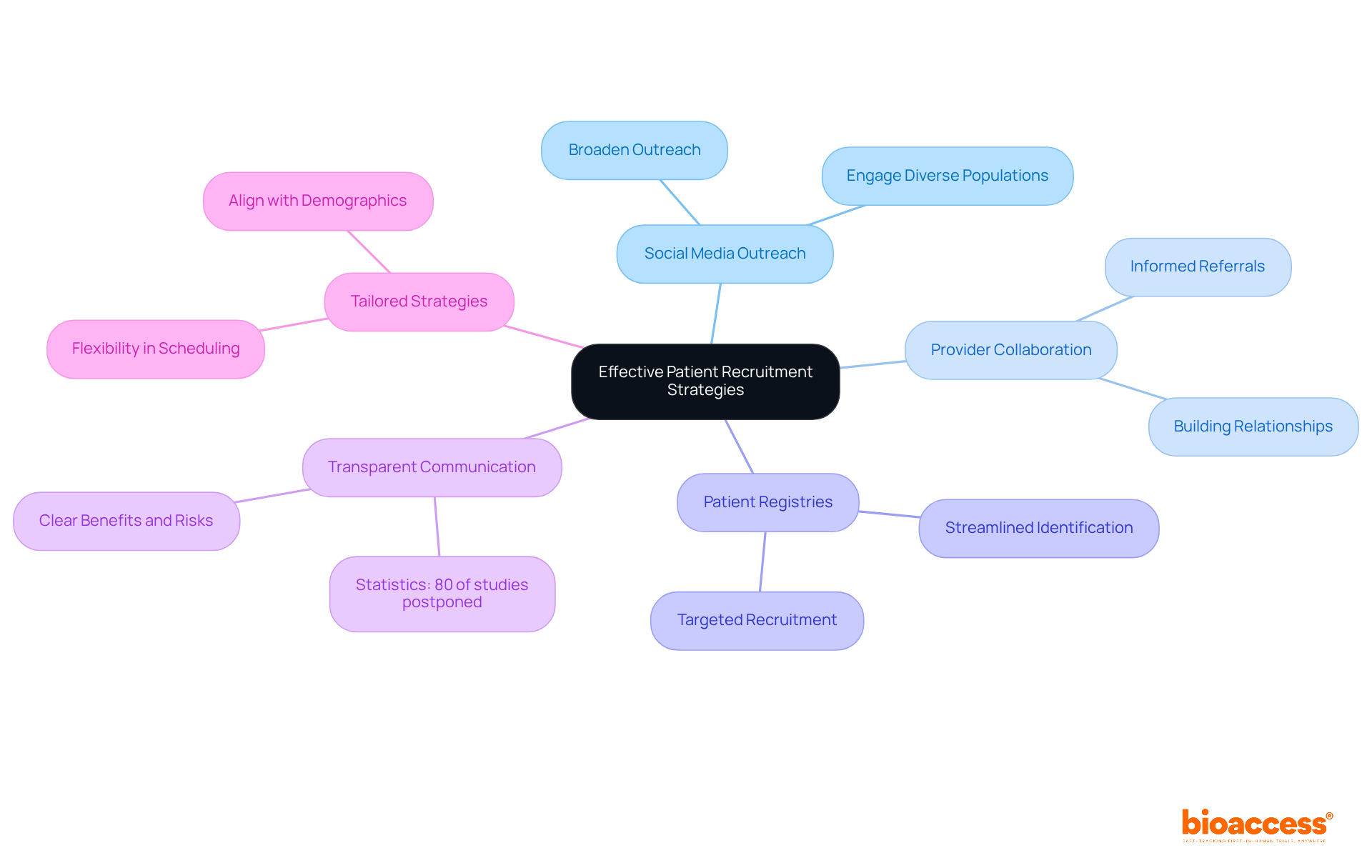
Recruiting patients for clinical trials for medical devices requires a strategic and multifaceted approach. Leveraging social media platforms significantly broadens outreach, enabling researchers to effectively connect with diverse populations. Collaborating with healthcare providers is essential, as informed providers can make valuable referrals, thereby enhancing enrollment rates. Utilizing patient registries streamlines the identification of potential participants, ensuring a more targeted recruitment process.
Statistics reveal that nearly 90% of research studies fail to meet their enrollment goals, underscoring the need for effective strategies. Transparent communication regarding the advantages and risks of participation is crucial; research indicates that 80% of studies are postponed due to difficulties in locating sufficient participants. Tailoring recruitment strategies to align with the demographics and preferences of the target population can lead to significant improvements in enrollment rates.
Successful patient recruitment examples include the integration of digital tools and online prescreening websites, which have proven effective in streamlining the recruitment process. The partnership between bioaccess™ and Caribbean Health Group to enhance research services in Barranquilla serves as a significant case study, illustrating how strategic alliances can yield better recruitment outcomes, achieving a 50% reduction in recruitment time and 95% retention rates. Furthermore, patient-centric approaches that prioritize the needs and concerns of potential participants can foster trust and encourage enrollment. As highlighted by industry specialists, adjusting recruitment strategies to the distinct characteristics of the patient group is vital for achieving success in clinical trials for medical devices.
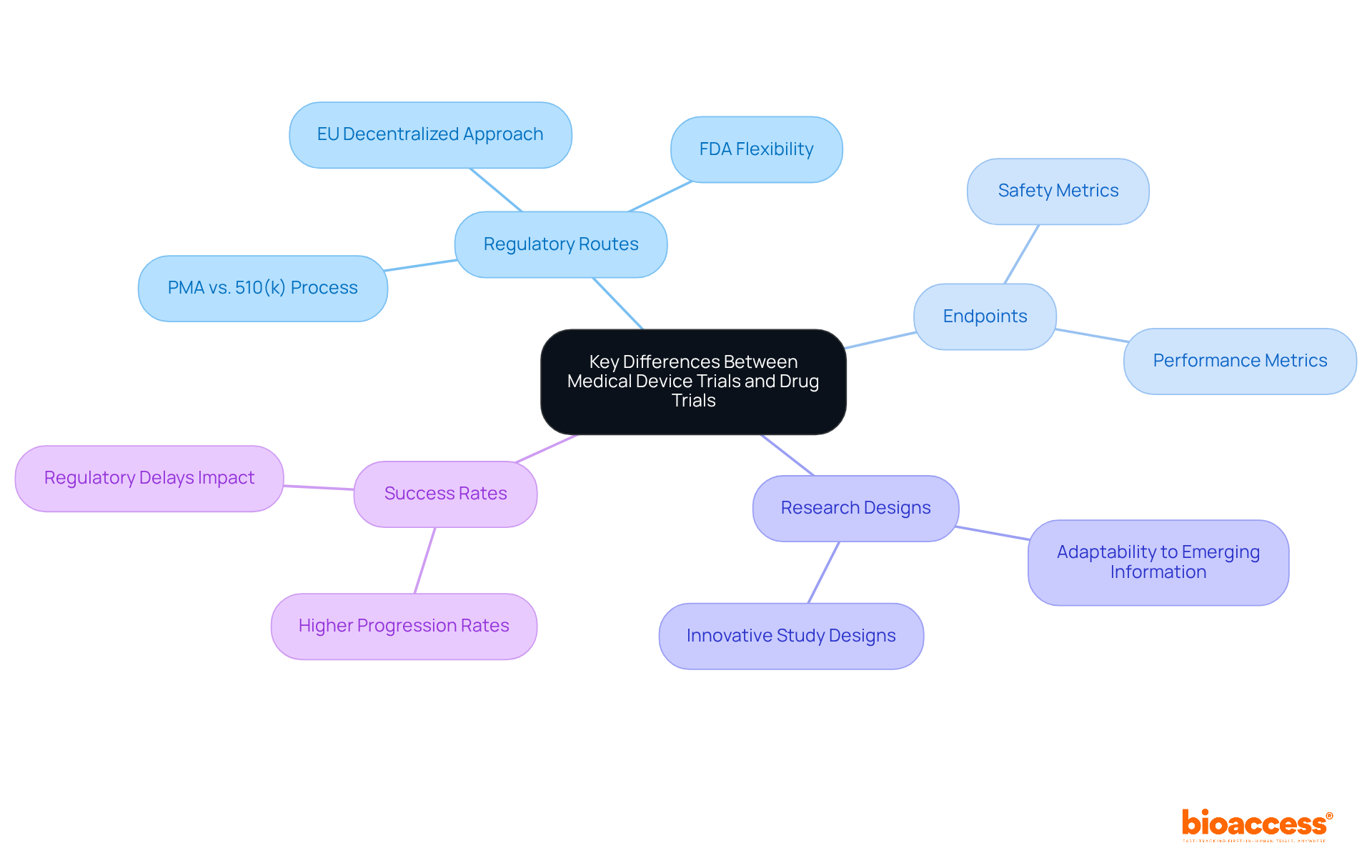
When compared to pharmaceutical studies, clinical trials for medical devices exhibit distinct characteristics, particularly regarding regulatory routes, endpoints, and research designs. While drug studies typically emphasize pharmacokinetics and pharmacodynamics, evaluations of medical equipment prioritize safety and performance metrics. Notably, the success rates of medical apparatus evaluations can be significantly higher; studies indicate that approximately 75% of initial clinical trials for medical devices have progressed beyond the United States due to regulatory delays, allowing for quicker approvals in other regions.
The regulatory landscape for medical devices is often more flexible, facilitating innovative study designs that can adapt to emerging information. This flexibility is crucial, as the FDA's centralized framework allows for faster processing of research applications, contrasting with the EU's more decentralized approach, which can lead to extended evaluation periods. Understanding these regulatory pathways is vital for developing effective strategies for clinical trials for medical devices.
Expert insights suggest that early collaboration with regulatory bodies, such as the FDA, can substantially enhance the probability of success in clinical trials for medical devices. Engaging in pre-submission meetings allows sponsors to negotiate study endpoints and designs, ultimately streamlining the approval process. Recent regulatory updates, including the introduction of the EU Medical Device Regulation (MDR), have established more stringent requirements, underscoring the necessity for thorough clinical evaluations and post-market surveillance.
In summary, recognizing the unique aspects of clinical trials for medical devices as opposed to drug studies is crucial for innovators in the Medtech, Biopharma, and Radiopharma sectors. By leveraging adaptable study frameworks and grasping regulatory complexities, companies can increase their chances of favorable study outcomes and expedite their path to market. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, clients can achieve accelerated research solutions, including 50% faster patient enrollment and significant cost reductions through FDA-ready information.
Efficient information management is essential in clinical trials for medical devices and requires meticulous planning and execution, particularly when partnering with a reputable CRO like bioaccess. Establishing clear information-gathering protocols forms the cornerstone of accurate and reliable data. The integration of electronic data capture (EDC) systems has revolutionized this process, enabling real-time data entry at study sites and significantly reducing errors associated with traditional paper-based methods. These systems bolster data integrity by providing standardized formats that facilitate seamless information collection and monitoring, as advocated by the Clinical Data Interchange Standards Consortium (CDISC).
Recent advancements in information management underscore the necessity of robust validation processes, which are vital for early detection of discrepancies during trials. This proactive approach not only safeguards data quality but also ensures compliance with regulatory standards—a primary focus for bioaccess as a leading Medtech clinical research partner in Latin America. Training personnel in information management practices is imperative, as it fosters familiarity with EDC systems and enhances overall efficiency. Adequate training minimizes the frequency of updates required by site personnel to access entry guidance documents, ultimately supporting the successful development of medical devices.
Moreover, safeguarding information security and patient confidentiality is paramount in healthcare information management. Implementing stringent data protection measures ensures that sensitive information is shielded from unauthorized access. By committing to comprehensive training and adhering to structured phases of medical information management, organizations can maintain high standards throughout the study, resulting in dependable research outcomes. For example, standardized electronic case report forms (eCRFs) can be reused without additional effort, streamlining the study build process and boosting overall productivity. As emphasized by experts in the field, effective information management is critical for the successful execution of clinical trials for medical devices, ensuring that high-quality data is readily available for analysis.

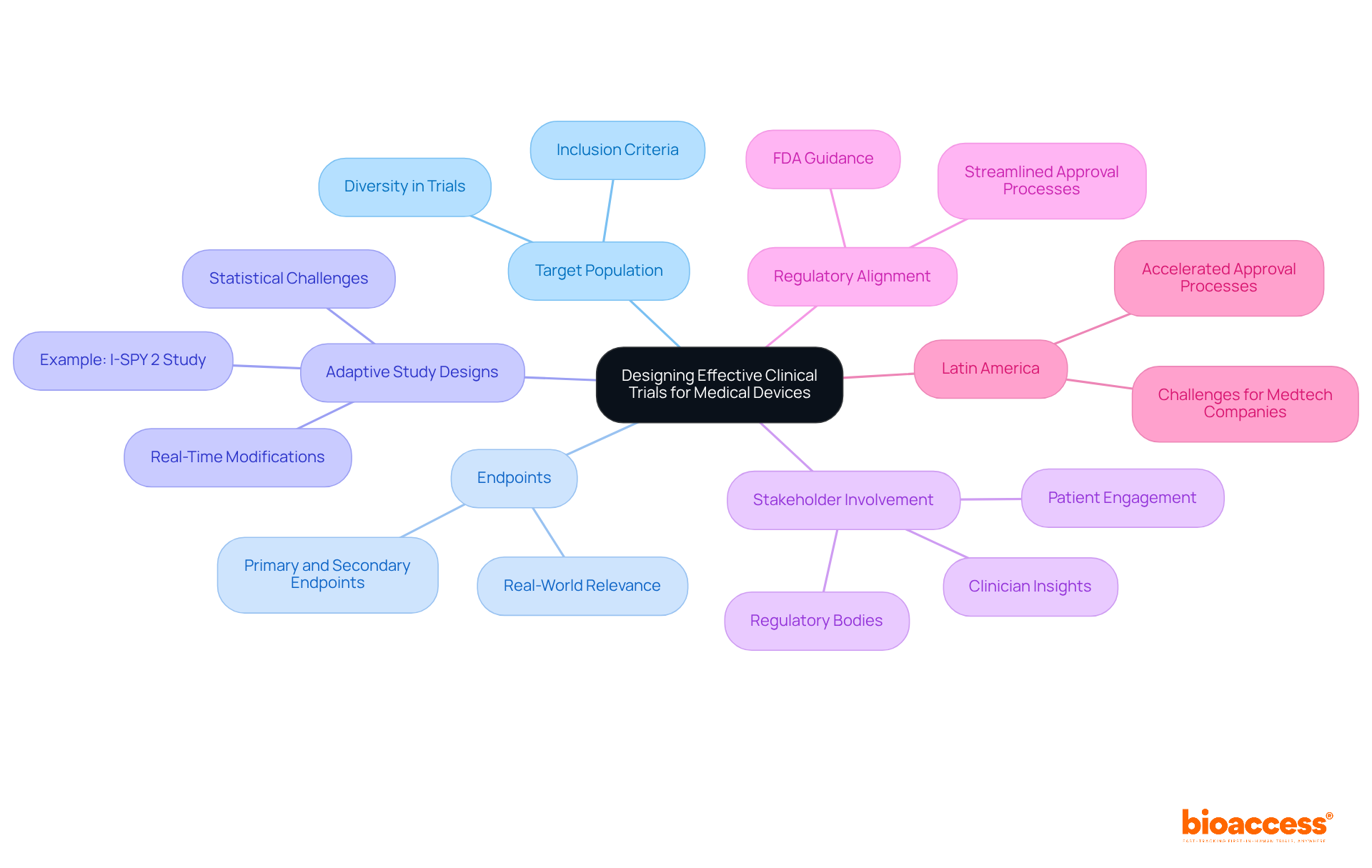
Creating efficient clinical trials for medical devices necessitates a thorough approach that considers the target population, endpoints, and overall study design. Recent trends underscore the growing acceptance of adaptive study designs, which offer enhanced flexibility and responsiveness to emerging data. These designs permit real-time modifications based on interim results, facilitating the quicker identification of effective treatments while minimizing participant exposure to ineffective therapies.
Involving stakeholders—such as clinicians, patients, and regulatory bodies—in the design process is vital. Their insights significantly enhance the relevance and applicability of the experiment, ultimately leading to higher success rates. For example, the I-SPY 2 study exemplifies how stakeholder collaboration can optimize treatment matching based on genetic profiles, thereby enhancing patient outcomes.
Moreover, aligning study designs with regulatory expectations is essential for successful approval. Regulatory agencies, including the FDA, have increasingly acknowledged the value of adaptive designs, providing guidance to streamline the approval process. This shift underscores the importance of flexible study designs in advancing clinical trials for medical devices, ensuring that novel solutions reach the market more effectively.
In Latin America, bioaccess® accelerates regulatory approval processes, achieving results in just 6-8 weeks compared to the typical 6-12 months in the US/EU. This capability enables the enrollment of treatment-naive cardiology or neurology cohorts 50% faster than Western sites, addressing common patient recruitment challenges faced by Medtech and biopharma startups. However, it is crucial to recognize the statistical challenges associated with adaptive studies, which require careful planning and expertise to maintain their integrity and validity. Engaging statisticians early in the planning process is essential to effectively define the necessity and scope of adaptive strategies.
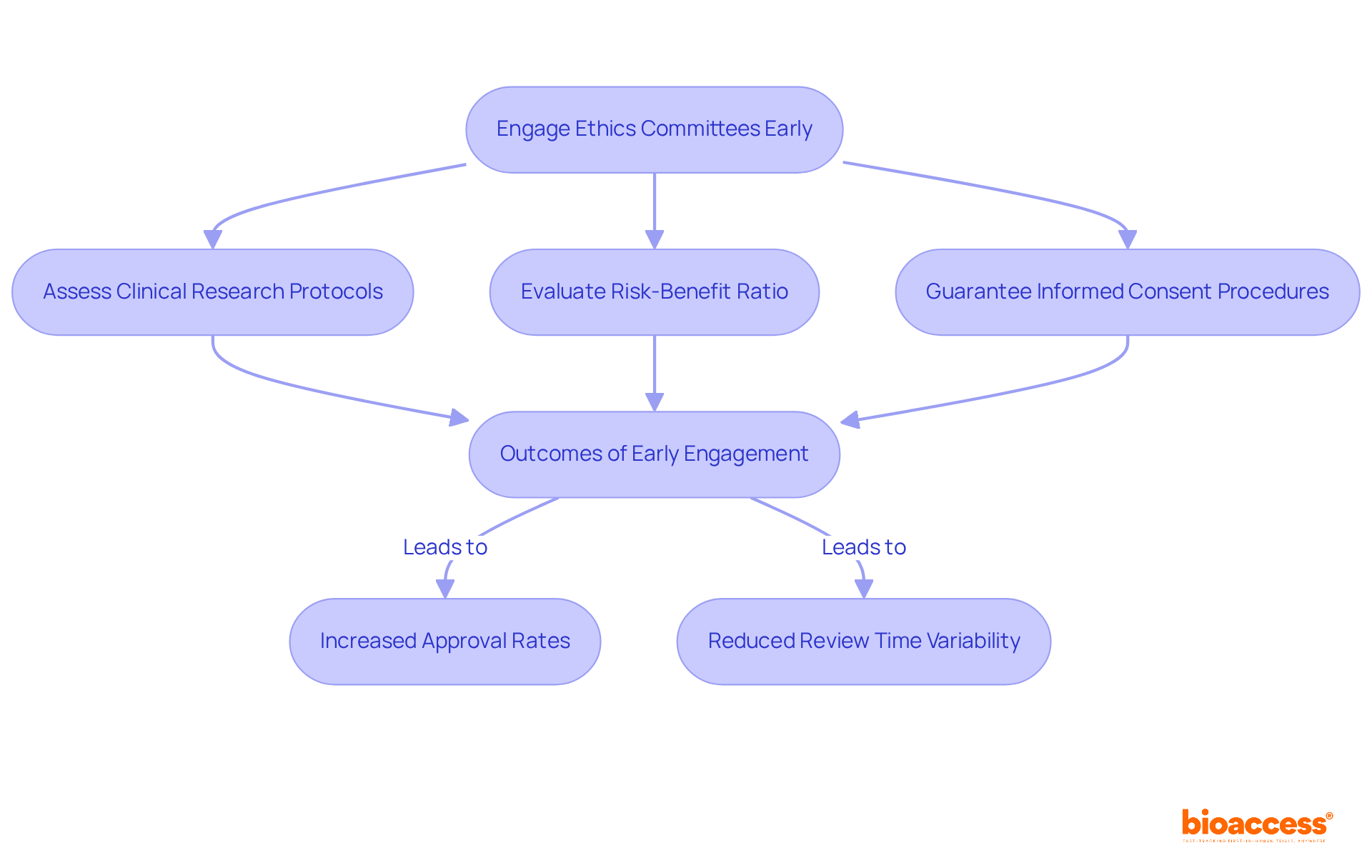
Ethics committees, known as Institutional Review Boards (IRBs), play a pivotal role in overseeing research studies, particularly in the realm of medical devices. Their primary responsibilities include:
Engaging ethics committees early in the study design process is not merely beneficial; it simplifies the approval procedure and bolsters the ethical integrity of the research. Studies indicate that early IRB involvement correlates with significantly higher approval rates, as proactive communication helps address potential concerns before formal submissions.
Moreover, the median time for ethics review across various IRBs can differ greatly, with some reviews taking as few as 32 days, while others may extend up to 396 days. This variability underscores the importance of cultivating a collaborative relationship with ethics committees to facilitate prompt approvals and enhance the overall effectiveness of research studies.
By leveraging bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, researchers can ensure their studies not only meet regulatory requirements but also uphold the highest ethical standards.
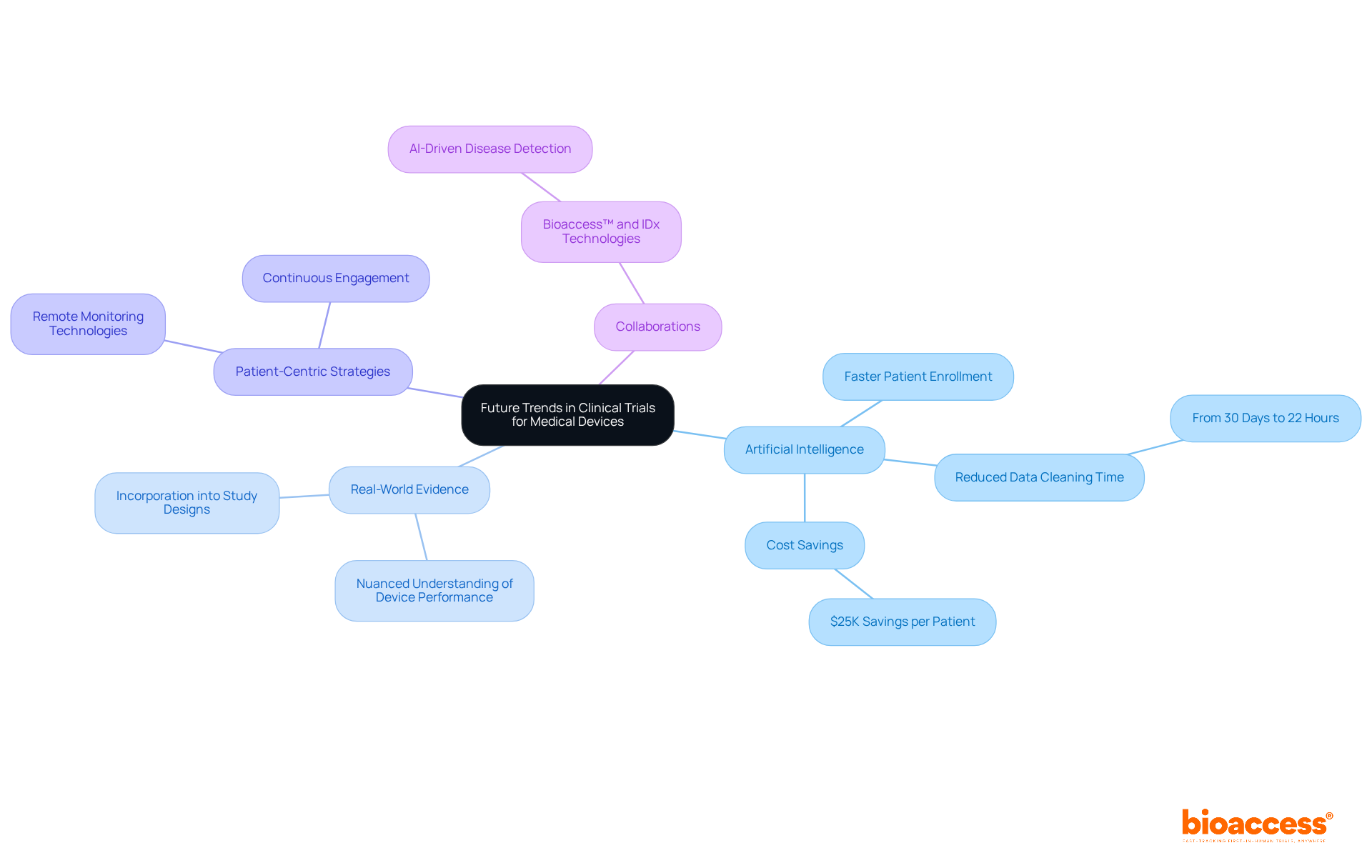
The future of clinical studies for medical devices is poised for significant transformation, driven by technological advancements, particularly the integration of artificial intelligence (AI) for data analysis and patient recruitment. Bioaccess® stands at the forefront of this evolution, demonstrating its innovative approach by achieving patient enrollment for treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites. This remarkable efficiency translates to substantial savings of $25K per patient through FDA-ready data, effectively eliminating rework and delays.
AI tools are increasingly utilized to streamline processes, with certain platforms reducing information cleaning time from 30 days to just 22 hours for over 40,000 participants. This enhanced efficiency not only accelerates timelines but also elevates the quality of data collected, which is crucial for regulatory compliance and successful outcomes, ultimately leading to cost savings in management.
Moreover, the incorporation of real-world evidence into study designs is gaining traction, allowing for a more nuanced understanding of device performance in everyday settings. This shift is vital as it addresses the rising demand for evidence that mirrors actual patient experiences and outcomes. For instance, GlobalCare Clinical Studies, in partnership with bioaccess™, has reported a reduction in study participant recruitment time by over 50%, alongside an impressive participant retention rate exceeding 95% in Colombia, showcasing the effectiveness of bioaccess's ambulatory services.
Patient-centric strategies, including remote monitoring technologies, are also revolutionizing the trial landscape. These innovations enable continuous engagement with participants, ensuring improved adherence to protocols and enhanced data collection. As industry experts note, "today's medical product sponsors must demonstrate not only safety and efficacy but also user-friendliness and compatibility with healthcare workflows." This comprehensive perspective is essential for securing regulatory approval and achieving market acceptance.
In conclusion, the integration of AI and real-world evidence, along with a focus on patient-centered methodologies, is set to redefine clinical trials for medical devices, making them more efficient, relevant, and aligned with the needs of both patients and healthcare providers. Furthermore, collaborations such as that of bioaccess™ with IDx Technologies for data licensing in Latin America further enrich the landscape by supporting AI-driven disease detection in ophthalmology.
The clinical trials landscape for medical devices is undergoing a significant transformation, particularly in Latin America, where bioaccess® excels in optimizing efficiency and accelerating research processes. By leveraging the region's regulatory advantages and diverse patient populations, bioaccess® empowers Medtech innovators to expedite market entry—a crucial factor in this competitive industry.
Key insights underscore the necessity of comprehending regulatory requirements and engaging local authorities early in the trial process. The structured phases of clinical trials, from safety assessments to larger efficacy evaluations, highlight the importance of thorough testing. Ethical considerations, particularly informed consent, are essential for maintaining participant trust and study integrity, while effective patient recruitment strategies are vital for overcoming enrollment challenges. Moreover, recognizing the unique aspects of medical device trials compared to drug trials is critical for developing tailored research approaches.
Looking ahead, the integration of innovations such as artificial intelligence and real-world evidence will prove essential in enhancing the efficiency and relevance of clinical trials. Stakeholders are urged to adopt patient-centric methodologies and cultivate collaborations that propel advancements in research. By prioritizing these strategies, the industry can address the increasing demand for faster evaluations while improving patient outcomes. The future of clinical trials promises significant enhancements in healthcare delivery, driven by innovation and collaboration.
What is bioaccess® and how does it optimize clinical trials for medical devices in Latin America?
bioaccess® is a company that accelerates clinical trials for medical devices by leveraging the regulatory efficiency of Latin America and its diverse patient populations. It secures ethical approvals in 4-6 weeks and has enrollment procedures that are 50% faster than traditional markets.
Why is the regulatory pace important for Medtech innovators?
The regulatory pace is essential for Medtech innovators as it helps maintain a competitive edge in the market. With R&D investments in medtech increasing by 10 percent annually, quicker evaluations are crucial for bringing products to market effectively.
What advantages does Colombia offer for conducting clinical trials?
Colombia offers a robust healthcare system, recognized as one of the best in Latin America, and has become a hub for research studies. It attracts significant foreign investment, generates nearly $500 million in economic benefits annually, and provides cost savings of over 30% compared to North America and Western Europe.
What are the tax incentives for R&D investments in Colombia?
Colombia offers R&D tax incentives including a 100% tax deduction and a 25% tax discount, which bolster investments in science, technology, and innovation projects.
How does bioaccess® address participant recruitment challenges in clinical trials?
bioaccess® expedites enrollment processes, which is crucial as 35 percent of study delays are attributed to inadequate participant recruitment.
What are the phases of clinical trials for medical devices?
Clinical trials are organized into three phases: Phase I: Focuses on safety and dosage with 20 to 80 participants. Phase II: Assesses effectiveness and monitors side effects with 100 to 300 participants. Phase III: Involves 300 to 3,000 participants and compares the new device against standard treatments to verify safety and effectiveness.
Why is understanding regulatory requirements important for clinical trials?
Understanding regulatory requirements is crucial as each country has distinct regulations that must be followed, including pre-market submissions and clinical trial applications. This knowledge helps in obtaining timely approvals and minimizing delays.
How can Medtech and Biopharma companies benefit from the regulatory environment in Latin America?
By engaging with local regulatory authorities early and capitalizing on the unique regulatory advantages in Latin America, these companies can expedite their clinical trials and bring their innovations to market more effectively.