


The article titled "10 Key Insights on In-Vivo Testing for Clinical Research Directors" presents a comprehensive examination of the pivotal aspects and advancements in in-vivo testing that are crucial for clinical research. It underscores the significance of in-vivo testing in drug development, illustrating how it bolsters the understanding of drug efficacy and safety. Furthermore, it addresses the regulatory, ethical, and technological considerations that are vital for the success of clinical trials.
In the evolving Medtech landscape, bioaccess plays a crucial role in tackling key challenges faced by clinical researchers. By integrating innovative solutions, stakeholders can enhance their research processes and outcomes. This article not only provides insights but also encourages readers to reflect on their current practices and consider potential improvements.
In conclusion, the importance of collaboration among researchers, regulatory bodies, and technology providers cannot be overstated. As the field advances, the next steps involve embracing these insights and fostering partnerships that drive clinical research forward.
In the rapidly evolving landscape of medical research, in-vivo testing stands out as a critical component for Clinical Research Directors who are navigating the complexities of drug development and regulatory compliance. This article presents ten key insights that illuminate the significance of in-vivo testing, demonstrating how it not only accelerates clinical trials but also enhances the efficacy and safety of innovative treatments.
As the field progresses, however, what pressing challenges and ethical considerations must be addressed to ensure responsible and effective research? By exploring these insights, we will reveal the pivotal role that in-vivo testing plays in shaping the future of personalized medicine and advancing healthcare solutions.
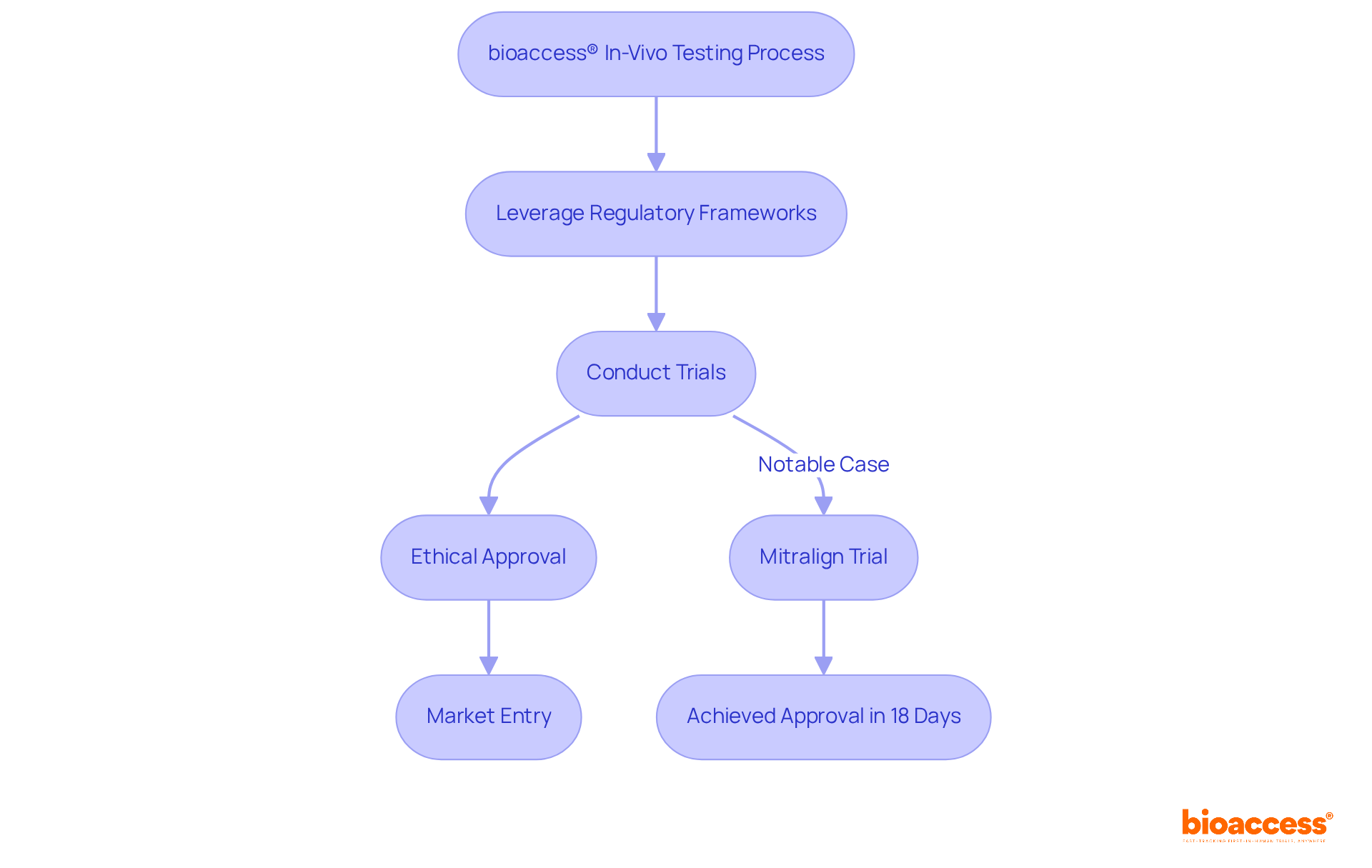
bioaccess® harnesses its extensive knowledge and local advantages to expedite live assessments for Medtech innovations. By leveraging the swift regulatory frameworks of Latin America, which allow First-in-Human (FIH) trial approvals in as little as 2-4 months, alongside the diverse patient populations in Colombia, bioaccess® facilitates effective live studies that are crucial for accelerating product development and market entry. This agility is essential for Medtech companies aiming to deliver groundbreaking solutions to patients promptly and effectively.
Noteworthy cases of successful live experimentation include:
By adeptly navigating the complexities of regulatory requirements set by organizations like Colombia's INVIMA and leveraging local expertise, bioaccess® not only enhances the speed of in-vivo testing but also positions Medtech innovators for success in a competitive landscape. Additionally, conducting trials in Latin America can yield cost savings of around 30% compared to the U.S. or EU, while the availability of R&D tax incentives further amplifies the strategic advantages of this region.
In-vivo testing refers to critical experiments conducted within living organisms, such as animal models or human subjects, to evaluate the effects of treatments or interventions. In-vivo testing is essential for understanding the pharmacokinetics, efficacy, and safety of new drugs and medical devices. It provides invaluable information that in-vitro studies cannot offer, establishing it as a cornerstone of medical research and development. For Medtech, Biopharma, and Radiopharma startups, partnering with bioaccess® can significantly accelerate clinical trials, ensuring rapid regulatory approval and efficient patient recruitment.
To stay informed about the latest developments in regulated products, Clinical Research Directors should actively follow the FDA on social media platforms like X and Facebook. Engaging with the FDA's NAMs roadmap and participating in upcoming workshops can yield valuable insights into best practices and regulatory updates related to in-vivo testing evaluations. This collaboration is not just beneficial; it is essential for navigating the complexities of clinical research in today's fast-paced environment.
In-vivo testing plays an integral role in various stages of drug development, encompassing several key applications that warrant attention.
Efficacy Testing serves as a critical process that evaluates the therapeutic effects of a drug within a living organism. This approach provides insights that in vitro models may not fully capture. Research has shown that while a medication may reduce cancer cell proliferation by 50% in a 2D model, its effectiveness can diminish to 40-45% in a 3D model. This discrepancy underscores the significance of realistic testing conditions, emphasizing the necessity for in-vivo experiments to accurately evaluate drug performance in environments that closely resemble human physiology.
Safety assessments are another crucial aspect of in-vivo testing, essential for identifying potential side effects and toxicities before progressing to human trials. The safety profile of medications such as Pulmozyne (dornase alfa), administered through the eRapid nebulizer system, has been thoroughly assessed in cystic fibrosis patients (NCT01712334). This highlights the importance of comprehensive safety evaluations in research.
Pharmacokinetics involves understanding how a drug is absorbed, distributed, metabolized, and excreted in the body. This knowledge is vital for predicting its behavior in humans. In-vivo testing delivers essential data that informs pharmacokinetic profiles, ensuring that drugs are both effective and safe. For instance, temperature stability is crucial for drugs delivered via inhalation devices, as fluctuations can significantly affect drug efficacy.
Dose Optimization is a critical step in drug development, determining the most effective and safe dosage for clinical trials. In-vivo testing enables researchers to fine-tune dosages based on real biological responses, maximizing therapeutic benefits while minimizing risks.
The importance of in-vivo testing in evaluating effectiveness and safety cannot be overstated. As Ariel Berlinski, a prominent expert in the field, noted, "Because there are no data, some third-party payers are starting to say, 'Sorry, I'm not paying for this.'" This statement highlights the necessity of robust biological data to support drug efficacy and safety claims.
Practical illustrations further demonstrate the significance of in-vivo testing for live subject evaluations. For example, the pairing of doramapimod with chemotherapy and immunotherapy has shown particularly successful outcomes against breast and pancreatic cancers. This highlights how biological studies can uncover synergistic effects that may not be evident in earlier evaluation stages. Furthermore, recent discoveries suggest that three times as many medications are expected to be effective on 3D microtumors compared to traditional 2D cell lines, emphasizing the evolving landscape of biological experimentation and its essential role in advancing drug development.

Addressing the several challenges that arise during in-vivo testing is crucial for clinical research.
Ethical Concerns: The humane treatment of animal subjects is paramount, necessitating strict adherence to ethical guidelines. Veterinarians play a crucial role in overseeing these practices, ensuring compliance with established ethical standards. The ethical implications of inducing suffering for scientific advancement are significant, and transparency in these processes is essential for maintaining public trust. To address these concerns, bioaccess provides compliance reviews and ethical oversight as part of its clinical trial management services.
Variability: Biological differences among subjects can significantly influence research outcomes. Variability in responses among genetically similar animals often requires larger sample sizes to achieve statistical significance. Implementing robust research designs and careful analysis is vital to manage this variability effectively. Bioaccess aids in this field by providing viability assessments and location selection services that assist in identifying the most appropriate research settings and groups.
Cost: In-vivo research can be significantly more costly than in-vitro options, mainly because of the requirement for specialized facilities, equipment, and animal care. The financial strain associated with in-vivo testing can pose a challenge for many research organizations, necessitating a thorough cost-benefit evaluation when planning experiments. Bioaccess aids in this process by providing project management services that help streamline operations and reduce unnecessary expenditures.
Regulatory Hurdles: Navigating the complex landscape of regulations governing animal research can delay study initiation and completion. Compliance with Good Laboratory Practice (GLP) and other regulatory requirements is essential for any new treatment or device aiming to enter the global market. Comprehending these regulations is essential for research directors to ensure timely project execution. To assist this process, bioaccess provides extensive trial management services, including compliance evaluations and trial preparation, which can help simplify the regulatory pathway and improve the efficiency of live subject assessments.

Regulatory considerations for in-vivo testing encompass several critical aspects:
Approval Processes: Securing necessary approvals from regulatory bodies, such as the FDA, is essential before initiating any clinical studies. The Investigational New Drug (IND) application serves as the primary route for obtaining approval, necessitating thorough preclinical data and research protocols.
Good Laboratory Practices (GLP): Adhering to GLP standards is crucial for ensuring the quality and integrity of data produced during in-vivo testing. Established by the FDA in 1978, GLP regulations mandate rigorous protocols for study conduct, including personnel training, facility maintenance, and equipment calibration. Compliance with GLP is vital, as it enhances the reliability of nonclinical safety data submitted to regulatory agencies.
Documentation: Maintaining meticulous records of all procedures, results, and communications with regulatory agencies is imperative. This documentation fosters transparency and accountability, facilitating smoother interactions with regulatory bodies during the approval process.
Reporting Adverse Events: Timely reporting of any unexpected adverse events to regulatory authorities is a critical responsibility. Regulatory agencies expect continuous monitoring of safety events during the trial phases, ensuring that any potential risks are addressed swiftly.
Statistics indicate that adherence to GLP can significantly impact the success of clinical trials, especially in-vivo testing. Research shows that over 90% of nonclinical investigations, particularly in-vivo testing, are subject to GLP requirements, underscoring the importance of compliance in securing regulatory approval. Furthermore, effective implementation of GLP can lead to enhanced data quality, ultimately improving the likelihood of successful drug development and market entry.
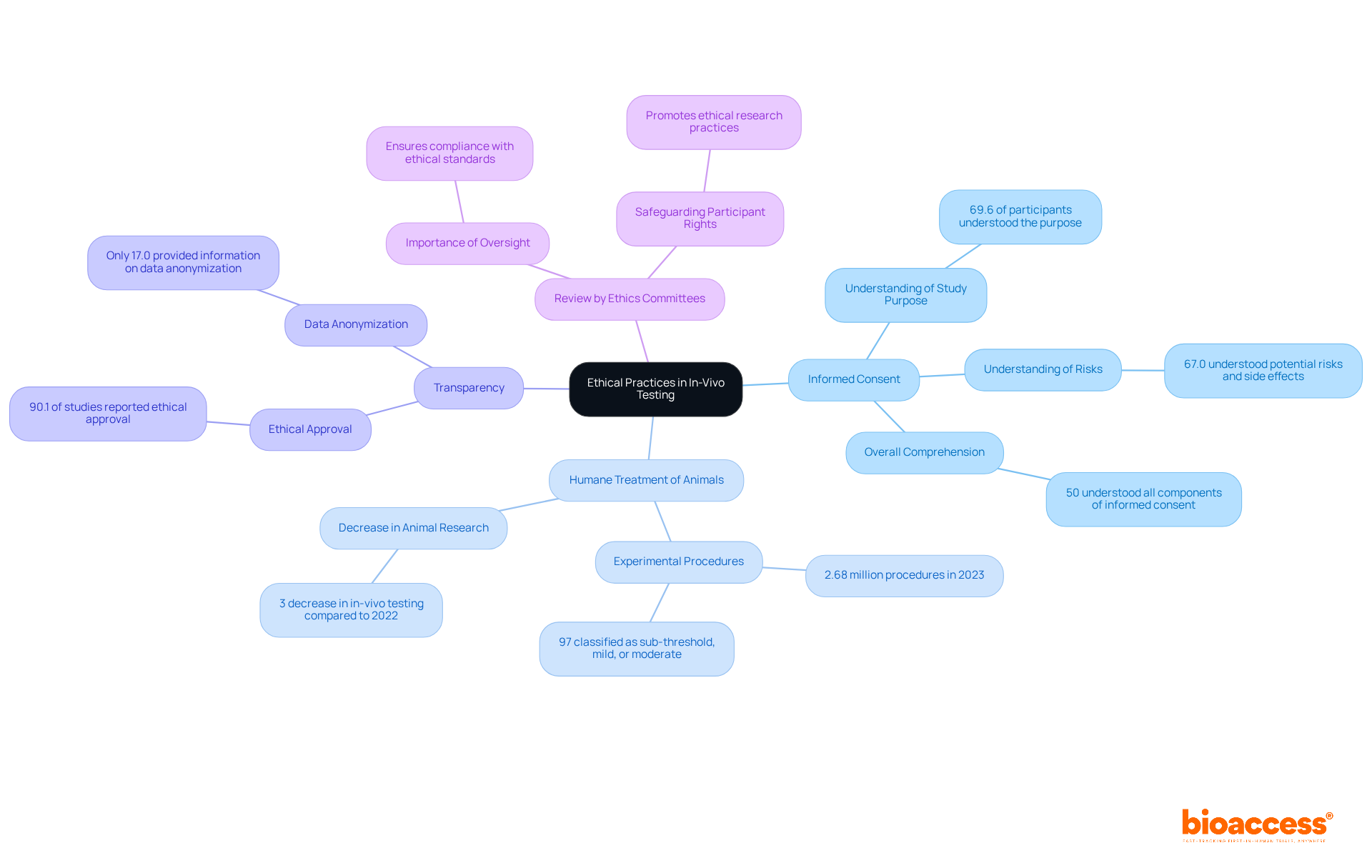
Ethical practices in in-vivo testing encompass several critical components:
Informed Consent: It is essential that human subjects are thoroughly informed about the study's nature, potential risks, and benefits before providing consent. Recent research indicates that while 74.7% of participants understood the nature of the investigation, comprehension of risks and side effects remains a concern, with only 67.0% grasping potential hazards. Furthermore, only 50% of participants properly understood all informed consent components, highlighting the ongoing need for improved communication strategies to enhance participant understanding.
Adhering to established guidelines is crucial for the humane treatment of animal subjects during in-vivo testing. In 2023, 97% of experimental procedures involving animals were classified as sub-threshold, mild, or moderate, reflecting a commitment to minimizing suffering. The total number of scientific procedures involving living animals in 2023 was recorded at 2.68 million, and the decrease of 3% in in-vivo testing compared to the previous year marks the lowest level of animal research activity since 2001.
Transparency: Keeping open communication with stakeholders regarding research objectives and outcomes is essential. Ethical approval was noted in 90.1% of research, yet only 17.0% offered information on data anonymization, highlighting a gap in transparency that requires attention.
Review by Ethics Committees: Submitting studies for review by ethics committees ensures compliance with ethical standards. This process is essential for safeguarding participant rights and promoting ethical research practices. Insights from ethics committee members highlight the significance of rigorous oversight in preserving the integrity of medical research.
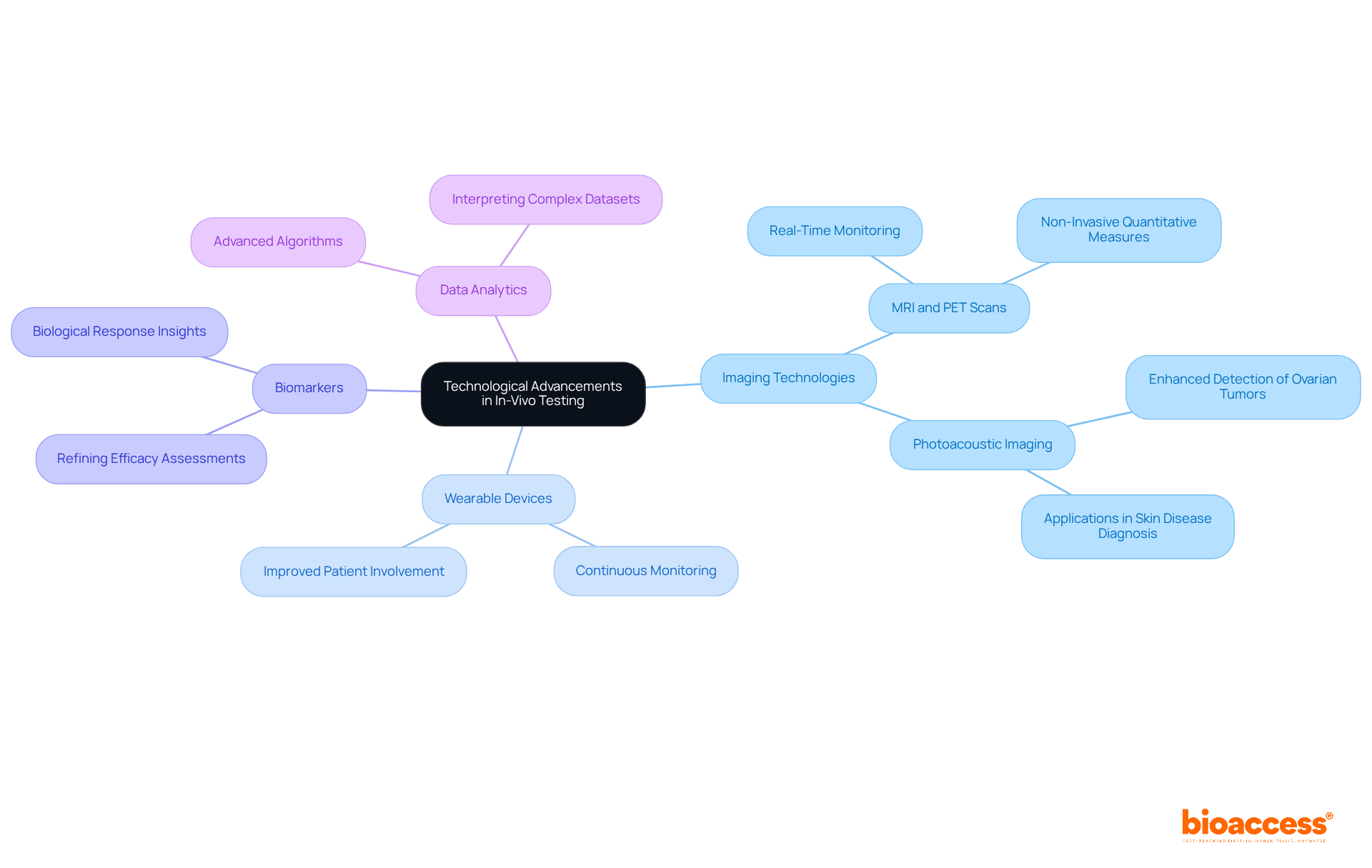
Recent technological advancements have profoundly transformed research methodologies in medicine, particularly in-vivo testing. Innovations in imaging technologies, including MRI and PET scans, enable real-time monitoring of biological processes, offering critical insights into treatment efficacy and safety. For instance, PET scans provide a non-invasive, quantitative measure of target distribution in tissues, which enhances the understanding of drug interactions and patient responses. Notably, only 20% to 25% of ovarian cancer cases can be diagnosed at an early stage, underscoring the necessity of advanced imaging techniques in improving early detection.
The integration of wearable devices has further revolutionized data gathering in medical trials. These devices facilitate continuous monitoring of subjects, allowing researchers to collect extensive data on drug effects in real-time. Research indicates that wearable technology can significantly enhance patient involvement and compliance, ultimately leading to improved health outcomes. As noted by a prominent researcher, "AI is transforming diagnostics, providing unmatched accuracy and speed," highlighting the critical role of technology in advancing medical research.
Moreover, the identification and utilization of biomarkers have proven essential in refining efficacy and safety assessments. Biomarkers offer valuable information regarding the biological response to treatments, enabling more precise evaluations of therapeutic effectiveness.
Advanced data analytics tools are also pivotal in interpreting the complex datasets generated by these technologies. By employing sophisticated algorithms, researchers can derive significant insights, leading to better-informed decision-making and enhanced trial designs. This integration of imaging methods, wearable technology, and data analysis is paving the way for more efficient and effective in-vivo testing in clinical research.
In-vivo testing is pivotal in advancing personalized medicine by enabling tailored treatments and enhancing therapeutic strategies. This methodology allows researchers to assess individual responses to treatments, considering genetic and phenotypic variations. For instance, studies have shown that live evaluations can lead to significant improvements in dosage optimization, ensuring that specific patient groups receive the most effective amounts of medication. This is particularly vital in oncology, where precise dosing can enhance treatment efficacy while reducing adverse effects.
Moreover, biological studies facilitate the identification of biomarkers that predict treatment responses, paving the way for more effective therapies. Recent advancements in blood-based biomarkers illustrate their potential in early cancer detection, enabling timely and personalized interventions.
As emphasized by leading researchers, "AI is the key to unlocking personalized medicine, tailoring treatments to individual patients." The integration of live experimentation outcomes into medical practice is transforming how therapies are customized. This shift not only improves patient outcomes but also aligns with the increasing focus on individualized medicine, where therapies are crafted to meet the unique needs of each patient. The continuous development of assessment methods for live organisms, particularly in-vivo testing, is driving innovation in treatment customization, underscoring its essential role in contemporary medical research.
To enhance the execution of live examination methodologies, research leaders are encouraged to explore successful case studies that demonstrate effective applications, such as advancements in early cancer identification, which highlight the tangible benefits of incorporating these practices into their research frameworks.

Future trends in in-vivo testing are poised to substantially transform medical research. Key developments include:
Increased Use of AI: Artificial intelligence is set to revolutionize data analysis and study design, enhancing efficiency and accuracy in clinical trials. AI's predictive analytics capabilities can streamline patient recruitment and monitor adherence to treatment regimens, addressing challenges that account for approximately 37% of trial delays. This integration of AI not only improves data handling but also supports compliance reviews and project management processes.
Integration of Genomics: The inclusion of genomic data into live research will enhance the comprehension of personal reactions to therapies. This approach allows for tailored therapies, improving patient outcomes and optimizing drug efficacy. AI-driven genomics analysis has already shown promise in predicting patient responses to specific medications, paving the way for personalized medicine. Advances in genomics and multi-omics data empower AI to design clinical trials with unprecedented precision, enhancing the overall effectiveness of treatments.
Advances in telemedicine and remote monitoring technologies will facilitate more flexible and patient-centered in-vivo testing. These innovations enable real-time data collection and patient engagement, enhancing retention rates and compliance throughout the trial process. AI tools can provide personalized experiences and timely reminders, significantly improving patient adherence and engagement.
Sustainability Practices: A growing emphasis on sustainability is likely to lead to the development of more ethical and environmentally friendly testing practices. This change corresponds with the growing need for responsible research methods that emphasize patient welfare and environmental factors, ensuring that trials are carried out with an emphasis on sustainability.
Accelerated Patient Enrollment: With bioaccess's expertise in managing research trials, including Early-Feasibility and First-In-Human Studies, the integration of advanced methodologies allows for patient cohorts to be enrolled 50% faster than conventional sites. This efficiency not only enhances trial timelines but also results in significant cost savings of $25K per patient, ensuring that trials are both effective and economically viable.
As these trends develop, the incorporation of AI and genomics into in-vivo testing methods will not only enhance the effectiveness of trials but also ensure they are more aligned with the needs of patients and the healthcare system.

In-vivo testing is an essential part of clinical research, crucial for understanding drug efficacy and safety. Studies indicate that most failures occur during Phase 2, which has a mere 32% success rate, and Phase 3, with a 60% success rate, primarily due to safety concerns and lack of efficacy. This underscores the importance of rigorous in-vivo testing during the development process.
Regulatory and ethical considerations play a paramount role in conducting studies. Compliance with established guidelines ensures both participant safety and data integrity. As highlighted by Jeffrey L. Evelhoch, 'Ensuring regulatory compliance is essential to the success of your research trials.' Bioaccess® emphasizes the necessity of adhering to regulatory standards, which is crucial for Medtech, Biopharma, and Radiopharma startups navigating the complexities of clinical trials.
Technological advancements, including in vivo imaging methods and the application of fusion proteins, are revolutionizing in vivo evaluation approaches. These innovations enable researchers to conduct more accurate and effective studies, enhancing the overall quality of clinical research.
The shift towards personalized medicine increasingly relies on live subject data, which is instrumental in tailoring treatments to meet individual patient needs. This trend highlights the significance of understanding pharmacokinetics and biological disposition, ensuring that therapies are both effective and safe for diverse patient populations.
Comprehensive trial management services are essential for effective in-vivo testing and overall research management. Services such as:
are essential components that contribute to the efficacy of clinical trials.
Finally, staying informed about future trends, particularly the ethical considerations surrounding animal welfare in research protocols, is crucial for effective clinical research management. By addressing these aspects, researchers can ensure that their studies are not only scientifically sound but also ethically responsible.

In-vivo testing is a cornerstone of clinical research, essential for evaluating the safety and efficacy of new medical treatments. By employing live subjects, researchers gain invaluable insights that in-vitro studies cannot provide. This methodology enhances the understanding of drug behavior in real biological systems and paves the way for personalized medicine, where treatments can be tailored to meet the unique needs of individual patients.
Key insights from the article highlight the critical role of in-vivo testing in drug development, emphasizing its applications in:
The swift regulatory frameworks in regions like Latin America, particularly through the services of bioaccess®, further accelerate the clinical trial process, allowing for faster market entry and significant cost savings. Additionally, the ethical considerations surrounding in-vivo testing underscore the importance of compliance with established guidelines, ensuring both participant safety and data integrity.
As the landscape of clinical research evolves, the integration of technological advancements such as AI and genomics will further enhance in-vivo testing methodologies. Embracing these innovations improves the efficiency of trials and aligns them with the growing emphasis on personalized healthcare. Researchers and clinical directors are encouraged to stay informed about these trends and actively engage with regulatory bodies to navigate the complexities of clinical trials effectively. By doing so, the potential for groundbreaking medical advancements can be unlocked, ultimately benefiting patients and the broader healthcare system.
What is bioaccess® and how does it support Medtech innovations?
bioaccess® accelerates in-vivo testing for Medtech innovations by leveraging swift regulatory frameworks in Latin America, allowing First-in-Human (FIH) trial approvals in as little as 2-4 months. It utilizes diverse patient populations in Colombia to facilitate effective live studies, crucial for product development and market entry.
What are some successful examples of live experimentation conducted by bioaccess®?
One notable example is the trial for Mitralign, which achieved ethical approval in just 18 days in Colombia. This demonstrates the region's potential for rapid trial approvals.
How does Colombia's healthcare system benefit Medtech trials?
Colombia's healthcare system is recognized as one of the best globally, ensuring high-quality patient care and recruitment, with approximately 95% of the population covered by universal healthcare.
What regulatory challenges does bioaccess® help navigate?
bioaccess® aids Medtech innovators in navigating regulatory requirements set by organizations like Colombia's INVIMA, enhancing the speed of in-vivo testing and positioning them for success in a competitive landscape.
What are the cost advantages of conducting trials in Latin America?
Conducting trials in Latin America can yield cost savings of around 30% compared to the U.S. or EU. Additionally, R&D tax incentives further enhance the strategic advantages of this region.
What is in-vivo testing and why is it important?
In-vivo testing refers to experiments conducted within living organisms to evaluate the effects of treatments or interventions. It is essential for understanding the pharmacokinetics, efficacy, and safety of new drugs and medical devices, providing information that in-vitro studies cannot offer.
How does in-vivo testing contribute to drug development?
In-vivo testing is integral to drug development, playing key roles in efficacy testing, safety assessments, pharmacokinetics, and dose optimization, ensuring that medications are effective and safe for human use.
What is the significance of efficacy testing in in-vivo studies?
Efficacy testing evaluates the therapeutic effects of a drug within a living organism, revealing insights that in vitro models may not capture. It emphasizes the need for realistic testing conditions to accurately evaluate drug performance.
Why are safety assessments crucial in in-vivo testing?
Safety assessments identify potential side effects and toxicities of medications before progressing to human trials, ensuring that the safety profile of drugs is thoroughly evaluated.
How does in-vivo testing inform pharmacokinetic profiles?
In-vivo testing provides essential data on how a drug is absorbed, distributed, metabolized, and excreted in the body, which is vital for predicting its behavior in humans.
What role does dose optimization play in drug development?
Dose optimization determines the most effective and safe dosage for clinical trials. In-vivo testing allows researchers to fine-tune dosages based on real biological responses, maximizing therapeutic benefits while minimizing risks.
What are some practical applications of in-vivo testing in drug development?
In-vivo testing has demonstrated successful outcomes, such as the pairing of doramapimod with chemotherapy and immunotherapy against cancers, revealing synergistic effects not evident in earlier evaluation stages. Additionally, it has shown that more medications are likely to be effective on 3D microtumors compared to traditional 2D cell lines.