


The article titled "10 Key Insights to Enhance Your Phase Research in Clinical Trials" provides essential strategies aimed at improving the effectiveness and efficiency of clinical trial research. It underscores the critical role of leveraging local advantages, such as regulatory efficiency and diverse patient populations, particularly in regions like Colombia. By doing so, it aims to accelerate patient enrollment and enhance the overall quality of clinical studies. This focus on local strengths is not merely a suggestion; it is a strategic imperative for achieving successful outcomes in clinical research.
The realm of clinical trials is rapidly evolving, as innovative strategies and technologies redefine how researchers approach phase research. Medtech companies must navigate this complex landscape, making it crucial to understand the key insights that can enhance their research processes. What challenges will they encounter, and how can they leverage opportunities for success? This article delves into ten pivotal insights that illuminate the path to more effective phase research while addressing the pressing questions that arise in the pursuit of groundbreaking medical advancements.
bioaccess® leverages its extensive expertise and local advantages to enhance phase research, particularly in Colombia, which offers significant competitive advantages for first-in-human studies. By taking advantage of Colombia's regulatory efficiency, where ethical approvals can be secured in just 90 to 120 days, alongside its diverse patient populations, bioaccess® accelerates patient enrollment by engaging treatment-naive cardiology or neurology cohorts 50% faster than Western sites. This rapid turnaround not only expedites the timeline for Medtech innovations but also substantially improves the overall effectiveness of phase research studies, allowing companies to bring their products to market much more swiftly than through traditional pathways.
As we approach 2025, the research landscape increasingly emphasizes innovation and adaptability, making bioaccess®'s phase research strategy particularly relevant for Medtech companies aiming to navigate these evolving dynamics effectively. Moreover, Colombia's healthcare system, recognized as one of the best globally, guarantees high-quality care and access to qualified researchers, further enhancing the value proposition for Medtech innovators.
Julio Martinez-Clark, CEO of bioaccess, underscores the benefits of conducting medical studies in Latin America, pointing to the region's cost-effectiveness, with savings surpassing 30% compared to North America and Western Europe, as well as the availability of R&D tax incentives that can significantly alleviate financial pressures for startups.

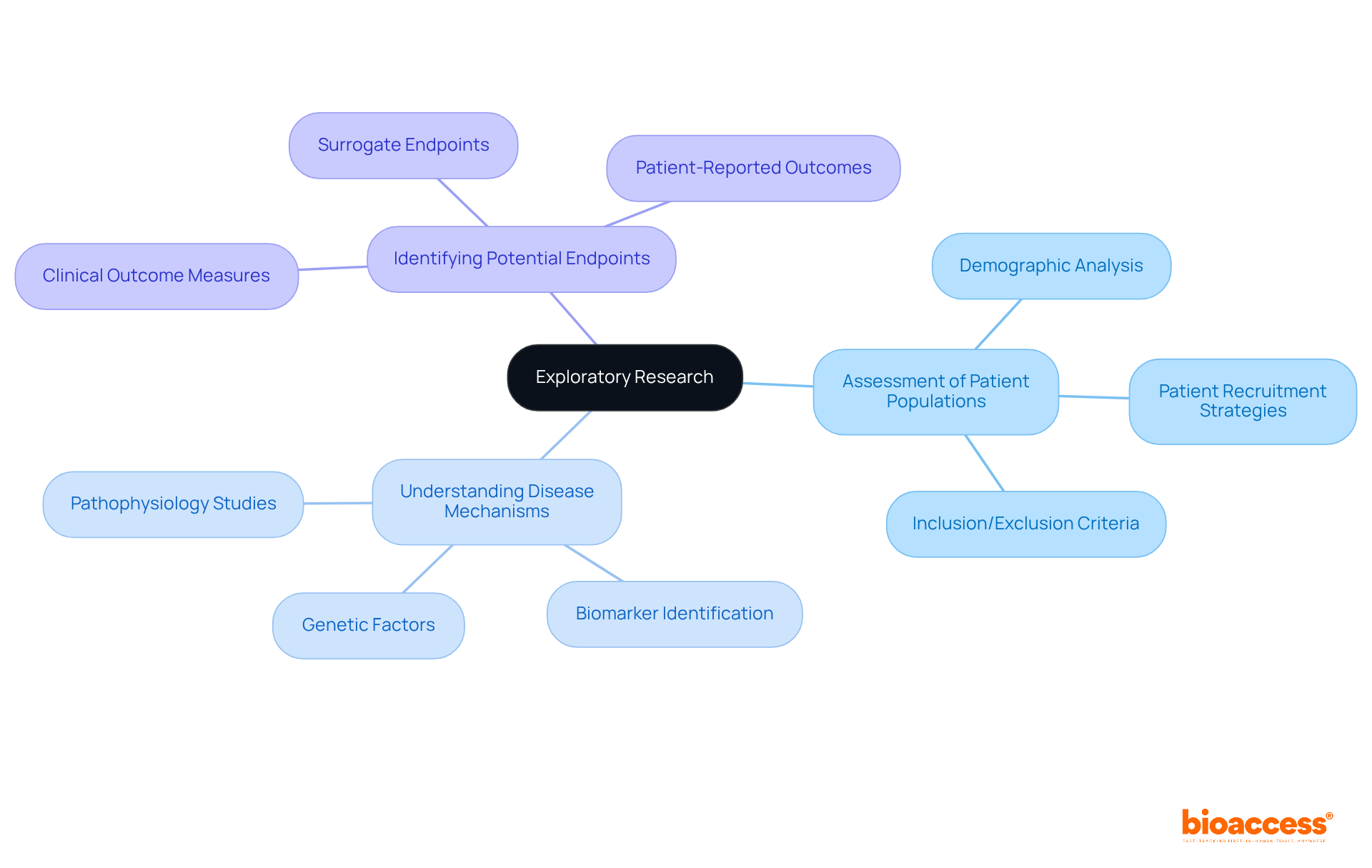
Conducting exploratory investigations is crucial for medical study teams as it allows them to gather vital information regarding the feasibility and potential effectiveness of new treatments. This phase research encompasses the assessment of patient populations, a thorough understanding of disease mechanisms, and the identification of potential endpoints. By investing time in exploratory research, teams can refine their hypotheses and design more effective medical studies, thereby significantly enhancing the likelihood of achieving successful outcomes.
Clinical studies often encounter a range of obstacles, including recruitment issues, regulatory intricacies, and budget limitations. As we look to 2025, the landscape is expected to become even more complex, with a projected decrease in the total number of medical studies launched in the US due to the Inflation Reduction Act. This shift is prompting pharmaceutical firms to concentrate on a smaller number of high-value therapeutic fields, highlighting the necessity for a proactive approach to navigate these challenges effectively.
Developing a comprehensive project plan is essential. Engaging stakeholders early and maintaining open lines of communication can significantly enhance collaboration and transparency. Dr. Stacie Bell emphasizes the critical importance of ensuring diverse populations are represented in medical research, which necessitates strategic planning and outreach initiatives.
To tackle recruitment difficulties, sponsors should consider unconventional methods, such as partnering with community organizations to reach underrepresented populations. Katrina Rice notes that 2025 is poised for significant advancements in diversifying research studies, underscoring the importance of tailored recruitment strategies.
Moreover, as sophisticated treatments are introduced into medical environments, the complexity and costs associated with focusing on smaller patient groups demand more intelligent study designs. A resurgence of adaptive studies, which allow modifications based on interim findings, is anticipated to alleviate some concerns regarding rising expenses and recruitment challenges. By anticipating potential obstacles and implementing contingency plans, teams can minimize disruptions and sustain project momentum, ultimately enhancing the success of medical studies.

Precision in clinical trial design is essential for yielding impactful results. Focused investigation entails the clear definition of objectives, careful selection of endpoints, and identification of the appropriate patient population. By refining the study scope, teams can significantly improve the credibility of their results, ensuring that the information gathered is both pertinent and practical. This method not only enhances study quality but also boosts the chances of regulatory approval, ultimately enabling quicker routes to market for innovative therapies.
Notably, bioaccess® offers an accelerated regulatory approval process, achieving approvals in just 6-8 weeks compared to the typical 6-12 months in the US/EU, and enrolls treatment-naive cardiology or neurology cohorts 50% faster than Western sites.
As W. Edwards Deming pointed out, 'Without information, you're just another individual with a viewpoint,' highlighting the essential role of information in study design.

Supporting studies are crucial in clinical assessments, as they entail conducting additional investigations or analyses to validate the results of primary evaluations. This may include:
By bolstering initial findings with supporting evidence, researchers can significantly enhance the credibility of their results, instilling greater confidence among stakeholders. For instance, post-hoc analyses can uncover clinically significant secondary outcomes when primary endpoints do not succeed, thereby providing deeper insights into the data. This practice is not only vital for obtaining regulatory approval but also plays a pivotal role in the successful commercialization of new therapies.
As we approach 2025, the emphasis on validation studies is more pronounced than ever, given that the average rate of drug withdrawals due to safety concerns has remained approximately 1.5 drugs per year since 1993. Such statistics underscore the necessity of rigorous validation to ensure that new treatments are both safe and effective.

Clinical studies are dynamic processes that necessitate ongoing research to adapt to emerging information and evolving circumstances. This requires meticulous observation of patient responses, conducting interim evaluations, and enacting essential modifications to protocols.
With bioaccess®'s FDA-ready data, research studies can achieve patient enrollment 50% faster than traditional methods, resulting in significant savings of $25K per patient. Emphasizing diversity in clinical studies is vital, as it facilitates the approval of new therapies applicable to various patient demographics.
By fostering a culture of adaptability, study teams can adeptly navigate challenges and improve trial outcomes. As Lindsay Kehoe emphasized, the alignment of flexibility and data quality is critical to meeting purpose-driven objectives. Continuous learning and improvement are paramount for successful phase research, ensuring that methodologies remain responsive to patient needs and regulatory standards.
Furthermore, partnerships like that of bioaccess™ and Caribbean Health Group position Barranquilla as a key hub for medical research in Latin America, supported by Colombia's Minister of Health. As the research study landscape evolves, particularly with advancements expected in 2025, embracing operational adaptability will be crucial for refining procedures and achieving reliable outcomes.

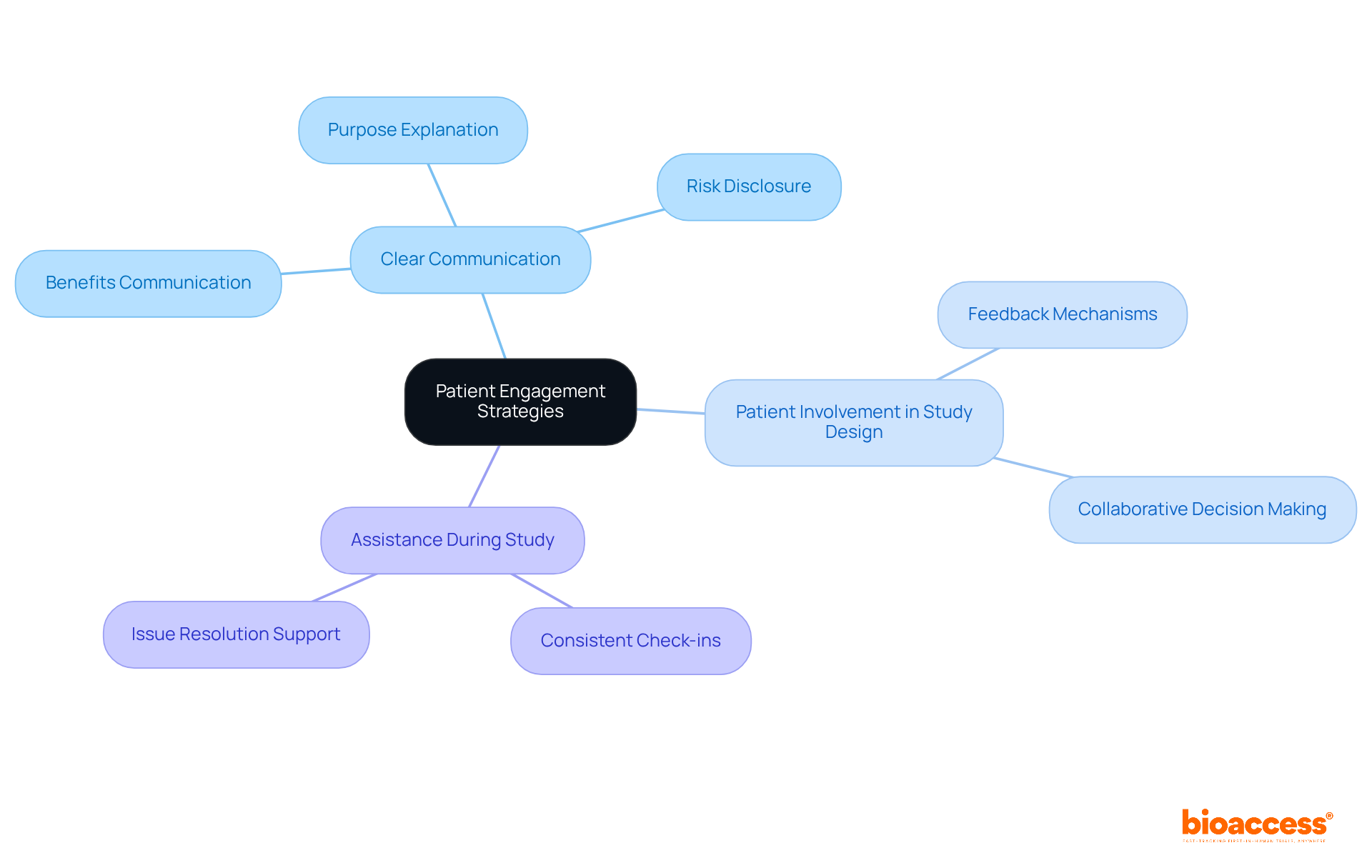
Effective patient engagement strategies are essential for enhancing participation in clinical studies. Clear communication regarding the study's purpose, benefits, and potential risks captures attention and builds interest. Involving patients in the study design fosters a patient-centered approach, increasing their willingness to participate. Furthermore, offering assistance during the study process—including consistent check-ins and issue resolution—boosts retention rates and enhances overall study results. These strategies are not just beneficial; they are critical for achieving successful outcomes in clinical research.
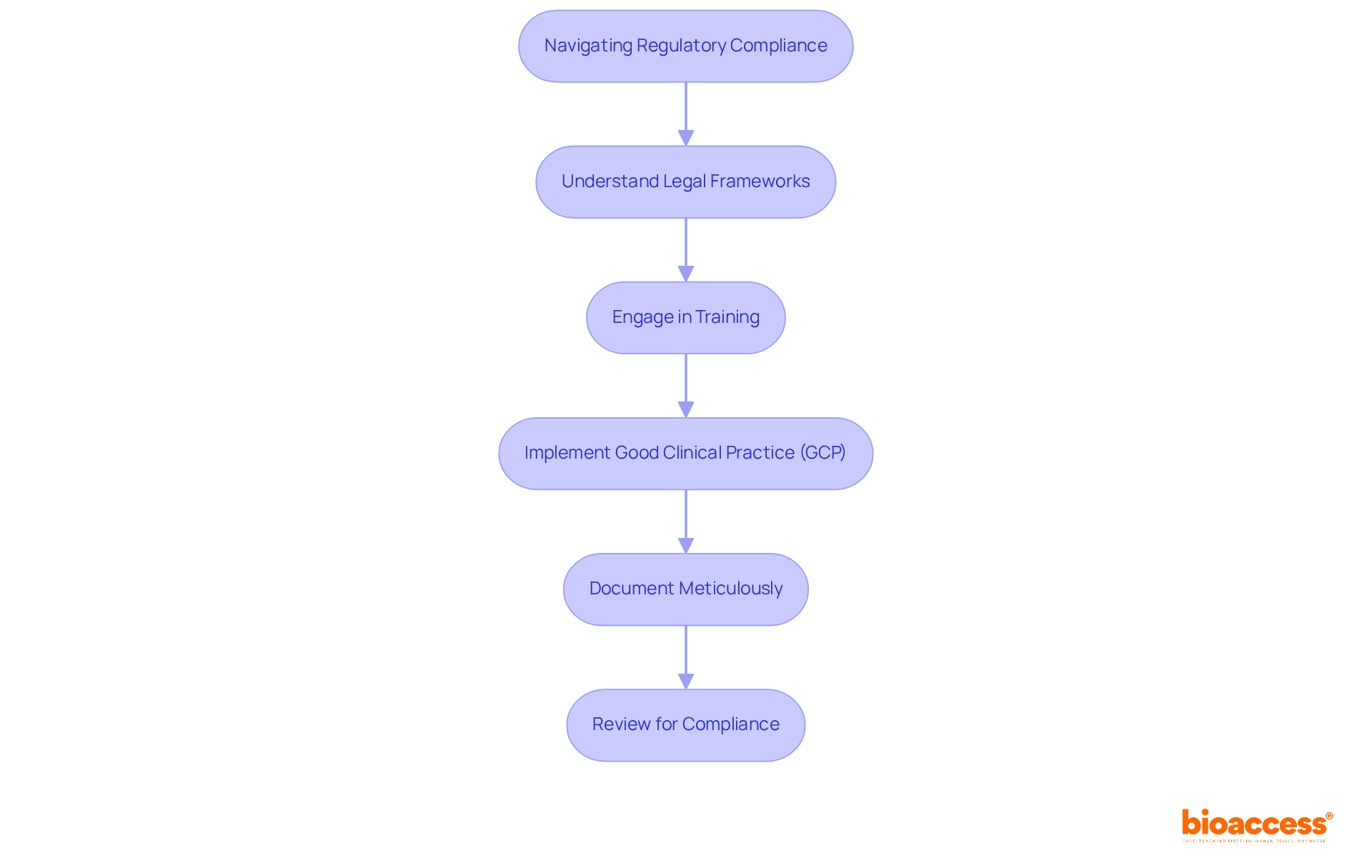
Navigating the intricate landscape of regulatory compliance is paramount for the success of clinical trials. Researchers must possess a thorough understanding of the legal frameworks governing their studies, which encompass ethical guidelines, informed consent processes, and stringent reporting requirements. Adhering to these regulations not only safeguards the integrity of the study but also mitigates the risk of costly delays and potential legal ramifications. A recent audit has underscored that compliance deficiencies can lead to significant setbacks, highlighting the necessity for meticulous documentation and strict adherence to Good Clinical Practice (GCP) guidelines.
To maintain compliance, research teams should engage in regular training and updates on evolving regulatory changes. The introduction of the revised E6(R3) Good Clinical Practice guidelines in 2025 will further impact study conduct, necessitating that sites implement robust systems and procedures to harness new efficiencies while ensuring participant safety and information integrity. Effective navigation of these frameworks has been demonstrated in various case studies, where organizations prioritizing compliance achieved timely database locks and improved information quality, ultimately enhancing study outcomes.
Moreover, the financial implications of regulatory compliance are substantial, with health systems and hospitals expending nearly $39 billion annually on administrative activities related to compliance across multiple domains. This underscores the critical importance of investing in quality management systems focused on risk identification and mitigation. By fostering a culture of adherence and continuous improvement, scientific teams can enhance their operational efficiency and contribute to the advancement of medical science.
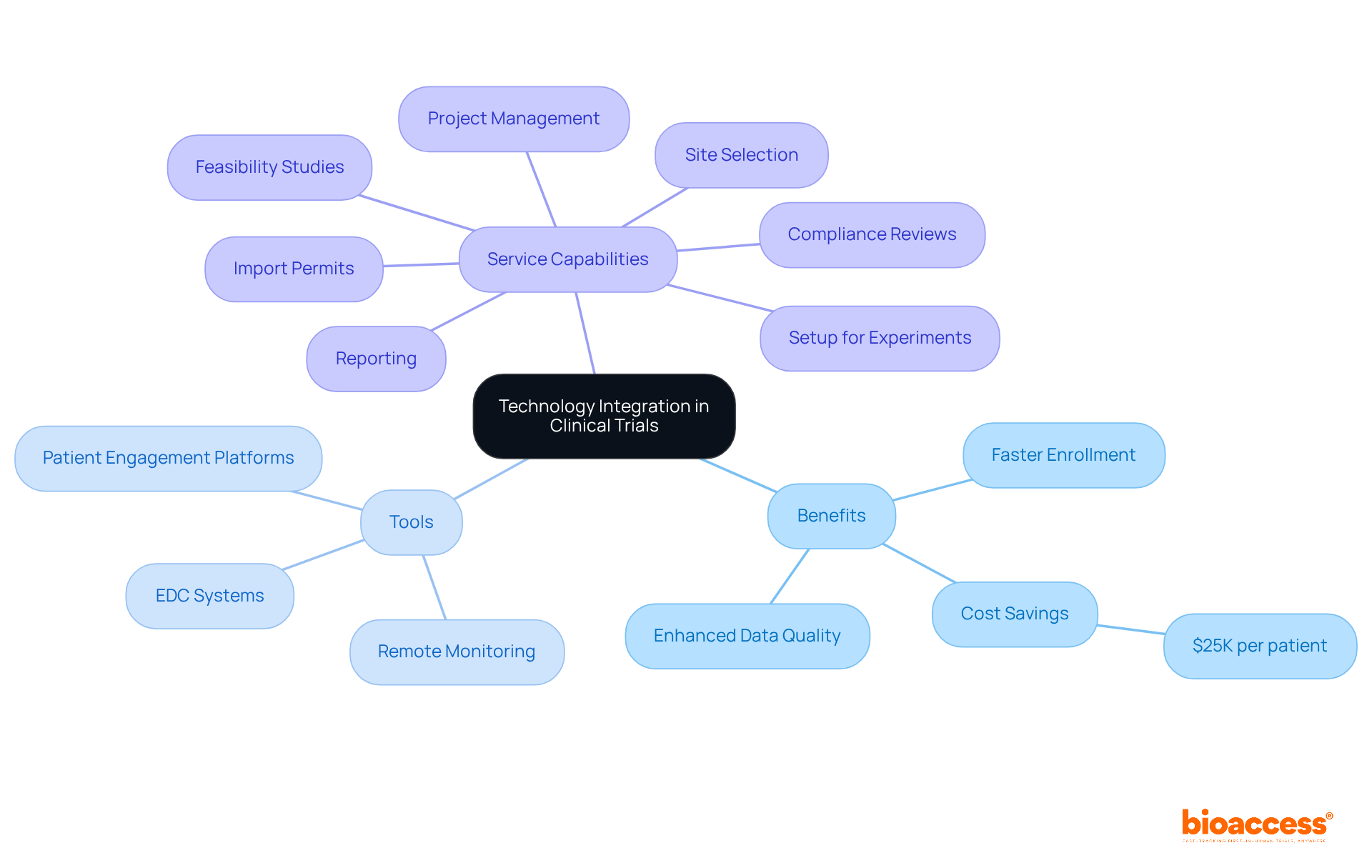
Incorporating technology into clinical trials is crucial for improving efficiency and information management. With bioaccess®, teams can enroll treatment-naive cardiology or neurology cohorts 50% faster than conventional Western sites, achieving significant cost savings of $25K per patient through FDA-ready data—eliminating rework and delays.
Electronic information capture (EDC) systems, along with remote monitoring and patient engagement platforms, play a pivotal role in streamlining processes and enhancing accuracy. By utilizing these advanced tools, study groups can significantly lessen administrative burdens, foster better communication, and ensure real-time access to information.
This technological integration not only accelerates the timeline for phase research but also enhances the overall quality of the data gathered, ultimately resulting in more dependable outcomes in medical studies.
Furthermore, bioaccess® provides extensive service capabilities, including:
These capabilities collectively improve the efficiency and effectiveness of research studies.
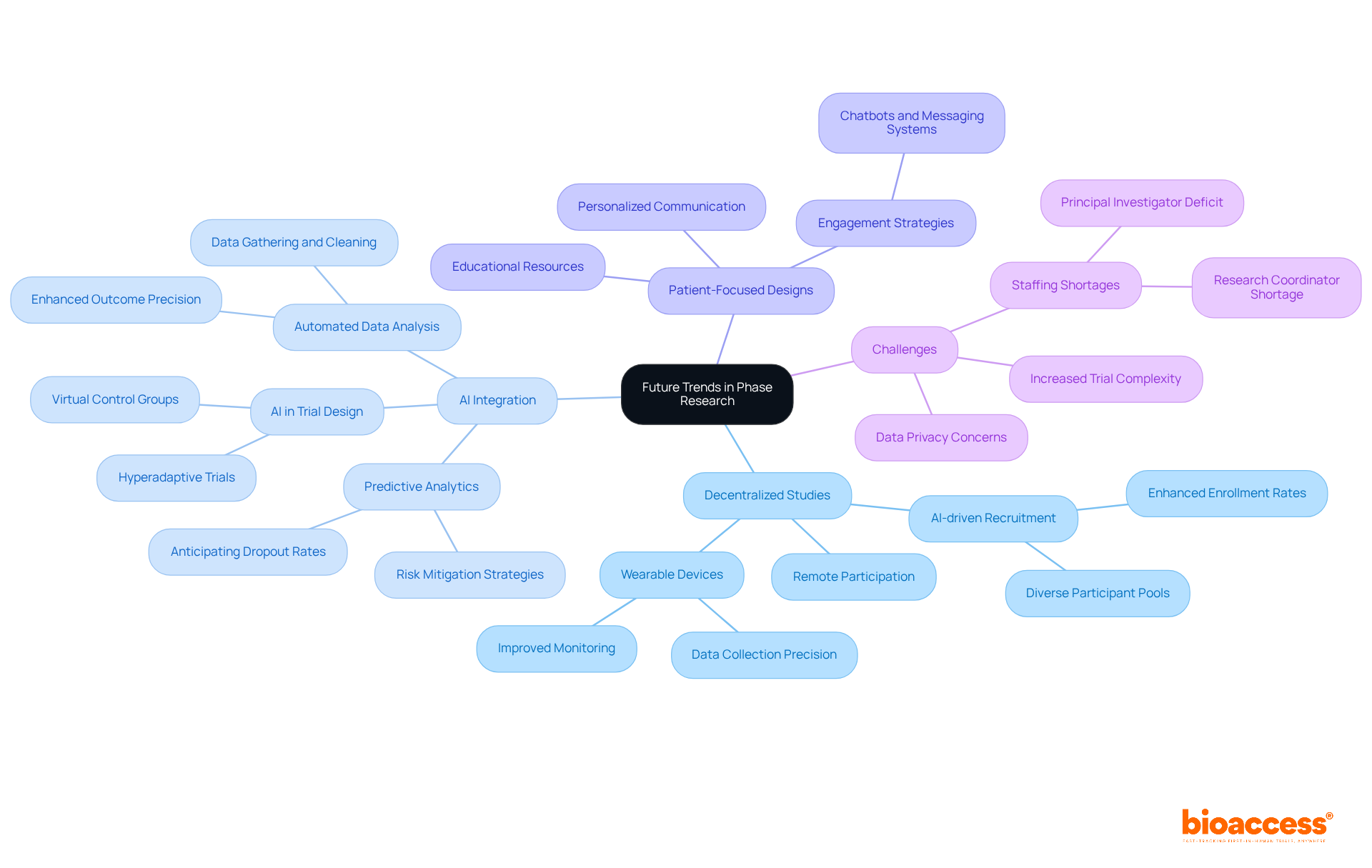
The landscape of clinical studies is undergoing a significant transformation, driven by innovations that are reshaping phase research. Decentralized studies are at the forefront, allowing for greater flexibility and accessibility, which is crucial in meeting diverse patient needs. These studies utilize technology to enable remote participation, thus improving recruitment and retention rates. For instance, AI-driven recruitment tools effectively identify and engage diverse participant pools, fostering inclusivity and improving the generalizability of results.
Artificial intelligence is also transforming information analysis in clinical studies. By automating essential processes like data gathering, cleaning, and interpretation, AI enhances the precision and speed of outcomes. Predictive analytics driven by AI can anticipate participant dropout rates and adverse events, enabling proactive risk mitigation strategies that significantly enhance study efficiency. As the market for AI in healthcare is anticipated to rise from $13.8 billion in 2022 to $164.1 billion by 2029, its incorporation into clinical studies is becoming increasingly essential.
Furthermore, the move towards patient-focused designs is transforming how studies are conducted. By prioritizing participant engagement through personalized communication and educational resources, research teams can enhance satisfaction and retention rates. Innovations like chatbots and automated messaging systems are employed to keep participants informed and involved throughout the testing process.
As we approach 2025, the incorporation of decentralized components and AI in phase research studies is expected to fulfill their promises, enabling sponsors to conduct more intelligent and quicker investigations. This evolution not only addresses the complexities of contemporary medical research but also aligns with the increasing demand for more efficient and effective healthcare solutions. Significantly, more than 40% of locations are facing a primary investigator deficit, and 70% of locations indicate that medical studies have become more difficult to oversee in the past five years, highlighting the necessity of implementing creative solutions. Furthermore, bioaccess's extensive management services for studies—including feasibility assessments, compliance evaluations, setup, import permits, project oversight, and reporting—are crucial in addressing these challenges. Automated data collection methods, such as real-time monitoring and sensor integration, are streamlining research efforts, further enhancing the efficiency of clinical trials.
The insights presented in this article underscore the critical importance of enhancing phase research in clinical trials, particularly within the Medtech sector. By leveraging innovative strategies and technologies, organizations can significantly improve the efficiency, effectiveness, and overall success of their clinical studies. The emphasis on Colombia’s unique advantages further highlights how geographical and regulatory factors can play a pivotal role in accelerating research timelines and patient enrollment.
Key arguments include:
Additionally, effective patient engagement strategies and rigorous regulatory compliance are essential in navigating the complexities of clinical research. The integration of technology, particularly AI and decentralized study designs, is positioned as a transformative force that can streamline operations and enhance data quality.
In conclusion, the evolving landscape of clinical trials necessitates a proactive approach to research methodologies. Embracing innovative practices addresses current challenges while positioning organizations to thrive in an increasingly competitive environment. By prioritizing adaptability, patient engagement, and technological integration, stakeholders can contribute to the advancement of healthcare solutions that are both efficient and effective. As the industry moves toward 2025, the call to action is clear: invest in these insights to ensure that clinical trials meet the demands of modern medicine and ultimately improve patient outcomes.
What is bioaccess® and how does it enhance early-phase clinical research?
bioaccess® leverages expertise and local advantages in Colombia to enhance phase research, particularly for first-in-human studies, by utilizing the country's regulatory efficiency and diverse patient populations.
What are the advantages of conducting clinical research in Colombia?
Colombia offers significant competitive advantages, including the ability to secure ethical approvals in 90 to 120 days and faster patient enrollment, particularly in treatment-naive cardiology or neurology cohorts, compared to Western sites.
How does bioaccess® impact the timeline for Medtech innovations?
By accelerating patient enrollment and reducing regulatory timelines, bioaccess® enables companies to bring their products to market more swiftly than through traditional pathways.
What is the significance of exploratory research in clinical trials?
Exploratory research is crucial as it helps medical study teams gather vital information on feasibility and effectiveness, assess patient populations, understand disease mechanisms, and identify potential endpoints.
What challenges do clinical studies face as we approach 2025?
Challenges include recruitment issues, regulatory complexities, and budget limitations, with a projected decrease in the number of medical studies launched in the US due to the Inflation Reduction Act.
How can project plans be improved to navigate obstacles in clinical trials?
Developing comprehensive project plans, engaging stakeholders early, and maintaining open communication can enhance collaboration and transparency in navigating challenges.
What strategies can be used to address recruitment difficulties in clinical studies?
Sponsors can consider unconventional methods, such as partnering with community organizations to reach underrepresented populations, and implementing tailored recruitment strategies.
What is the anticipated trend for study designs in response to rising costs and recruitment challenges?
A resurgence of adaptive studies, which allow modifications based on interim findings, is expected to address concerns regarding costs and recruitment challenges in clinical trials.