

The article titled "10 Key Pharmacokinetics (PK) Concepts Every Research Director Should Know" serves as an essential guide for research directors in clinical studies, focusing on crucial pharmacokinetic principles. It underscores the importance of grasping concepts such as:
These factors are not merely academic; they directly influence drug development, dosing strategies, and patient outcomes. Thus, the article highlights the pivotal role of pharmacokinetics in optimizing therapeutic effectiveness, making it a fundamental aspect of clinical research.
Pharmacokinetics (PK) is crucial in the development and administration of medications, yet its complexities often go unnoticed by many research directors. A solid grasp of key concepts—absorption, distribution, metabolism, and excretion—can greatly enhance the effectiveness of clinical studies and improve patient care. With the rapid advancements in pharmacogenetics and the unique challenges presented by critically ill patients, how can directors ensure they are prepared to navigate these intricate dynamics? This article delves into ten essential pharmacokinetics concepts that every research director must understand to optimize their clinical research strategies.
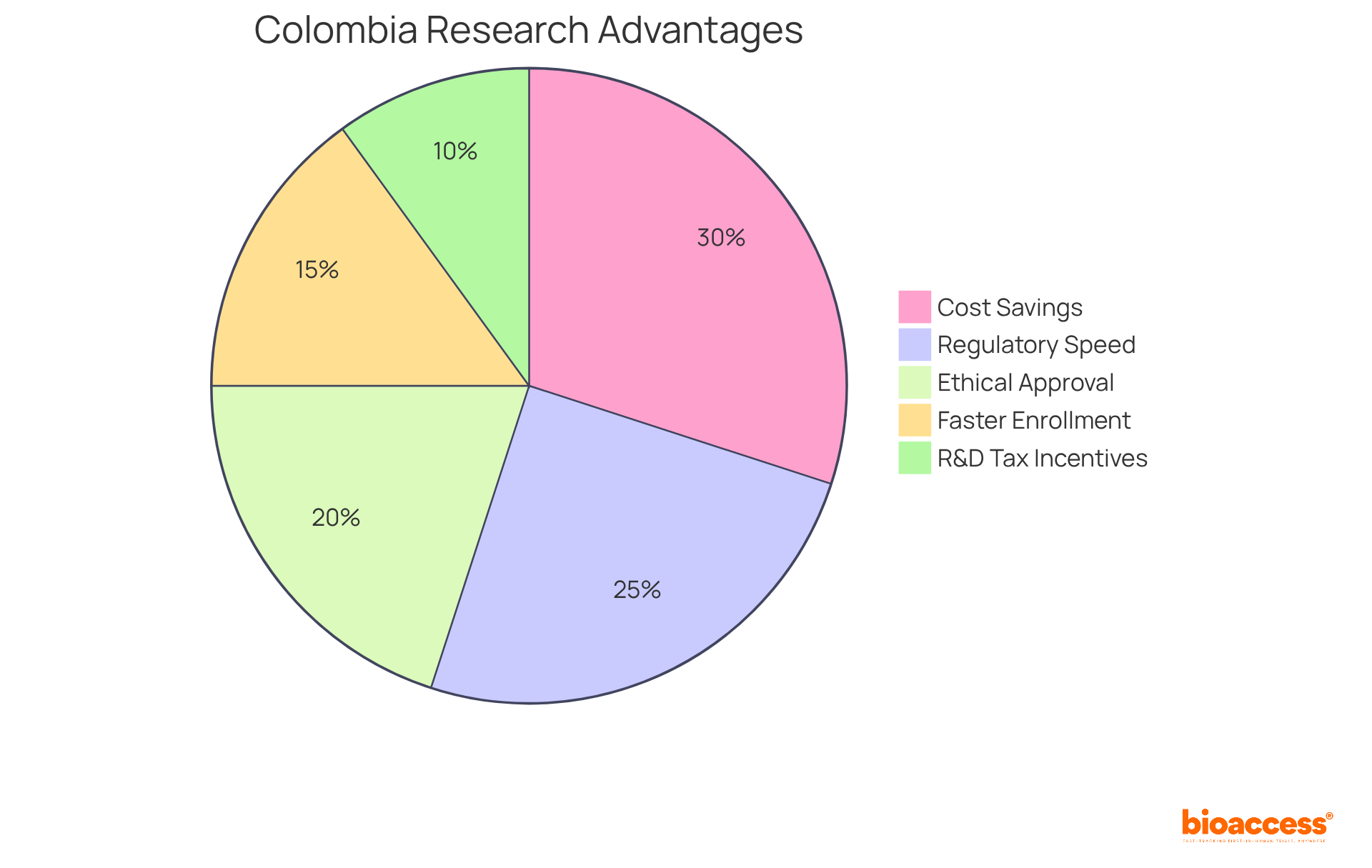
bioaccess® excels in streamlining clinical research processes, particularly in pharmacokinetics (PK), by leveraging its strategic presence in Latin America, especially Colombia. This country offers remarkable cost savings of over 30% compared to North America and Western Europe, coupled with a swift regulatory review process that typically spans only 90-120 days.
With ethical approvals secured in a rapid 4-6 weeks and enrollment rates that are 50% faster than traditional markets, bioaccess® provides a substantial competitive advantage for researchers. Such operational flexibility enables swift data gathering and evaluation, essential for studies that require prompt outcomes to inform medication development and regulatory filings.
Clinical research directors emphasize the significance of these expedited processes, noting that efficient regulatory pathways not only enhance study timelines but also improve overall project viability. Successful studies on medication absorption conducted in Colombia further illustrate the effectiveness of bioaccess®'s approach, showcasing how strategic location and regulatory speed can lead to groundbreaking advancements in treatment research and development.
Additionally, Colombia's healthcare system is ranked #22 by the World Health Organization, and hospitals must undergo rigorous ICH/GCP certification, ensuring high-quality standards in clinical research. The country's population of over 50 million, with 95% covered by universal healthcare, provides a robust patient recruitment base.
Moreover, R&D tax incentives, including a 100% tax deduction for investments in science and technology, enhance the attractiveness of conducting trials in Colombia.
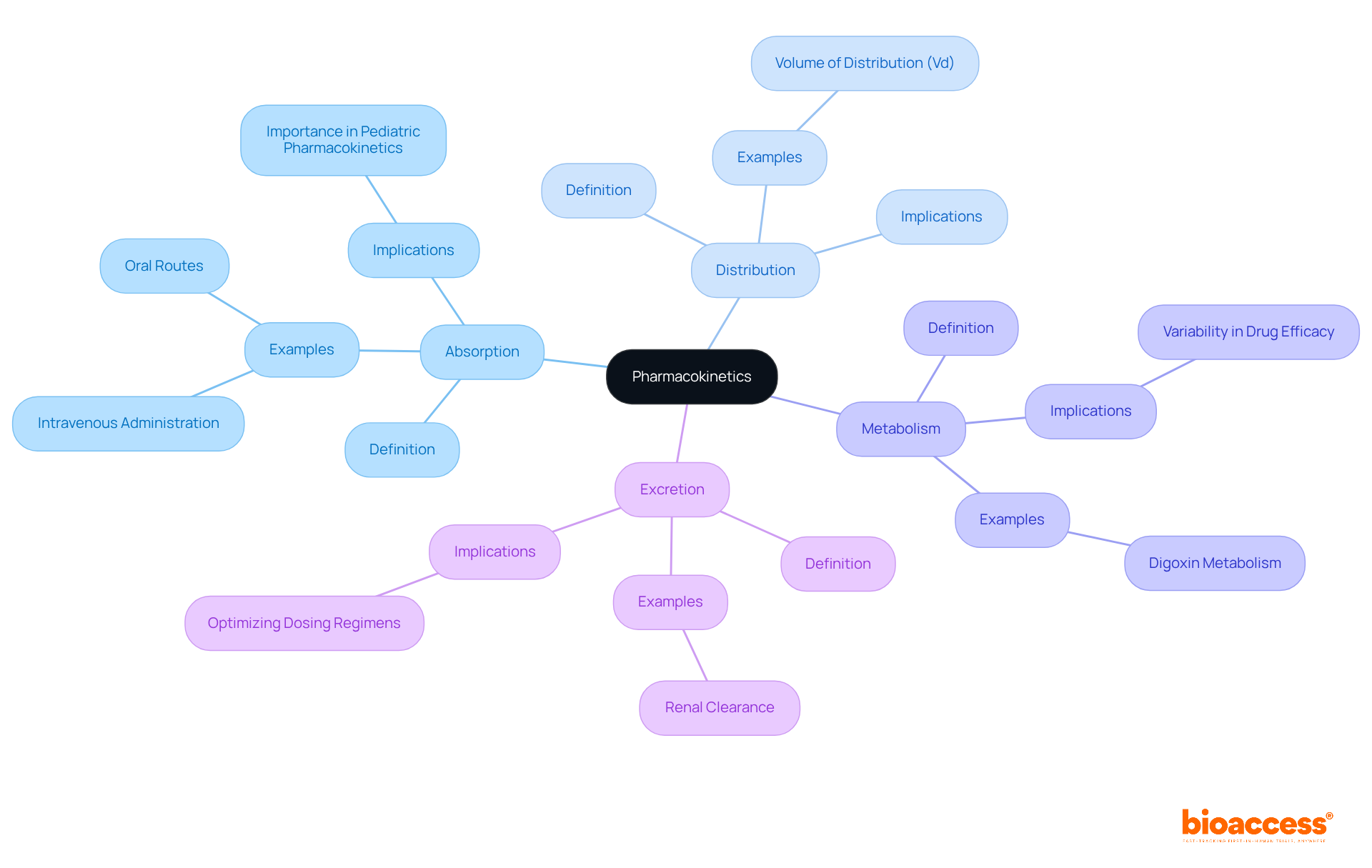
Key pharmacokinetics (pk) terms are essential for understanding medication behavior in the body.
Absorption: This is the process by which a substance enters the bloodstream, significantly influenced by the method of administration. For instance, intravenous administration ensures 100% bioavailability, while oral routes may vary based on factors like food intake and gastrointestinal health. A recent £1.5m Wellcome Trust Health Innovation grant aims to develop a child-friendly formulation of 13-cis-retinoic acid, highlighting the importance of absorption in pediatric pharmacokinetics (pk).
Distribution: This refers to how the substance disperses throughout the body, which can be affected by its chemical properties and the individual's physiology. For instance, the volume of distribution (Vd) is an essential parameter that describes the quantity of medication in the body relative to plasma concentration, influencing dosing strategies.
Metabolism: This involves the biochemical alteration of substances, primarily occurring in the liver. Variability in metabolism can result in differences in medication efficacy, as observed with digoxin, where gut bacteria affect its effectiveness by metabolizing the substance differently in various individuals. The case study "Deciphering Digoxin Deactivation" illustrates this variability, providing insights for improving therapeutic use.
Excretion: The elimination of substances from the body is essential for sustaining therapeutic levels and reducing toxicity. Understanding the pathways of excretion, such as renal clearance, is vital for optimizing dosing regimens.
Understanding these concepts is essential for any research director engaged in pharmacokinetics (PK), as they directly influence medication development and care for individuals. As Dr. Gareth Veal observes, tracking individual patient medication exposures can result in more suitable dosing schedules, especially in specialized groups such as children with cancer. This emphasizes the significance of drug movement terminology in research and its use in practical situations. Furthermore, advancements such as a new microsensor capable of tracking pharmacokinetics (PK) and pharmacodynamics in live animals represent current trends in pharmacokinetics research, further highlighting the importance of these concepts.
Fundamental formulas in pharmacokinetics are essential for understanding the behavior of substances within the body and for designing effective clinical studies. The Volume of Distribution (Vd) is calculated as:
This indicates the extent to which a substance disperses throughout various body compartments.
Clearance (Cl) is defined by the formula:
This reflects the efficiency of substance removal from the body.
The Half-life (t1/2), representing the time required for plasma levels of a substance to decrease by 50%, can be estimated using the equation:
This relationship underscores that substances with a high Vd typically exhibit longer half-lives, as a greater proportion remains outside the central compartment, thereby influencing the rate of decline in plasma levels during elimination.
Furthermore, loading doses can be calculated using the formula:
where Cp signifies the desired plasma concentration and F denotes bioavailability.
Understanding pharmacokinetics pk formulas is crucial for optimizing medication therapy and ensuring safety for patients, particularly in diverse populations where factors such as age, body composition, renal function, and genetic variations in enzyme metabolism significantly impact clearance rates and medication distribution.

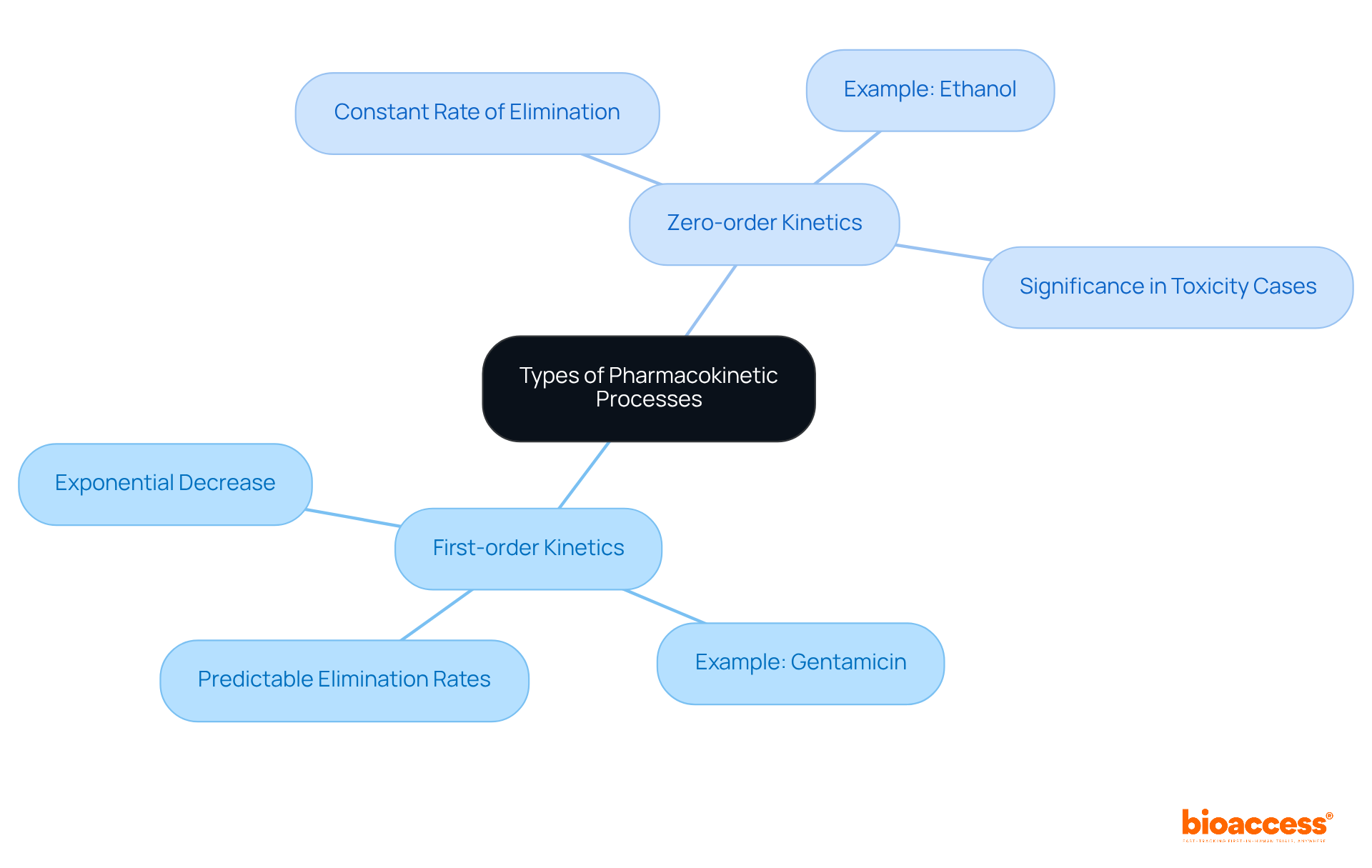
Understanding pharmacokinetics (pk) processes is vital for grasping how medications function within the body, particularly across diverse patient populations. Two primary types of kinetics are crucial to comprehend:
First-order kinetics: This process involves an exponential decrease in drug concentration over time, indicating that the rate of elimination correlates with the drug's concentration. For instance, gentamicin undergoes first-order kinetics, where its elimination rate is directly proportional to its level. This characteristic facilitates predictable pharmacokinetics pk and medication elimination rates, which is essential for dosage adjustments and therapeutic monitoring.
Zero-order kinetics: Here, the substance's level diminishes at a constant rate, irrespective of its initial amount. This phenomenon arises when metabolic pathways become saturated, as observed with substances like ethanol, where the elimination rate remains consistent regardless of the initial concentration. Understanding zero-order kinetics within pharmacokinetics pk is particularly crucial in toxicity cases, such as methanol ingestion, where the time elapsed since ingestion becomes more significant than the quantity consumed. This comprehension aids clinicians in making informed treatment decisions.
Moreover, substances like phenytoin exhibit non-linear elimination kinetics, which underscores the importance of pharmacokinetics pk for meticulous monitoring and minor dose adjustments. As C. Borowy emphasized, "When administering medications, one must fully understand the mechanism of action and elimination to reduce unwanted adverse effects." As research on medication absorption progresses in 2025, it continues to shed light on the complexities of substance dynamics, enhancing the ability to tailor therapies to individual patient needs.
Substance clearance is defined as the volume of plasma from which a compound is entirely eliminated per unit time, establishing it as a vital parameter in pharmacokinetics (pk) studies. Understanding pharmacokinetics (pk), especially medication clearance, is crucial for establishing suitable dosing intervals and quantities, which directly influences therapeutic effectiveness and safety.
Several factors influence drug clearance, including:
Recent research highlights that variations in metabolic enzyme activity, often influenced by genetic factors, can significantly affect clearance rates. For instance, research indicates that older individuals may encounter decreased clearance due to impaired organ function, necessitating careful dosage modifications.
Furthermore, drug-drug interactions can lead to altered clearance profiles, complicating treatment regimens. Accurate assessment of these factors is essential for effective pharmacokinetics (pk) modeling, ensuring that dosing strategies are tailored to individual patient needs and improving overall clinical outcomes.
As noted by J.P. Garnier, former Chief Executive of GlaxoSmithKline, the pharmaceutical sector faces challenges that require a nuanced understanding of medication clearance, particularly in the context of evolving healthcare environments and regulatory pressures. Additionally, globalization has introduced complexities in medication clearance practices, as variations in ethnic origin, diet, and environmental factors can create differences in medicine demand and clearance rates across regions.
Incorporating insights from recent case studies, such as those examining R&D outsourcing trends, can further clarify the implications of medication clearance in the broader pharmaceutical context.

Bioavailability refers to the fraction of an administered dose that successfully reaches systemic circulation. This critical concept is influenced by several factors, including:
Understanding these influences is essential for evaluating bioavailability, as it plays a pivotal role in comparing various medication formulations. Ultimately, this evaluation ensures that patients receive effective doses, thereby enhancing the overall efficacy of clinical treatments.
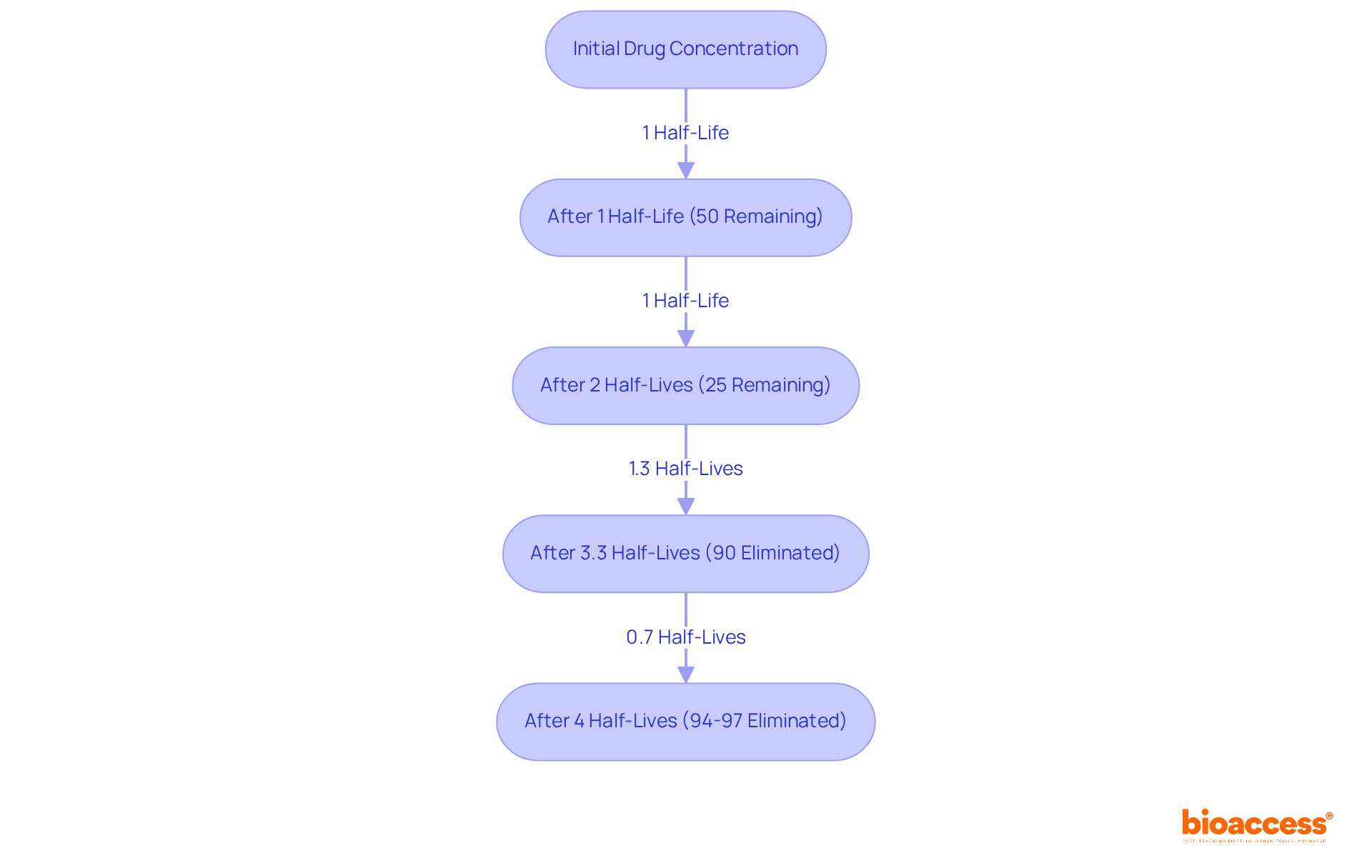
Half-life (t1/2) is defined as the time required for the plasma concentration of a substance to decrease by 50%. This pharmacokinetics (PK) parameter is crucial for establishing dosing intervals and comprehending medication accumulation within the body. For example, substances with a long half-life can often be administered less frequently due to their prolonged effects, whereas those with a short half-life may require more frequent dosing to sustain therapeutic levels.
Recent research indicates that approximately 90% of a medication is eliminated after about 3.3 half-lives, with 94% to 97% cleared after 4 to 5 half-lives. This knowledge is vital for clinicians when designing dosing regimens, as it directly influences patient adherence and treatment efficacy. Furthermore, the relationship between half-life and dosing frequency is underscored by the fact that most clinically significant substances follow first-order pharmacokinetics (PK), where the elimination rate is proportional to the substance level. This principle emphasizes the necessity of accurately calculating half-life to optimize dosing schedules and minimize the risk of toxicity.
In practice, the steady-state concentration of a medication—achieved after approximately 4 to 5 half-lives—plays a pivotal role in determining the frequency of administration. For instance, a recent survey revealed that 97% of oncologists are open to discussing flexible dosing options with patients, reflecting a growing recognition of the need for personalized treatment plans based on pharmacokinetics (PK) principles. Notably, 85% of oncologists do not believe that a higher dose of a cancer treatment is always more effective, highlighting the importance of personalized dosing strategies.
Understanding pharmacokinetics (PK), particularly half-life, not only aids in effective dosing strategies but also empowers patients to engage in discussions about their treatment, ultimately leading to improved health outcomes. Moreover, the clinical significance of half-life is particularly evident in situations involving toxicity, such as overdose or incorrect dosages, where precise dosing can prevent adverse effects. Factors such as renal or hepatic disease can significantly influence medication half-life, necessitating careful consideration in dosing decisions. Additionally, elimination curves graphically represent the half-life of substances, providing a visual aid in understanding substance elimination over time. Lastly, patient age can influence the precise half-life of a medication, particularly in pediatric and geriatric populations, underscoring the necessity for a nuanced approach to pharmacokinetics (PK).
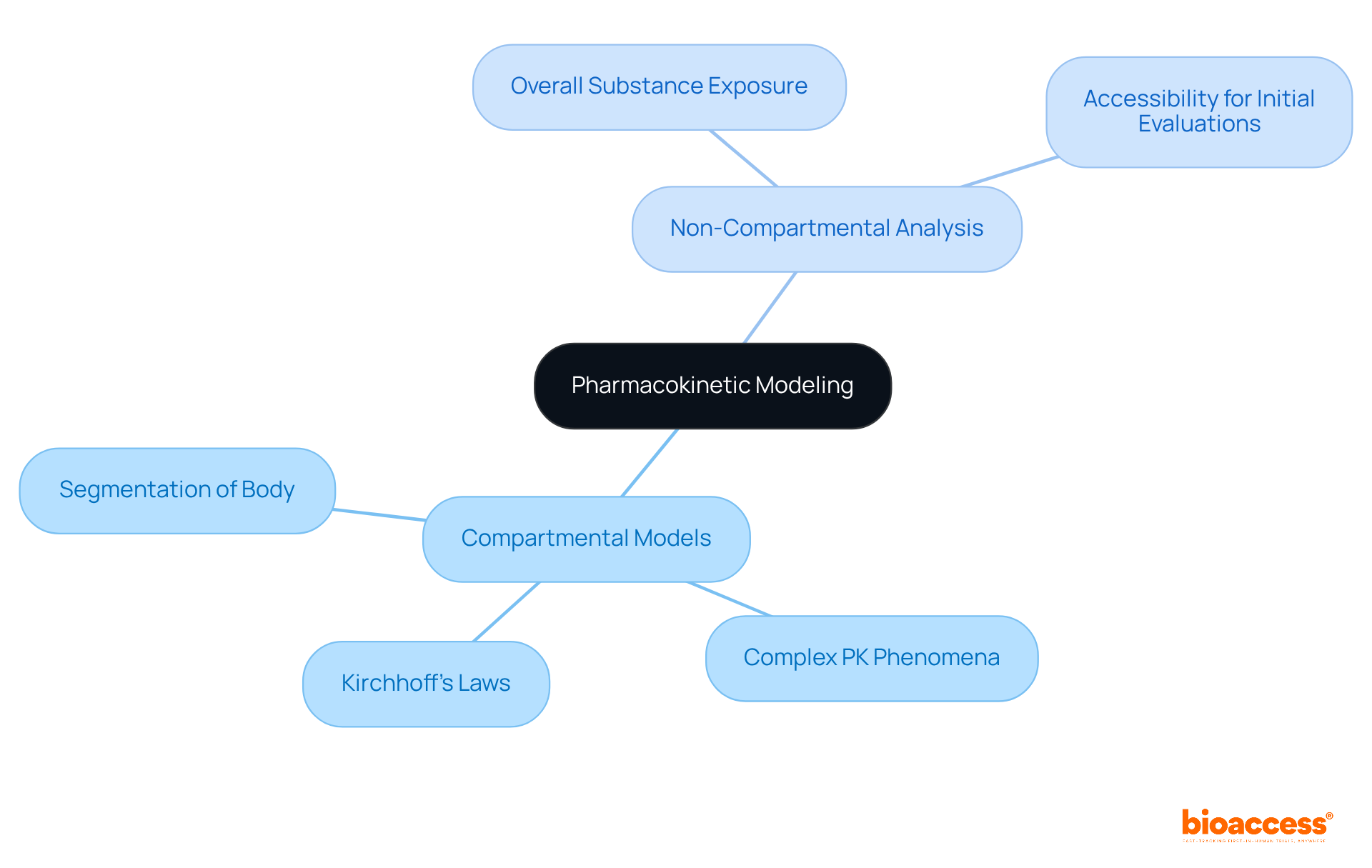
Pharmacokinetics (PK) modeling employs mathematical frameworks to predict the behavior of substances within the body, playing a crucial role in medication development. Two primary modeling approaches exist: compartmental models and non-compartmental analysis.
Compartmental models segment the body into distinct sections, enabling comprehensive simulations of substance distribution and elimination. This method is particularly advantageous for understanding complex pharmacokinetics (PK) phenomena, as it captures the dynamics of substance movement between compartments. For instance, the application of Kirchhoff’s Laws in pharmacokinetics (PK) research has deepened the understanding of medication clearance mechanisms, offering a more precise depiction of substance behavior.
Conversely, non-compartmental analysis presents a more straightforward approach, focusing on overall substance exposure without necessitating compartmentalization. This method streamlines the analysis, making it accessible for initial evaluations and situations where detailed compartmental data may not be essential.
Recent advancements in modeling techniques related to medication absorption and distribution have significantly enhanced the accuracy of substance behavior predictions. The integration of machine learning algorithms, for example, has simplified the development of population pharmacology (PopPK) models, enabling researchers to analyze diverse datasets effectively. This automation not only reduces the time required for model development but also enhances the reproducibility of results. In fact, studies indicate that the implementation of model-informed treatment development (MIDD) can save up to four years in clinical trial processes, underscoring the efficiency of these models in treatment development.
Researchers highlight the critical role of compartmental models in pharmacokinetics (PK). As one remarked, 'Population medication models are essential for comprehending substance behavior across groups, yet conventional development is frequently laborious and sluggish.' This underscores the ongoing need for innovative modeling methods that can adapt to the complexities of modern pharmaceutical development.
Ultimately, the evolution of pharmacokinetics (PK) modeling continues to transform the landscape of clinical research, providing vital tools for predicting medication behavior and optimizing therapeutic outcomes.
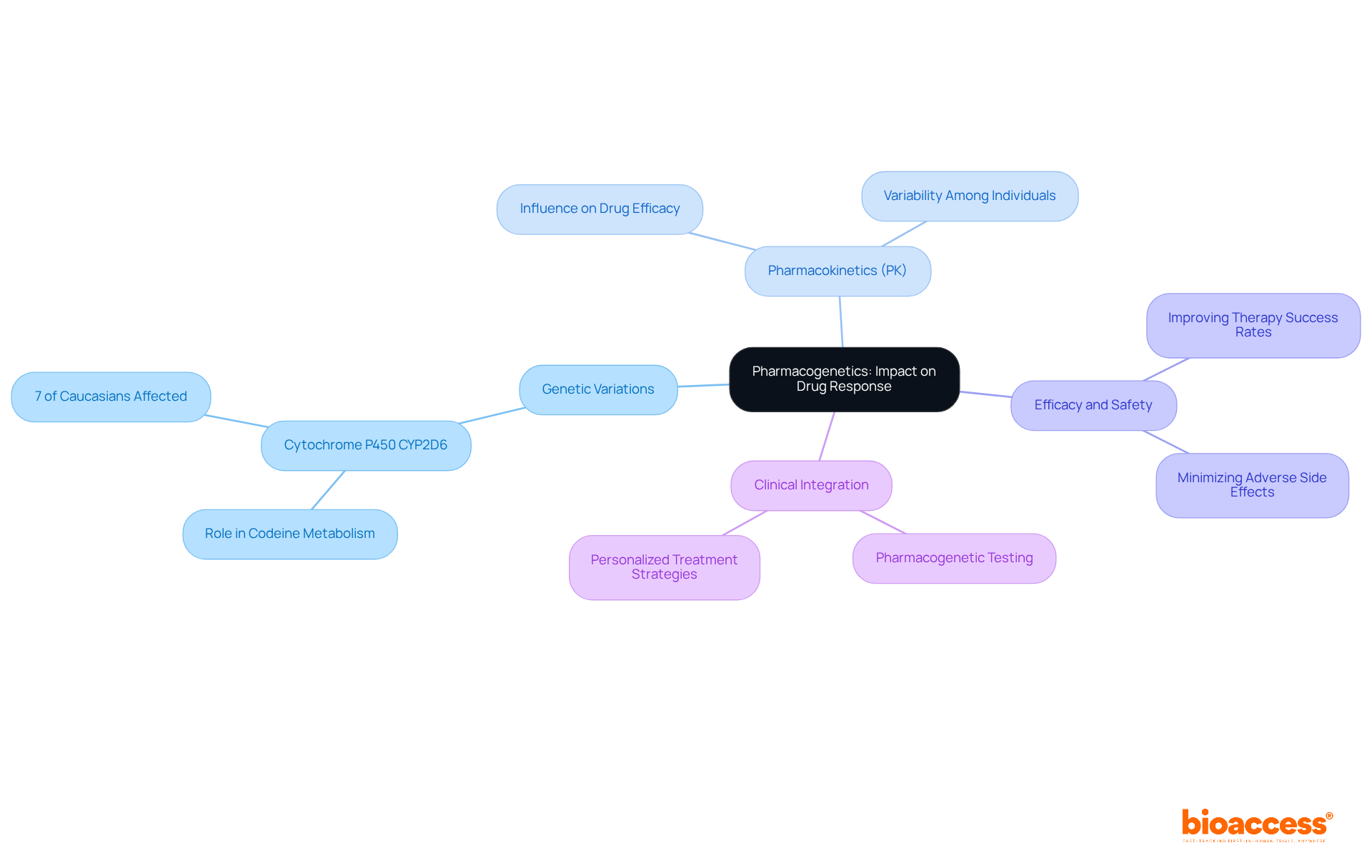
Pharmacogenetics investigates how genetic elements affect pharmacokinetics (PK) and medication response. This field demonstrates that variations in the genes responsible for metabolizing enzymes can lead to significant differences in efficacy and safety among individuals. For example, approximately 7% of Caucasians lack one copy of the Cytochrome P450 CYP2D6 gene, which is crucial for metabolizing codeine, rendering the substance ineffective for these individuals. Such findings underscore the necessity of integrating pharmacogenetic data into clinical trials, as this integration can enhance the understanding of pharmacokinetics (PK) and optimize treatment strategies.
Recent studies emphasize the potential of pharmacogenetic testing to anticipate individual responses to medications, thereby improving therapy success rates and minimizing adverse side effects. Experts in the field assert that customizing treatments according to genetic profiles not only enhances outcomes for individuals but also fosters a more personalized approach to medicine. By leveraging these insights, researchers can navigate the complexities of pharmacokinetics (PK) and medication metabolism more effectively, ultimately leading to safer and more effective therapeutic interventions.
In critically ill individuals, variability in pharmacokinetics is significantly influenced by factors such as organ dysfunction, altered blood flow, and changes in body composition. Acute kidney injury, for instance, can lead to substance accumulation and altered pharmacokinetics, necessitating careful monitoring and potential dose adjustments to prevent toxicity. Research indicates that as many as 74% of ICU patients required modifications in their beta-lactam dosages based on assessed medication levels, underscoring the necessity for personalized pharmacotherapy. Furthermore, monitoring of antibiotic levels in patients undergoing renal replacement therapy revealed subtherapeutic amounts 15% of the time, highlighting the challenges of dosing adjustments in this population.
Moreover, the impact of organ dysfunction on medication absorption and distribution is profound. Gastrointestinal complications can impair drug absorption during enteral feeding, while fluctuations in plasma protein levels can affect drug binding and free drug concentrations, increasing the risk of adverse effects. As articulated by Dr. Morales Castro, "For the aforementioned reasons, critically ill individuals require a high degree of individualisation to achieve appropriate pharmacotherapy." Understanding these pharmacokinetic variations is crucial for optimizing treatment in this vulnerable population.
Modifying medication dosages according to individual variability is essential for reaching therapeutic objectives. Hydrophilic medications, for example, often necessitate larger initial loading doses in critically ill individuals due to an increased volume of distribution. This individualized approach is vital for ensuring effective pharmacotherapy while taking pharmacokinetics into account to minimize the risk of therapeutic failures. The integration of therapeutic medication monitoring (TDM) into clinical practice further supports this tailored strategy, as TDM is essential for safe pharmacotherapy in the ICU, allowing for real-time adjustments based on patient-specific factors. Additionally, the complexity of drug-drug interactions in critically ill patients cannot be overlooked; the risk of interactions increases fourfold when seven medications are used and up to eightfold when more than ten drugs are administered.

Understanding pharmacokinetics (PK) is essential for research directors who aim to enhance medication development and improve patient outcomes. This article has explored key concepts in pharmacokinetics, emphasizing the importance of grasping how drugs are absorbed, distributed, metabolized, and excreted. By integrating insights from bioaccess® and the unique advantages of conducting clinical research in Colombia, the discussion highlights the significant impact of regulatory efficiency and cost-effectiveness on the research landscape.
Key arguments presented include:
The exploration of drug clearance, bioavailability, half-life, and pharmacogenetics underscores the complexity of medication behavior and the necessity for personalized treatment approaches. Furthermore, the challenges faced in critically ill patients illustrate the need for tailored pharmacotherapy, reinforcing the significance of understanding patient variability in pharmacokinetics.
Ultimately, the insights shared in this article serve as a call to action for research directors to prioritize pharmacokinetic principles in their studies. By leveraging these concepts, researchers can optimize drug development processes, enhance therapeutic effectiveness, and contribute to safer and more effective medical interventions. Engaging with the evolving landscape of pharmacokinetics will not only advance clinical research but also ensure that treatments are tailored to meet the diverse needs of patients.
What is bioaccess® and how does it contribute to clinical research in pharmacokinetics?
bioaccess® is a platform that streamlines clinical research processes in pharmacokinetics, particularly in Latin America, with a strong focus on Colombia. It offers significant cost savings, a fast regulatory review process, and quicker ethical approvals, enhancing the efficiency of clinical trials.
What are the cost advantages of conducting clinical research in Colombia through bioaccess®?
Conducting clinical research in Colombia can result in cost savings of over 30% compared to North America and Western Europe, making it a financially attractive option for researchers.
How quickly can ethical approvals be obtained in Colombia for clinical trials?
Ethical approvals in Colombia can be secured in a rapid timeframe of 4-6 weeks.
What enrollment advantages does bioaccess® provide?
Enrollment rates in Colombia are 50% faster than in traditional markets, allowing for quicker participant recruitment for clinical studies.
What is the significance of Colombia's healthcare system for clinical research?
Colombia's healthcare system is ranked #22 by the World Health Organization and mandates rigorous ICH/GCP certification for hospitals, ensuring high-quality standards in clinical research. The population of over 50 million, with 95% covered by universal healthcare, provides a strong patient recruitment base.
Are there any tax incentives for conducting research and development in Colombia?
Yes, Colombia offers R&D tax incentives, including a 100% tax deduction for investments in science and technology, which enhances the appeal of conducting clinical trials in the country.
What are some key pharmacokinetic terms that researchers should know?
Key pharmacokinetic terms include absorption, distribution, metabolism, and excretion, all of which are crucial for understanding how medications behave in the body.
How is the Volume of Distribution (Vd) calculated in pharmacokinetics?
The Volume of Distribution (Vd) is calculated using the formula: Vd = Amount of substance in the body / Plasma level.
What does the clearance (Cl) formula represent in pharmacokinetics?
Clearance (Cl) is defined by the formula: Cl = Rate of elimination / Plasma level, reflecting the efficiency of substance removal from the body.
How is the half-life (t1/2) of a substance estimated?
The half-life (t1/2) can be estimated using the equation: t1/2 = 0.693 × Vd / Cl, indicating how long it takes for plasma levels to decrease by 50%.
Why is understanding pharmacokinetics formulas important for patient safety?
Understanding pharmacokinetics formulas is crucial for optimizing medication therapy and ensuring safety, especially in diverse populations where various factors can affect drug clearance and distribution.