


The article titled "10 Key Strategies to Enhance Your Safety Trial Success" presents effective methods aimed at improving outcomes in safety trials within clinical research. It underscores the significance of engaging diverse patient populations, implementing robust monitoring systems, and training teams on compliance. These strategies are not just theoretical; they are backed by evidence that highlights their effectiveness in enhancing trial efficiency, ensuring participant safety, and maintaining data integrity.
In the evolving Medtech landscape, these strategies play a crucial role in addressing key challenges faced by researchers. By focusing on inclusivity and rigorous monitoring, clinical trials can achieve better results and foster trust among participants. This approach not only enhances the quality of data collected but also contributes to the overall success of the trials.
Ultimately, collaboration among stakeholders is essential for navigating the complexities of clinical research. By adopting these strategies, researchers can significantly improve their trial outcomes and ensure a safer environment for participants. The next steps involve integrating these practices into existing frameworks to drive meaningful change.
In the increasingly complex landscape of clinical research, the success of safety trials relies heavily on strategic approaches that streamline processes and enhance participant engagement. By implementing targeted strategies, researchers can accelerate trial timelines, ensure compliance, and improve outcomes. Yet, as the regulatory environment evolves, a critical question arises: how can clinical research teams effectively navigate these challenges to maximize the success of their safety trials? This article delves into ten key strategies designed to enhance safety trial success, offering actionable insights for researchers eager to lead the way in medical innovation.
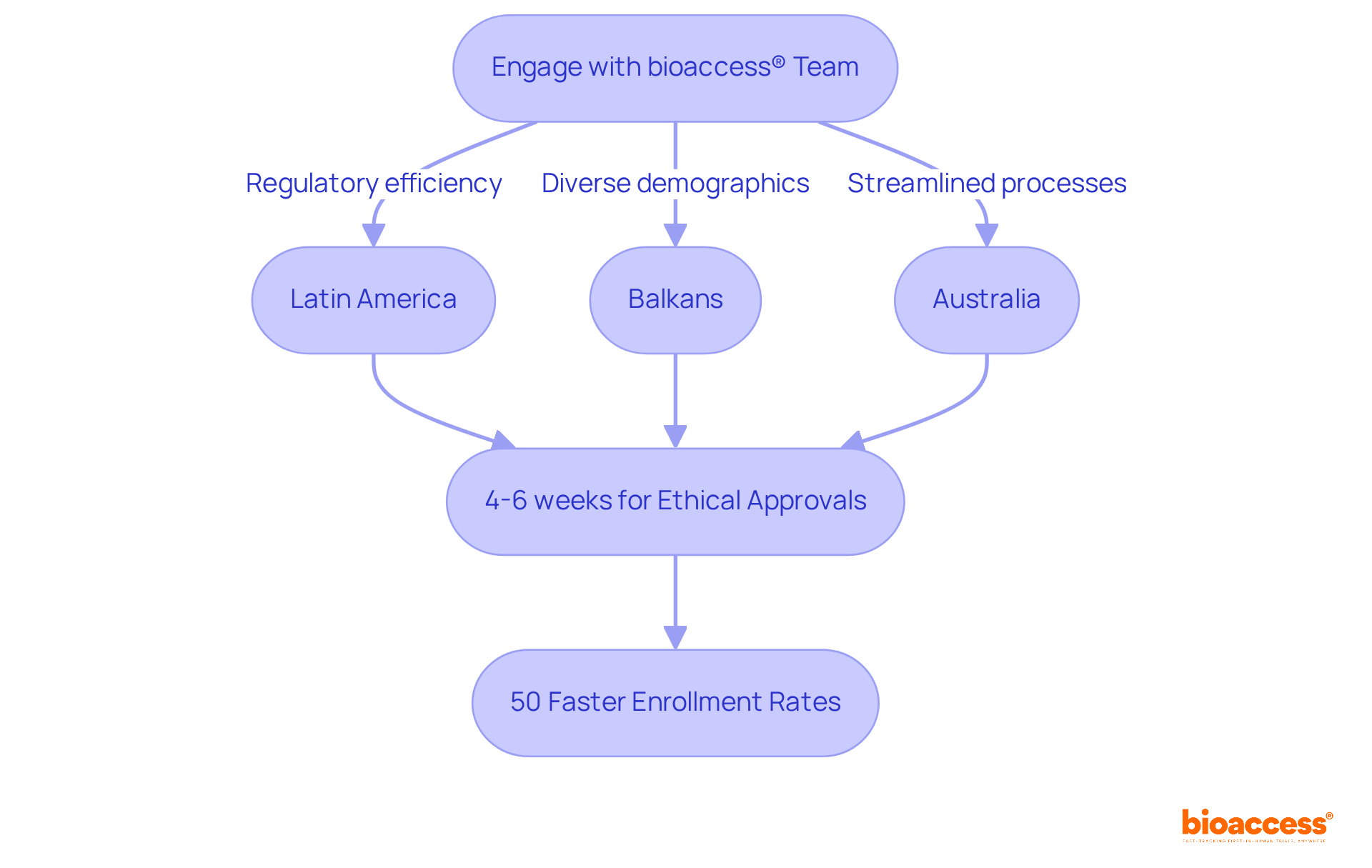
bioaccess® stands out in delivering customized research services that significantly expedite security assessments. By harnessing the regulatory efficiency found in Latin America, the diverse patient demographics of the Balkans, and the streamlined processes in Australia, bioaccess® secures ethical approvals in an impressive timeframe of just 4-6 weeks. This remarkable agility leads to enrollment rates that are 50% faster than traditional methods, enabling evaluations to begin promptly. Such efficiency not only accelerates the pace of medical research but also enhances the potential for timely insights and innovations in medical technology.
Successful examples of protection assessments utilizing these rapid ethical approvals underscore the effectiveness of this approach, illustrating how bioaccess® is at the forefront of advancing evaluations in the Medtech and Biopharma sectors. To fully leverage the advantages of bioaccess®'s comprehensive services, clinical research directors are urged to engage early with the team. This collaboration allows for tailored solutions that align with specific study requirements, ensuring a smoother and more efficient process.
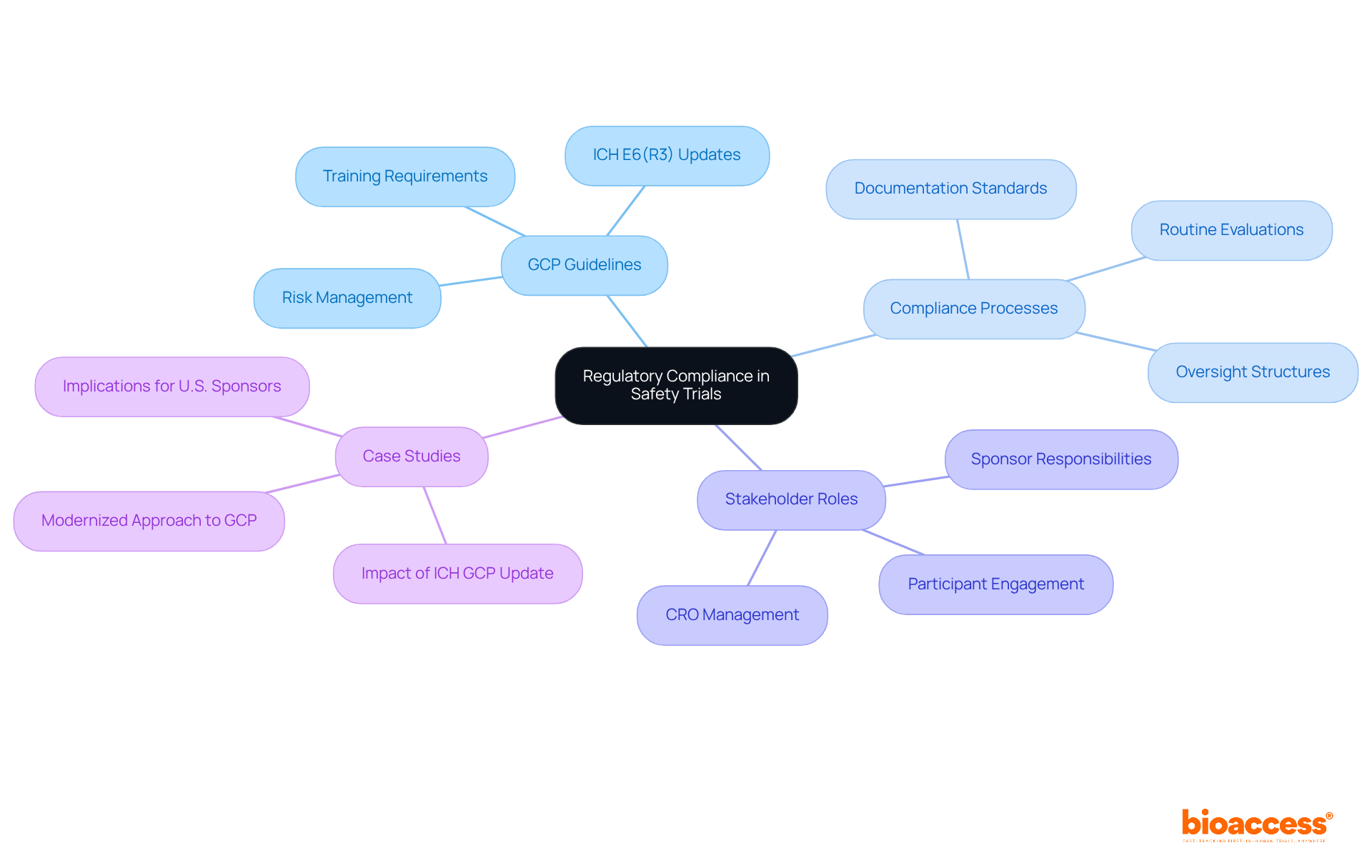
Navigating the complex environment of regulatory adherence is crucial for the success of testing procedures. A thorough understanding of both local and international regulations, particularly the updated Good Clinical Practice (GCP) guidelines, is essential. The updated ICH E6(R3) guidelines, effective from July 2025, introduce significant enhancements that emphasize risk-based quality management and proportional oversight. These changes are vital for improving study integrity and participant safety, signifying a strategic overhaul in study management. Sponsors are urged to create and oversee studies with greater intention and responsibility.
To ensure compliance, all study protocols must be meticulously documented, and team members should receive comprehensive training on these requirements. Routine evaluations and revisions to testing procedures are essential to uphold compliance and mitigate potential risks that could jeopardize the endeavor's success. Organizations like bioaccess, which provide extensive clinical study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—can enhance operational efficiency and build trust among subjects. This ultimately leads to more dependable outcomes in risk evaluations.
Furthermore, successful navigation of GCP compliance has been demonstrated in various case studies, highlighting the benefits of early patient involvement and clear stakeholder roles in improving collaboration and data integrity. Additionally, maintaining control over CROs, vendors, and technology providers is crucial as part of the defined oversight structures emphasized in the ICH E6(R3) guidelines.
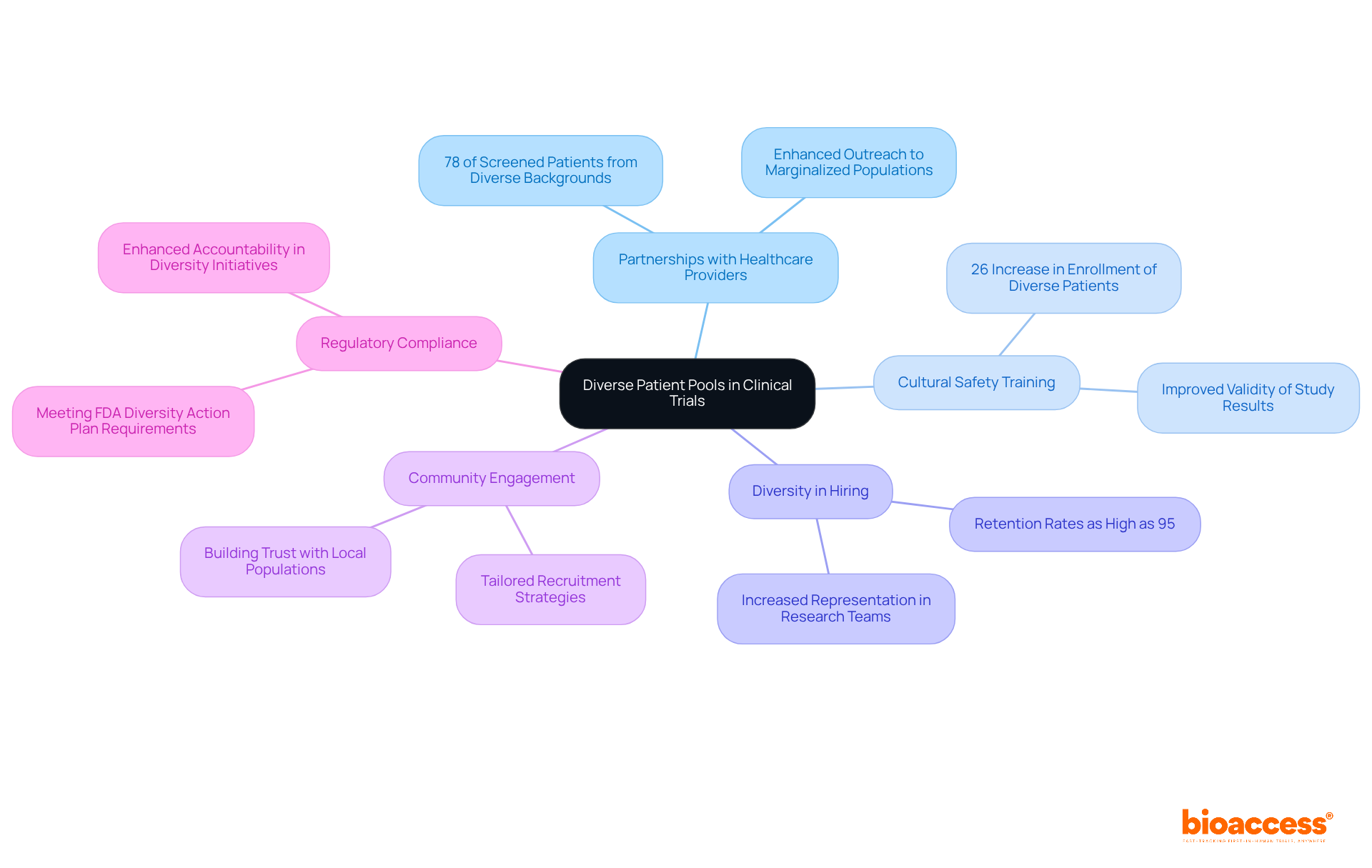
Engaging varied patient groups is crucial for enhancing the strength of effectiveness studies in clinical research. By enlisting individuals from diverse backgrounds, researchers can capture a broader spectrum of treatment responses, leading to more precise evaluations of safety and efficacy. Research has shown that clinical studies involving varied groups yield better health outcomes, reflecting the complexities of real-world scenarios.
Partnering with local healthcare providers and community organizations significantly boosts outreach to marginalized populations, ensuring that studies benefit from a diverse range of participants. This strategy not only enhances the validity of study results but also meets regulatory expectations for demographic diversity, ultimately contributing to more equitable healthcare outcomes. For instance, sites that engaged in cultural safety training saw a remarkable 26% increase in the enrollment of diverse patients.
Moreover, collaborations with Federally Qualified Healthcare Centers (FQHCs) have demonstrated that 78% of screened patients came from diverse backgrounds. By emphasizing diversity in hiring initiatives, research studies can achieve retention rates as high as 95%, as evidenced by a partnership with GlobalCare Clinical Studies.
Comprehensive research management services provided by bioaccess™, including feasibility studies, site selection, compliance reviews, and project management, enhance the overall effectiveness of these studies. The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for clinical studies in Latin America, supported by Colombia's Minister of Health. This initiative highlights the potential for job creation and economic development in the region.
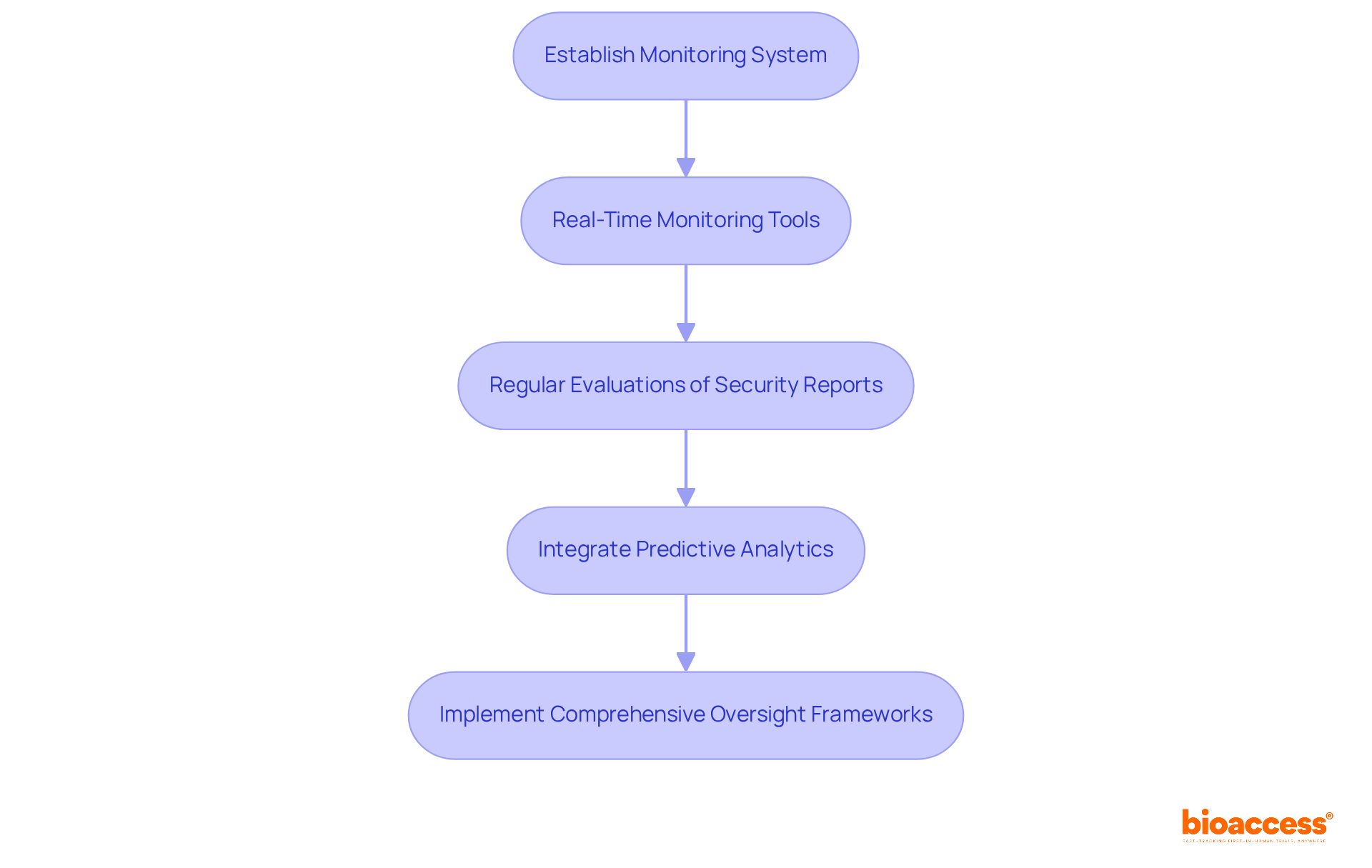
Establishing a robust monitoring system is essential for maintaining the integrity of evaluations in clinical research. Real-time monitoring tools enable continuous tracking of adverse events and safety-related data throughout the safety trial, ensuring that any emerging issues are swiftly addressed. Regular evaluations of security reports facilitate necessary modifications to procedures, fostering a proactive approach that not only safeguards individuals but also enhances the reliability of research outcomes. For example, integrating predictive analytics can help identify expected adverse events and improve participant retention, while electronic data capture systems streamline data collection and monitoring processes.
As industry specialists emphasize, access to continuous and real-time information is vital for enhancing security measures in medical studies. By implementing comprehensive oversight monitoring frameworks, stakeholders can instill trust in the research process and demonstrate a commitment to upholding the highest standards of scientific rigor. This commitment not only addresses key challenges in the Medtech landscape but also paves the way for collaborative efforts that drive innovation and involve a safety trial to improve patient safety.
Employing sophisticated statistical techniques is essential for efficiently examining data related to security in medical studies. Bayesian analysis, in particular, offers a robust framework for interpreting complex data sets, allowing researchers to update their beliefs about treatment effects as new evidence emerges. For instance, in the pivotal BNT162b2 vaccine study, Bayesian techniques revealed a likelihood exceeding 98.6% that the actual vaccine effectiveness was above 30%. This highlights their capacity to provide detailed perspectives on treatment efficacy and security.
Collaborating with biostatisticians skilled in Bayesian techniques ensures that your data analysis is both rigorous and statistically valid, ultimately strengthening the foundation of your conclusions. Furthermore, understanding the role of prior distributions—whether skeptical, neutral, or optimistic—can significantly influence the outcomes of your analysis. As Estelle Russek-Cohen noted, "One particular appeal of Bayesian methods is the use of sequential learning and Bayesian interim decision-making."
As the landscape of medical research evolves in 2025, adopting these innovative approaches will be crucial for enhancing the effectiveness of assessments. However, it is also important to recognize the potential challenges and limitations of Bayesian methods, such as the need for careful prior selection and the computational demands they may impose. By addressing these challenges head-on, researchers can leverage Bayesian analysis to its fullest potential.
Comprehensive instruction on study protocols is essential for ensuring adherence and safeguarding the integrity of research, particularly within Brazil's regulatory framework overseen by ANVISA. Regular training sessions for all team members are crucial, focusing on key aspects such as:
This proactive approach cultivates a culture of adherence and responsibility, significantly enhancing the success rates of clinical studies. Organizations that implement structured training programs have reported improved adherence to protocols and a decrease in compliance-related issues.
As bioaccess® emphasizes, "By adopting a systematic method for documentation, we enable simple retrieval during audits, a vital component for monitoring for ANVISA compliance." With bioaccess®'s comprehensive services—including rapid site activation, with over 50 sites activated in under 8 weeks—and FDA/EMA/MDR-ready datasets featuring centralized monitoring, organizations can ensure compliance and efficiency in their research processes. Furthermore, effective training can lead to quicker project completion and improved overall quality in clinical studies, underscoring the importance of investing in team development to achieve optimal results.
Creating clear communication pathways among all participants is essential for the efficient functioning of testing procedures during the safety trial in clinical research. Effective communication not only promotes teamwork but also ensures that all parties are aligned on goals and expectations. As safety expert Jeff Cooper aptly noted, "Safety is something that happens between your ears, not something you hold in your hands," highlighting the necessity for a proactive communication strategy.
Employing project management tools can significantly enhance efficiency by streamlining information sharing and monitoring progress. These tools facilitate regular updates and documentation, which are crucial for maintaining clarity among team members. A well-organized Communication Plan (CP) should encompass defined roles, responsibilities, and communication channels, ensuring that all stakeholders are informed about project developments and challenges.
Encouraging open dialogue is vital for swift problem-solving and fostering a collaborative environment. Case studies have shown that understanding the functions of different stakeholders improves communication efficiency, ultimately aiding the successful implementation of research studies. By prioritizing communication, organizations can navigate the complexities of safety trials more effectively, which leads to improved outcomes and compliance.
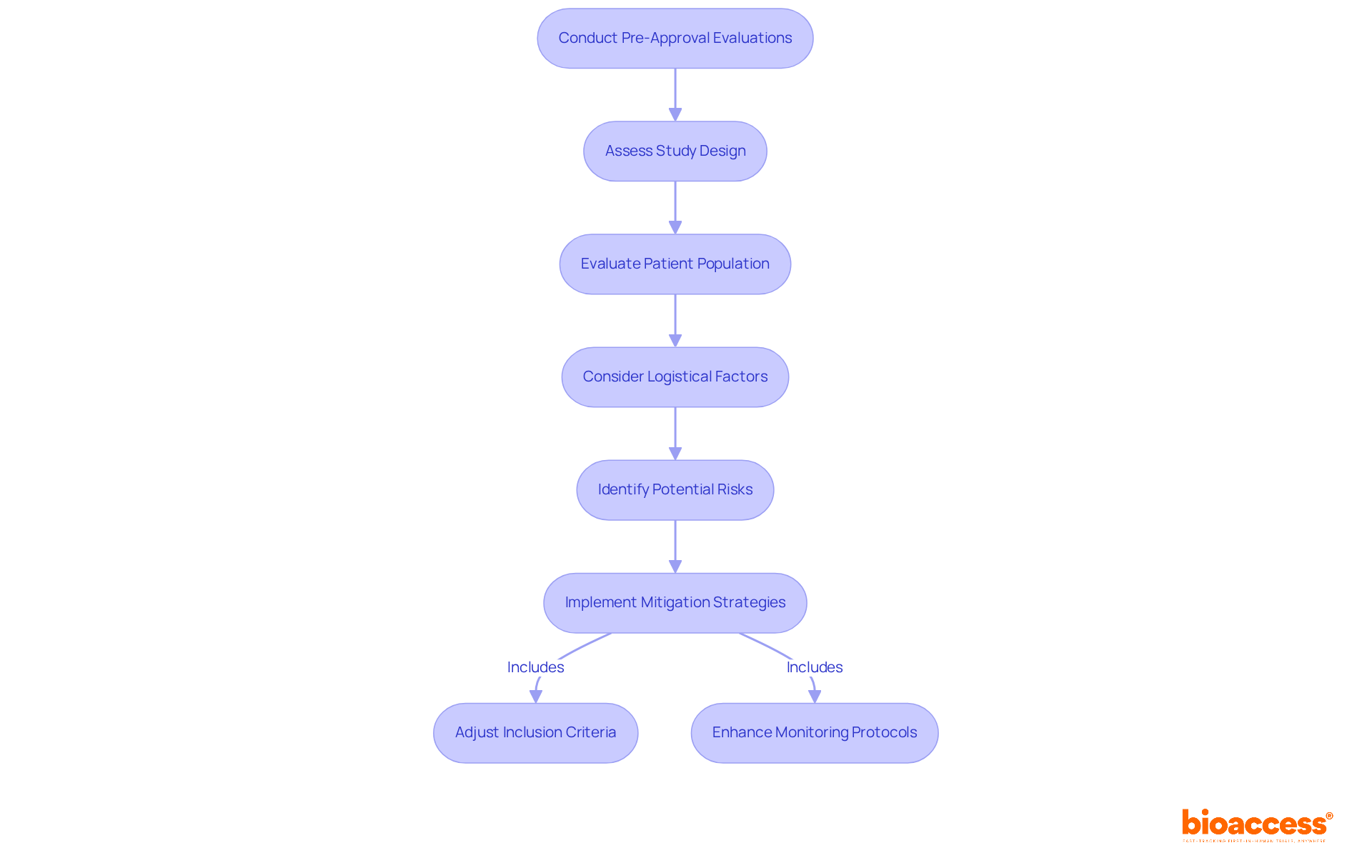
Conducting thorough pre-approval evaluations is essential for identifying potential risks that could jeopardize the success of research studies. At bioaccess®, we recognize the critical need for a comprehensive assessment of study design, patient population, and logistical considerations to uncover challenges that may arise. Our extensive experience in feasibility studies and site selection equips us with a deep understanding of the demographics and health profiles of the patient population, revealing specific vulnerabilities that must be addressed.
By proactively identifying these risks, you can implement effective mitigation strategies—like adjusting inclusion criteria or enhancing monitoring protocols—to ensure a smoother research process. The significance of risk evaluation in medical studies has never been more apparent, especially as we approach 2025. The evolving landscape of health research demands increased vigilance and flexibility to safeguard patient well-being and maintain study integrity.
With the expertise of our Clinical Study Managers, including Oswaldo Amaya, MD, and Dr. Sergio Alvarado, we are committed to facilitating medical device clinical studies in Latin America. Our focus is on ensuring compliance and effective project management throughout the study lifecycle. Collaboration is key; let’s work together to navigate these challenges and drive successful outcomes in clinical research.
Effective patient involvement is crucial for enhancing recruitment and retention in safety trials. Clear and compelling communication strategies are vital to convey the purpose, benefits, and procedures of the assessment. By leveraging social media, engaging with communities, and collaborating with patient advocacy organizations, outreach to potential contributors can significantly expand. For instance, patient-centered studies have been shown to attract up to twice the number of participants, underscoring the effectiveness of these strategies.
Once individuals are enrolled, maintaining regular communication is essential. This includes providing updates on trial progress, addressing any concerns, and fostering a sense of community among participants. Research indicates that retention rates in resource-challenged nations can soar to 95%-100% when individuals feel supported and engaged. Furthermore, implementing personalized engagement tactics, such as reminder calls and educational newsletters, can further enhance participant commitment and satisfaction.
As we look ahead to 2025, the landscape of medical studies is evolving, making it imperative to emphasize innovative engagement strategies to tackle recruitment challenges. By prioritizing patient needs and preferences, study sponsors can not only boost recruitment but also ensure higher retention rates, ultimately leading to more successful efficacy evaluations.
Post-experiment follow-up is crucial for ensuring the long-term well-being of individuals involved in safety trials. A structured follow-up plan that includes regular check-ins and assessments is vital for identifying any delayed adverse effects or long-term outcomes. This ongoing involvement not only safeguards the well-being of participants in the safety trial but also generates valuable data that enhances our understanding of the intervention's impact. Such comprehensive oversight significantly boosts the credibility of safety trial outcomes, reflecting a commitment to volunteer welfare and ethical research practices.
For example, systematic reviews indicate that effective follow-up methodologies after a safety trial can lead to improved retention rates and more reliable long-term data, ultimately contributing to the overall integrity of clinical research. bioaccess® specializes in managing:
This ensures that your follow-up processes are not only compliant but also effective in monitoring participant safety.
A systematic review protocol underscores the necessity for clear guidelines to navigate ethical issues in post-trial care, which can significantly enhance the credibility of safety trial results. However, challenges such as logistical constraints and participant engagement must be addressed to ensure successful follow-up. Clinical researchers are encouraged to develop a structured follow-up plan based on best practices, integrating community feedback and ethical considerations to bolster participant trust and improve data quality in the safety trial.
The strategies outlined in this article underscore the essential components for enhancing safety trial success in clinical research. By leveraging expert services, understanding regulatory compliance, engaging diverse patient populations, and implementing robust monitoring systems, researchers can significantly elevate the integrity and outcomes of their trials. The integration of advanced statistical methods and comprehensive training for teams further solidifies the foundation for effective safety evaluations.
Key insights highlight the importance of:
Establishing clear communication channels and conducting thorough pre-trial assessments are crucial steps that contribute to a streamlined trial process and improved participant retention. Moreover, a focus on post-trial follow-up ensures that long-term safety monitoring is prioritized, reinforcing a commitment to ethical research practices.
Ultimately, the landscape of safety trials is evolving, and adopting these strategies is paramount for achieving successful outcomes. Researchers are encouraged to embrace these best practices and collaborate with expert services like bioaccess® to navigate the complexities of clinical trials effectively. By doing so, they can enhance the quality of their studies and contribute to the advancement of medical knowledge and patient safety in the long run.
What services does bioaccess® provide for safety trials?
bioaccess® offers customized research services that expedite security assessments, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
How quickly can bioaccess® secure ethical approvals for clinical trials?
bioaccess® can secure ethical approvals in an impressive timeframe of just 4-6 weeks.
What advantages does bioaccess® offer in terms of enrollment rates for safety trials?
bioaccess® achieves enrollment rates that are 50% faster than traditional methods, allowing evaluations to begin promptly.
Why is understanding regulatory compliance important for safety trials?
A thorough understanding of regulatory compliance, especially updated Good Clinical Practice (GCP) guidelines, is crucial for ensuring the success of testing procedures and improving study integrity and participant safety.
What are the key updates in the ICH E6(R3) guidelines effective from July 2025?
The ICH E6(R3) guidelines introduce enhancements that emphasize risk-based quality management and proportional oversight, which are vital for improving study integrity and participant safety.
How can organizations ensure compliance with study protocols?
Organizations should meticulously document all study protocols, provide comprehensive training for team members, and conduct routine evaluations and revisions to maintain compliance and mitigate risks.
How does engaging diverse patient groups benefit clinical research?
Engaging varied patient groups enhances the strength of effectiveness studies, leading to more precise evaluations of safety and efficacy and better health outcomes that reflect real-world scenarios.
What strategies can improve outreach to diverse patient populations?
Partnering with local healthcare providers and community organizations can significantly boost outreach to marginalized populations, ensuring a diverse range of participants in studies.
What impact does cultural safety training have on patient enrollment?
Sites that engaged in cultural safety training saw a 26% increase in the enrollment of diverse patients.
How can bioaccess™ contribute to the establishment of clinical studies in Latin America?
bioaccess™ collaborates with Caribbean Health Group to establish Barranquilla as a premier location for clinical studies in Latin America, highlighting potential job creation and economic development in the region.