


The article identifies ten medical device consulting firms that can significantly expedite research and development processes for medical device companies. Each firm is distinguished by its unique strengths, including:
These attributes collectively enhance clinical trial efficiency and compliance within a complex regulatory landscape, underscoring their critical role in the Medtech sector. By collaborating with these firms, medical device companies can navigate challenges more effectively, ultimately driving innovation and improving patient outcomes.
In the rapidly evolving landscape of medical technology, the race to bring innovative devices to market has never been more competitive. Medical device consulting firms play a crucial role in this process, offering expertise that accelerates research and development while navigating complex regulatory environments. This article explores ten standout consulting firms that provide essential services, from clinical trial management to regulatory compliance, enabling companies to streamline their operations and achieve market readiness. As the demand for efficient and effective consulting solutions grows, the question arises: which firms will rise to the challenge and redefine the standards of excellence in the medical device industry?
Bioaccess® stands out among medical device consulting firms by delivering exceptional clinical research agility. By harnessing the regulatory speed of Latin America, the diverse patient populations in the Balkans, and the efficient pathways in Australia, the company secures ethical approvals in an impressive 4-6 weeks. This swift turnaround results in enrollment that is 50% faster than traditional sectors, establishing the company as a vital partner for medical device consulting firms and innovators in Medtech, Biopharma, and Radiopharma looking to expedite their research and development processes.
With over 15 years of industry experience, bioaccess® offers high-quality clinical research services tailored to the specific needs of each client, facilitating the rapid progression of medical devices from first-in-human studies to market readiness. Core services encompass:
Recent collaborations, including the partnership with Welwaze Medical Inc. for the Celbrea® launch in Colombia and alliances with GlobalCare Clinical Trials to enhance ambulatory services, underscore the organization’s commitment to accelerating clinical trials. These initiatives have achieved a remarkable 50% reduction in recruitment duration and 95% retention rates, illustrating the effectiveness of this approach.
As the global clinical trials sector is projected to expand significantly, reaching USD 104.41 billion by 2032 with a CAGR of 6.8%, the demand for agile and effective medical device consulting firms remains critical. This underscores the importance of collaboration and the next steps towards enhancing clinical research outcomes.
The company stands out in delivering comprehensive adherence to standards and quality assurance services specifically tailored for medical device consulting firms. Their expert team collaborates closely with clients to develop and uphold robust Quality Management Systems (QMS) that comply with FDA regulations, ISO standards, and EU directives. By implementing customized solutions, the company aids businesses in optimizing adherence procedures, ensuring their products meet critical legal standards for market entry. This commitment to quality assurance not only mitigates risks but also enhances product safety, facilitating successful product launches.
As the medical device sector faces increasing oversight and complexity, medical device consulting firms play a vital role in navigating these challenges. Their services include:
Medical device consulting firms offer solutions that address the multifaceted obstacles that medical device startups confront, such as regulatory challenges, competition, recruitment issues, and financial constraints. With the market projected to grow significantly, reaching an estimated USD 3.5 billion in 2024 and USD 7.2 billion by 2033, and a forecasted CAGR of 8.5% from 2026 to 2033, the organization establishes itself as an indispensable ally for companies aiming to thrive in this evolving landscape, particularly in Colombia, where the INVIMA oversees medical devices as a Level 4 health authority by PAHO/WHO.

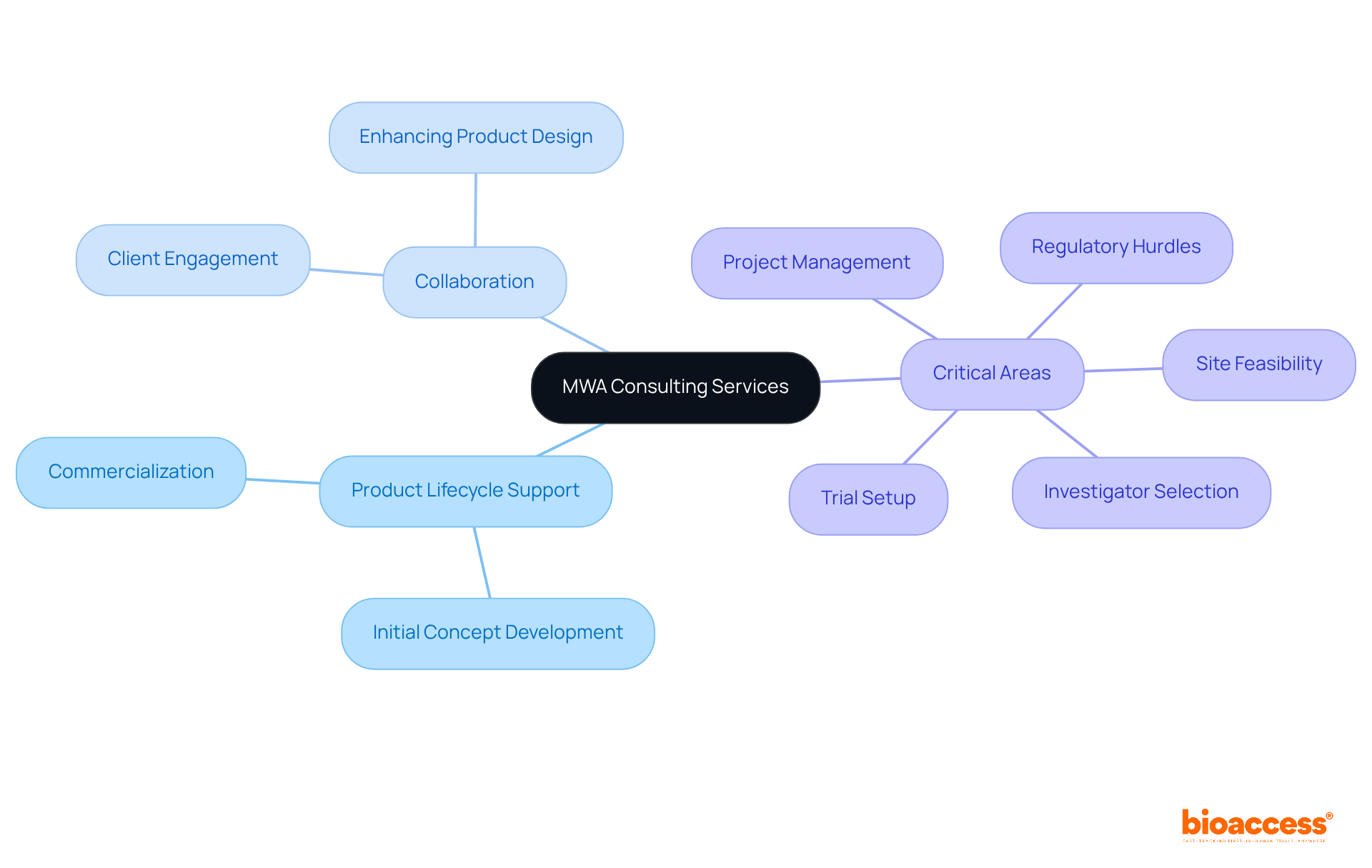
The company provides a comprehensive suite of services meticulously tailored for the medical device industry through medical device consulting firms. Their expertise spans the entire product lifecycle, from initial concept development to commercialization, ensuring that clients receive robust support at every stage.
By collaborating closely with clients, the team enhances product design, improves functionality, and guarantees adherence to standards across diverse regions, including Latin America. They focus on critical areas such as:
All of which are essential for accelerating clinical trials. Additionally, the platform provides thorough reporting on study status, inventory, and adverse events.
By merging strategic planning with technical expertise, this organization collaborates with medical device consulting firms to empower innovators in effectively tackling the complexities of medical device development, ultimately reducing time-to-market and enhancing market access opportunities.
The organization specializes in delivering comprehensive quality management and compliance support services tailored specifically for medical device consulting firms. Their expertise encompasses the development and implementation of Quality Management Systems (QMS), alongside compliance consulting and training programs provided by medical device consulting firms.
With a team of specialists from medical device consulting firms, the organization assists clients in navigating the complexities of FDA regulations and global standards, ensuring that their products meet the highest quality and safety criteria. As regulatory frameworks evolve in 2025, companies will encounter increased scrutiny; notably, 725 data breaches were reported in 2023 alone, compromising over 133 million records.
The organization collaborates with medical device consulting firms to offer a thorough approach to advancing medical device trials, including:
Medical device consulting firms provide customized solutions that not only facilitate compliance but also enhance the likelihood of successful product introductions and access to the industry, positioning clients for success in a competitive landscape.

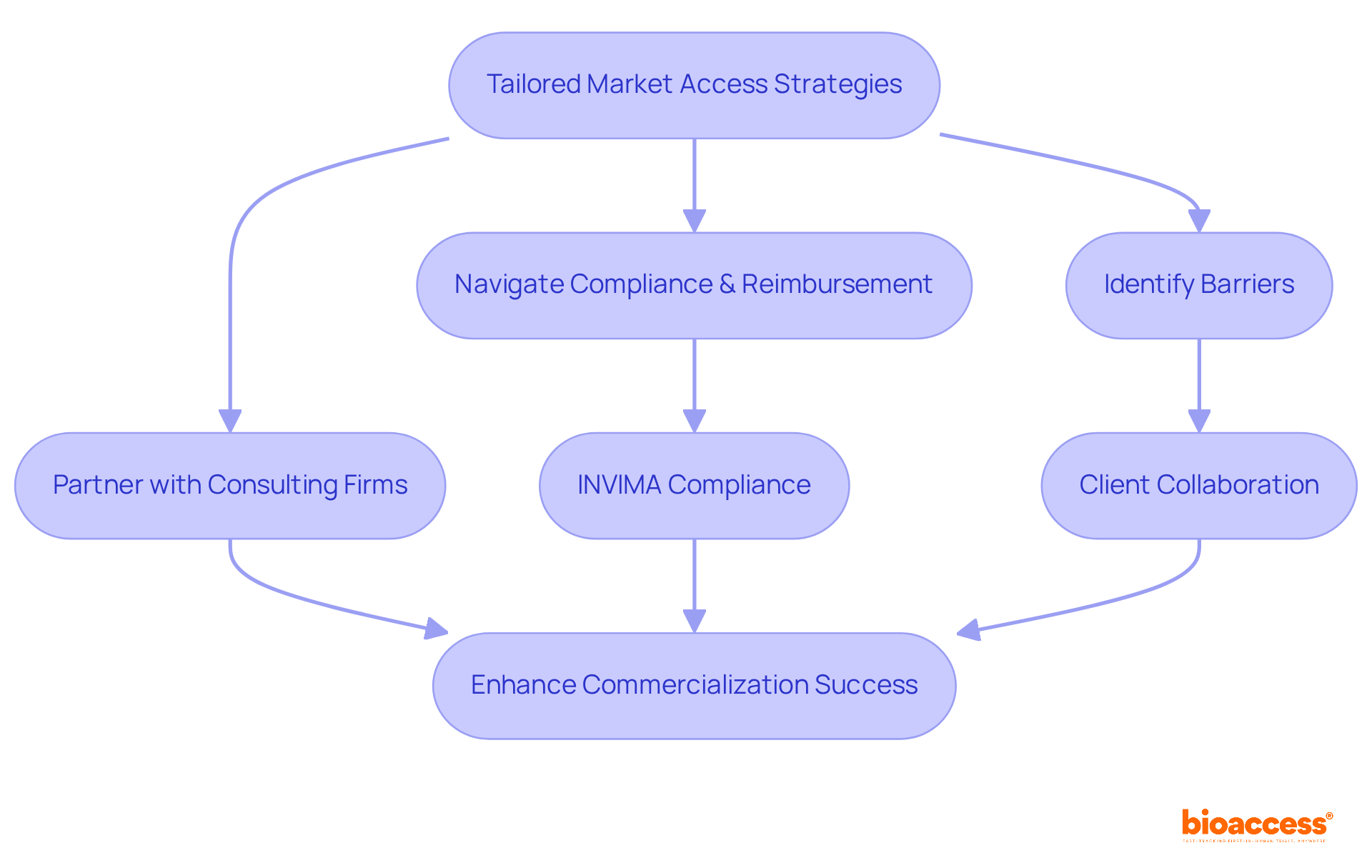
Bioaccess excels in developing tailored access strategies for medical device producers, often partnering with medical device consulting firms to navigate the complex environment of compliance and reimbursement in Latin America. Their comprehensive services navigate reimbursement frameworks, regulatory compliance, and strategic entry planning, particularly in countries like Colombia, where medical device consulting firms must consider INVIMA, a Level 4 health authority recognized by PAHO/WHO, for medical device regulation. By collaborating closely with clients, medical device consulting firms help the company identify potential barriers and formulate effective strategies to mitigate them, significantly enhancing the likelihood of successful commercialization.
This proactive strategy accelerates product launch schedules and prepares clients for sustainable growth in increasingly competitive environments. As reimbursement strategies evolve, bioaccess remains at the forefront, ensuring that clients are well-equipped to adapt and thrive. Notably, public payers held a 66.30% share in 2024, underscoring the critical importance of effective reimbursement strategies in the current landscape. Furthermore, the increase in fully paid claims indicates a properly operating reimbursement system that promotes financial stability for healthcare providers, essential for manufacturers aiming for successful entry into the industry.
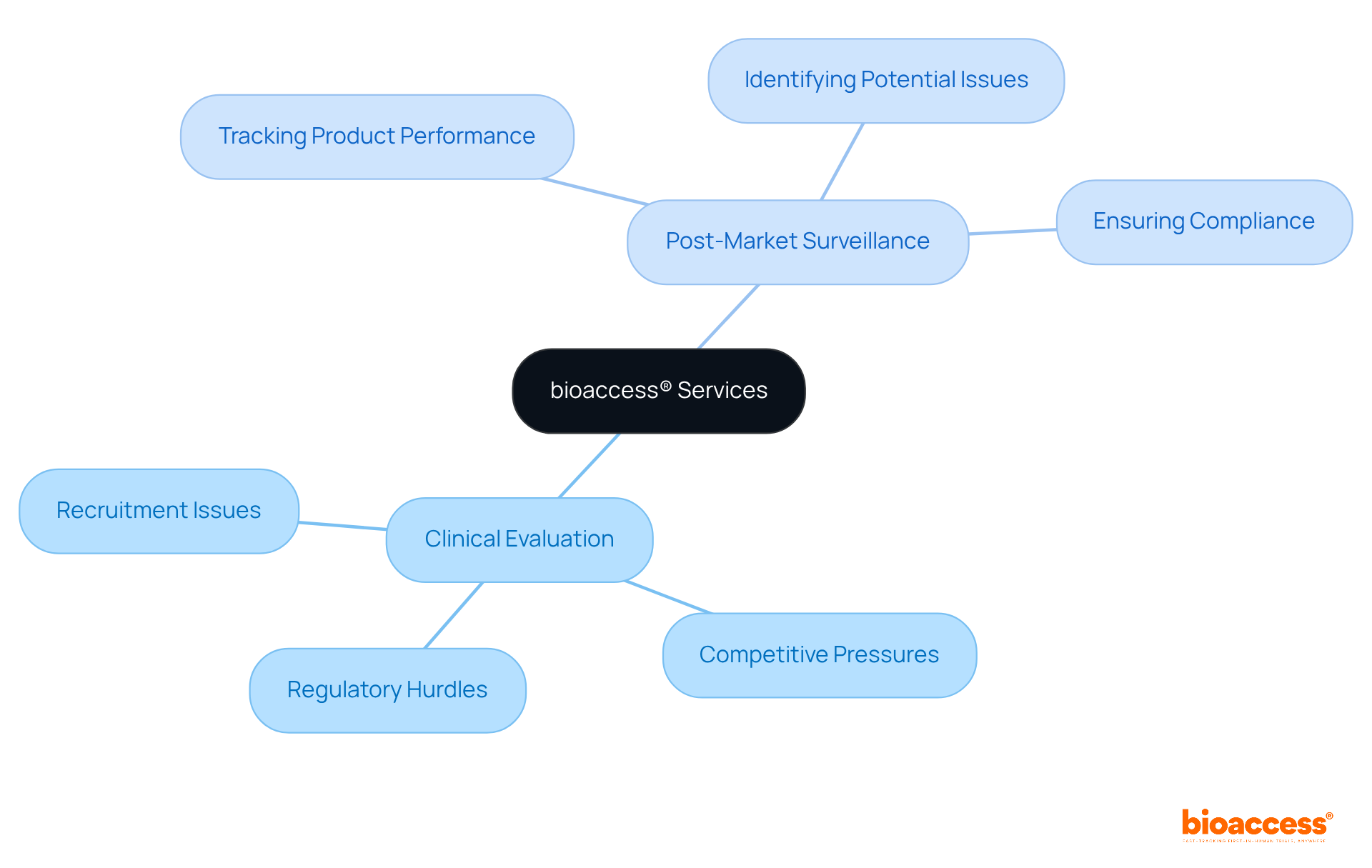
bioaccess® delivers expert clinical evaluation and post-market surveillance services through medical device consulting firms specifically designed for medical device companies navigating the Latin American market. Our dedicated team assists clients in managing the complexities of regulatory compliance, ensuring that clinical evaluations demonstrate product safety and effectiveness—essential elements for successful submissions to authorities.
We understand the unique challenges faced by medical device startups, including:
We are committed to facilitating expedited site activation and compliance for clinical trials across LATAM in collaboration with medical device consulting firms. Furthermore, bioaccess® provides thorough post-market surveillance services to track product performance once it is launched, ensuring that any potential issues are swiftly identified and resolved.
By focusing on both pre-market and post-market activities, bioaccess® empowers clients to uphold regulations and enhance product quality throughout the entire product lifecycle.
The organization stands out as a leading service provider in clinical research, delivering integrated solutions tailored specifically for medical device consulting firms. Their comprehensive approach encompasses:
This ensures clients experience a seamless journey throughout the product development lifecycle. By prioritizing compliance and optimization, the team of specialists works closely with clients to enhance clinical trial design and execution. This all-encompassing model not only expedites the path to market but also markedly boosts project efficiency, effectively addressing the increasing complexities of clinical trials within the Medtech sector.
Notably, a health organization has partnered with Caribbean Health Group to position Barranquilla as a premier hub for clinical trials in Latin America, with the backing of Colombia's Minister of Health. As the Medical Device Clinical Trials Market is projected to reach USD 33.5 billion by 2034, the demand for tailored solutions from medical device consulting firms is more critical than ever, enabling companies to efficiently navigate compliance landscapes while meeting the growing needs for expedited drug approvals and personalized medicine.

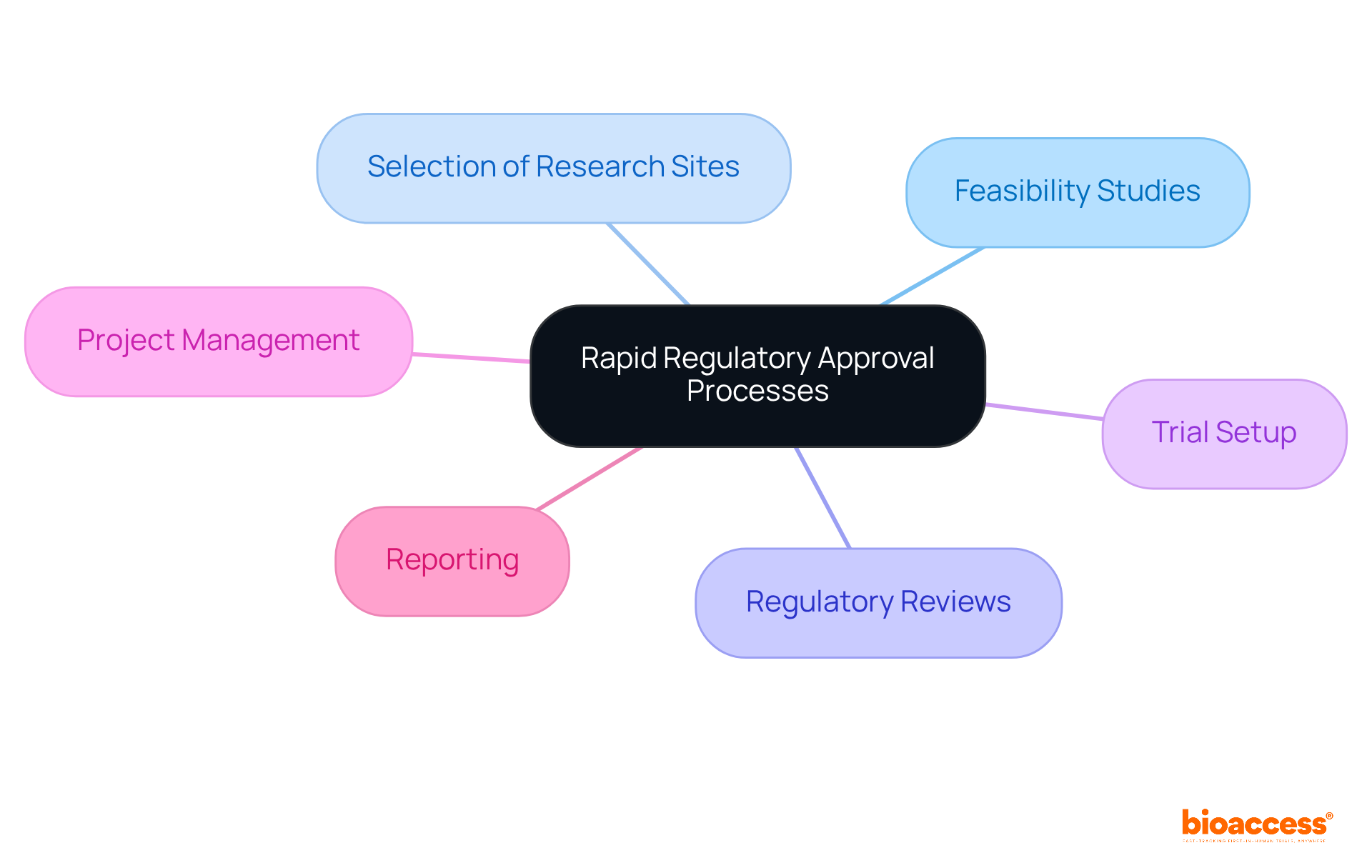
The company specializes in streamlining approval procedures for medical device producers. Medical device consulting firms have a skilled team that provides an extensive range of consulting services aimed at simplifying the submission process, including:
As the need for oversight affairs services expands due to the challenges of managing approval processes across different areas, the company's emphasis on quality and compliance becomes progressively essential. By reducing delays and increasing the chances of successful approvals, the company enables clients to introduce innovative products more swiftly, ultimately benefiting patients and healthcare professionals. With the medical device compliance affairs market anticipated to expand at a CAGR of 7.93% from 2024 to 2032, the need for medical device consulting firms offering specialized services is more essential than ever.
The firm is dedicated to assisting medical device businesses in navigating the intricate landscape of regulatory adherence with the support of medical device consulting firms. Their seasoned team at medical device consulting firms offers invaluable insights into FDA regulations and international standards, ensuring clients are thoroughly equipped to meet regulatory demands.
This organization employs a comprehensive approach that encompasses:
Such a framework not only aids medical device consulting firms in maneuvering through compliance complexities but also mitigates risks associated with product launches.
Furthermore, Bioaccess provides essential services, including:
This ensures a thorough comprehension of the compliance landscape. Current trends indicate that a proactive oversight strategy is crucial for medical device consulting firms, as these companies face increasing pressure to adapt to evolving regulations and industry expectations.
For instance, early engagement with oversight agencies has proven effective in streamlining submission processes, facilitating quicker approvals and market access. As a leading authority in the field noted, 'Adhering to regulations is not merely an obstacle to overcome; it’s a continuous dedication to patient safety and product quality.'
Bioaccess exemplifies this approach, demonstrating how medical device consulting firms can leverage strategic oversight planning to transform compliance challenges into opportunities for innovation and growth. Additionally, smaller firms often struggle to navigate international compliance frameworks, underscoring the necessity of expertise in achieving successful market entry.
The company distinguishes itself as a premier provider of integrated compliance and quality consulting services specifically designed for medical device consulting firms in the sector. Their comprehensive approach encompasses:
This guarantees that clients receive a robust solution to their development challenges. As underscored in industry insights, "Creating a successful medical device compliance strategy is a team effort," a principle that the company embodies through its collaborative efforts with clients. The talented team at the organization adeptly navigates the intricate oversight landscape, ensuring compliance while enhancing product quality and safety. This collaborative effort not only streamlines the path to commercialization but also significantly improves patient outcomes.
With the consulting outsourcing services sector projected to grow at a CAGR of approximately 8.36% from 2024 to 2030, the company is well-prepared to support clients in meeting the evolving demands placed by medical device consulting firms within the medical device industry. By prioritizing effective regulatory strategies and quality management, bioaccess empowers clients to achieve timely submissions and successful market entry.

The exploration of top medical device consulting firms reveals a dynamic landscape where agility and expertise are paramount for accelerating research and development. These firms play a crucial role in navigating the complexities of regulatory compliance, quality assurance, and market access, ultimately facilitating the successful launch of innovative medical devices.
Key insights from the article highlight the strengths of various firms, such as:
Each firm brings unique services to the table, addressing the multifaceted challenges faced by medical device innovators and ensuring that they can effectively navigate the intricate regulatory frameworks that govern the industry.
In a rapidly evolving market projected to grow significantly in the coming years, the importance of partnering with skilled consulting firms cannot be overstated. Companies looking to thrive in the medical device sector should consider leveraging these specialized services to enhance their research and development processes, streamline compliance, and ultimately improve patient outcomes. By embracing collaboration with these consulting experts, innovators can position themselves for success in an increasingly competitive landscape.
What is bioaccess® and what services does it provide?
Bioaccess® is a medical device consulting firm that delivers exceptional clinical research agility. It offers high-quality clinical research services tailored to clients' needs, including feasibility studies, investigator selection, trial set-up, regulatory compliance, project management, and comprehensive reporting.
How quickly can bioaccess® secure ethical approvals for clinical trials?
Bioaccess® can secure ethical approvals in an impressive 4-6 weeks, resulting in enrollment that is 50% faster than traditional sectors.
What recent collaborations has bioaccess® engaged in?
Bioaccess® has partnered with Welwaze Medical Inc. for the Celbrea® launch in Colombia and collaborated with GlobalCare Clinical Trials to enhance ambulatory services, achieving a 50% reduction in recruitment duration and 95% retention rates.
What is the projected growth of the global clinical trials sector?
The global clinical trials sector is projected to expand significantly, reaching USD 104.41 billion by 2032 with a compound annual growth rate (CAGR) of 6.8%.
What services does Rook Quality Systems provide?
Rook Quality Systems delivers comprehensive adherence to standards and quality assurance services for medical device consulting firms, including feasibility studies, site selection, compliance reviews, trial setup, import permits, nationalization of investigational devices, project management, and reporting.
How does Rook Quality Systems help businesses with regulatory compliance?
Rook Quality Systems collaborates with clients to develop and uphold robust Quality Management Systems (QMS) that comply with FDA regulations, ISO standards, and EU directives, optimizing adherence procedures and enhancing product safety.
What is the market outlook for medical device consulting firms?
The medical device market is projected to grow significantly, reaching an estimated USD 3.5 billion in 2024 and USD 7.2 billion by 2033, with a forecasted CAGR of 8.5% from 2026 to 2033.
What services does MWA Consulting offer to the medical device industry?
MWA Consulting offers a comprehensive suite of services for the medical device industry, including site feasibility, investigator selection, trial setup, project management, and navigating regulatory hurdles.
How does MWA Consulting support clients throughout the product lifecycle?
MWA Consulting collaborates closely with clients to enhance product design, improve functionality, and ensure adherence to standards across diverse regions, thus reducing time-to-market and enhancing market access opportunities.