


The article outlines ten pivotal medical device conferences in 2025 that innovators must attend to remain at the forefront of the industry. Each conference addresses critical themes, including advancements in technology, regulatory compliance, and networking opportunities. These events offer invaluable insights and collaboration possibilities, which are essential for fostering innovation in the medical device sector. By participating, attendees can not only enhance their knowledge but also connect with key industry players, driving progress in clinical research.
As the medical device industry evolves at an unprecedented pace, conferences in 2025 promise to be pivotal for innovators seeking to stay ahead. These events not only offer a platform for networking and collaboration but also provide insights into the latest trends, regulatory challenges, and technological advancements shaping the future of healthcare. However, with numerous options available, which conferences will truly deliver the most value and opportunities for growth? This article highlights ten must-attend medical device conferences in 2025, ensuring that industry leaders and emerging players alike can navigate the complexities of this dynamic landscape effectively.
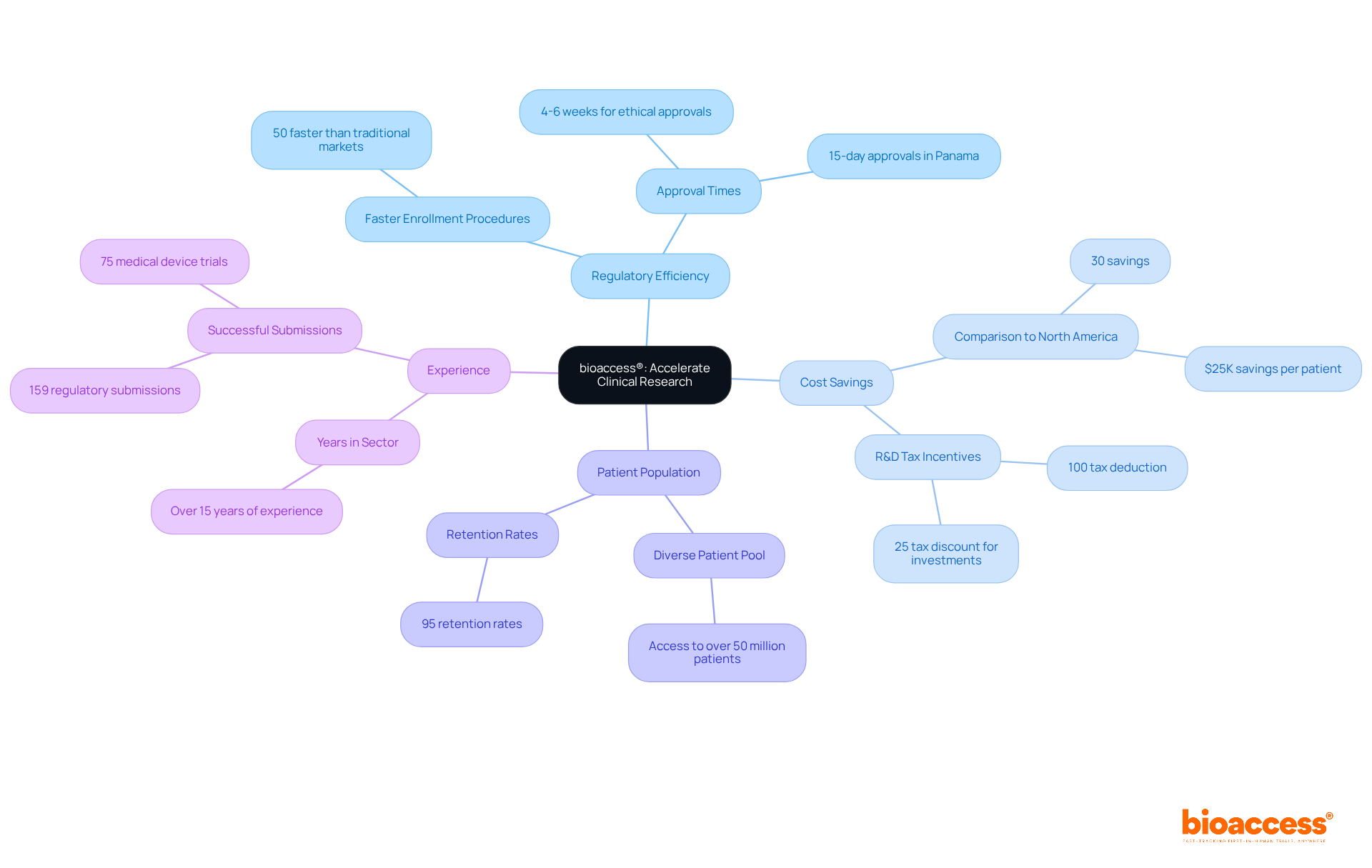
bioaccess® spearheads the acceleration of research for medical devices throughout Latin America, particularly in Colombia, which offers significant competitive advantages for first-in-human trials. By leveraging the region's regulatory efficiency, bioaccess® secures ethical approvals in an impressive 4-6 weeks, greatly surpassing conventional sectors. This rapid process is complemented by Colombia's cost savings of over 30% compared to North America and Western Europe, alongside a robust healthcare system ranked among the best globally.
With access to a diverse patient population exceeding 50 million, bioaccess® empowers innovators to conduct first-in-human and early-feasibility studies with remarkable efficiency. Furthermore, Colombia's R&D tax incentives enhance the appeal of conducting trials in the region.
With more than 15 years of sector experience, bioaccess® delivers top-notch research services that enable Biopharma and Radiopharma firms to expedite their innovations to market. Industry leaders acknowledge that the swift regulatory environment in Latin America not only reduces time to market but also enhances the overall success of clinical trials, establishing it as an ideal destination for research and development.
Scheduled for January 13-16, 2025, in San Francisco, the J.P. Morgan Healthcare Conference is a pivotal event for leaders in medical technology, particularly in the context of medical device conferences 2025. This premier gathering at the medical device conferences 2025 attracts top executives, investors, and innovators, creating a unique platform for networking and collaboration. Participants at the medical device conferences 2025 will engage in discussions about the latest trends in the medical technology sector, including new investment opportunities and strategic partnerships that can significantly enhance their businesses in a competitive landscape. With over 500 companies represented and nearly $11 trillion in market capitalization, the conference is positioned to facilitate meaningful connections and foster discussions that could lead to transformative advancements in healthcare.
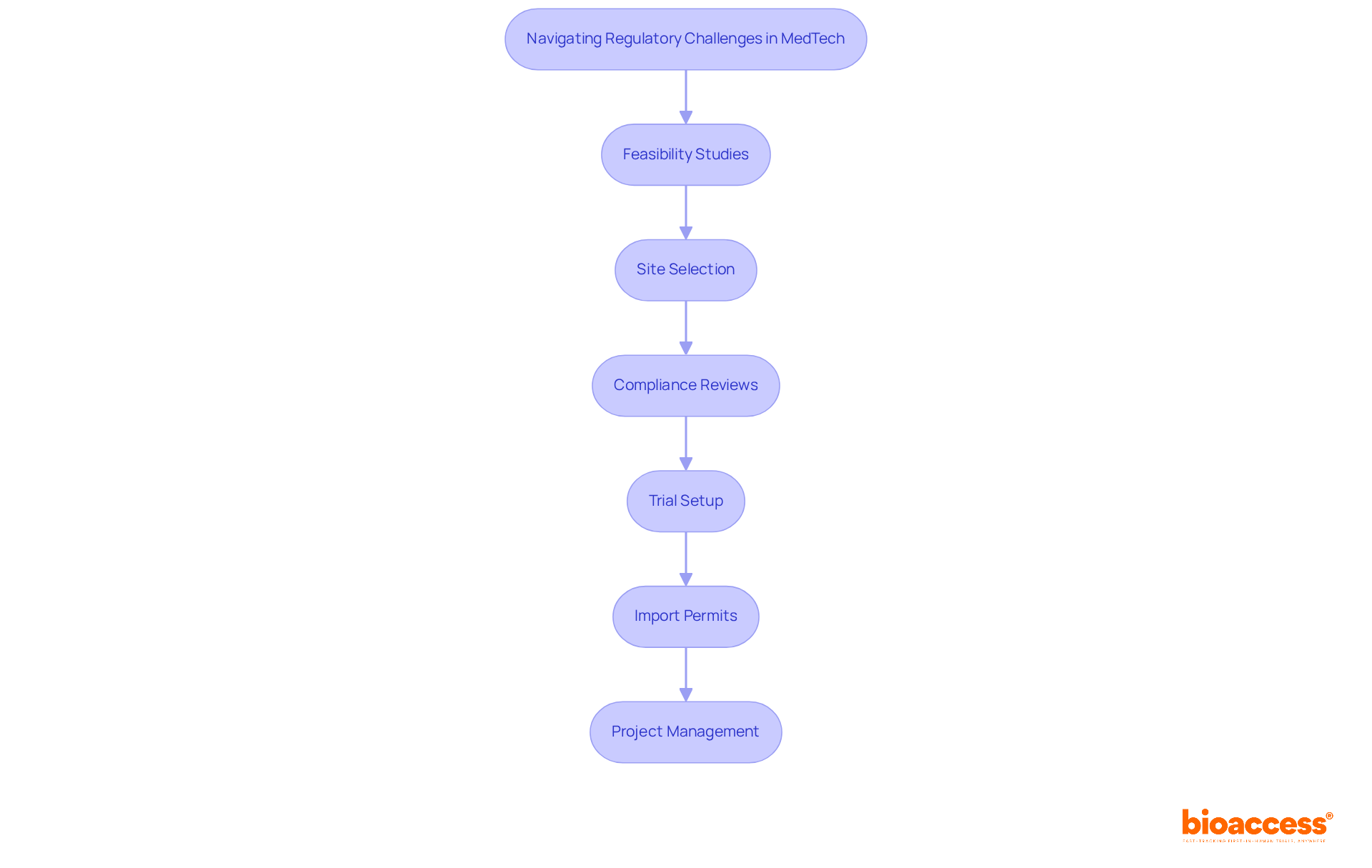
As the industry anticipates swift changes and an increase in deal flow within the cell and gene therapy sector, the conference will serve as a critical moment for industry leaders to explore these advancements. Furthermore, the extensive trial management services provided by bioaccess—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—will be vital topics of discussion. These services not only ensure the successful implementation of clinical trials but also contribute to job creation, economic development, and healthcare enhancement. This underscores the importance of global cooperation and innovation in the medical technology field.

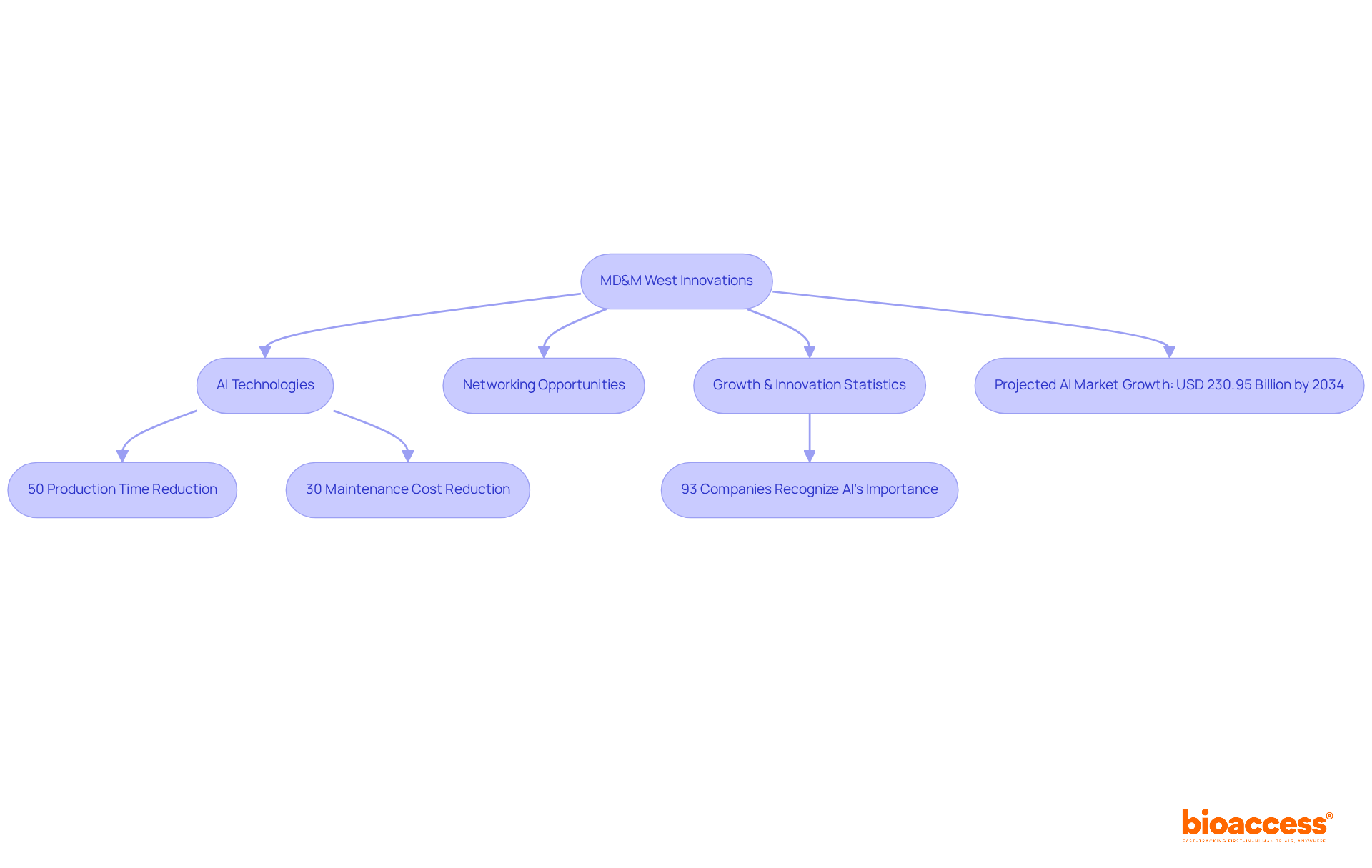
Scheduled for February 4-6, 2025, in Anaheim, CA, MD&M West is a standout event among the medical device conferences 2025 for manufacturers. This conference will host a diverse range of exhibitors presenting cutting-edge innovations in manufacturing technologies, materials, and processes. Attendees can expect to gain insights into advancements that streamline production, reduce costs, and improve product quality. Notably, AI technologies showcased at the event are projected to enhance efficiency, with manufacturers experiencing up to a 50% reduction in production time. Furthermore, AI-enabled predictive maintenance solutions can lower maintenance costs by as much as 30% and decrease unexpected downtime by 45%.
Industry leaders emphasize the importance of networking and knowledge sharing at MD&M West, with many reporting successful strategies for engaging new customers and fostering collaborations. In fact, 93% of companies recognize AI as key for propelling growth and innovation in manufacturing. This event is crucial for anyone engaged in the medical technology manufacturing sector, especially in light of the medical device conferences 2025, which provide invaluable opportunities to remain at the forefront in a rapidly changing industry. The AI in manufacturing market is projected to reach around USD 230.95 billion by 2034, highlighting the significance of advancements discussed at MD&M West.
The MedTech Forum, one of the key medical device conferences 2025, stands as a pivotal event for Medtech professionals, focusing on compliance challenges and innovations. This forum brings together industry leaders to deliberate on the latest governance developments, compliance strategies, and innovative practices that can significantly enhance product development. Attendees will acquire invaluable insights into navigating the evolving regulatory landscape, ensuring their products meet the stringent standards required for market entry.
Furthermore, bioaccess® will showcase its expertise in accelerating trials, offering comprehensive services such as:
This forum is part of the medical device conferences 2025 and presents an exceptional opportunity for startups in the medical technology, biopharma, and radiopharma sectors to discover strategies for expediting their clinical research processes.
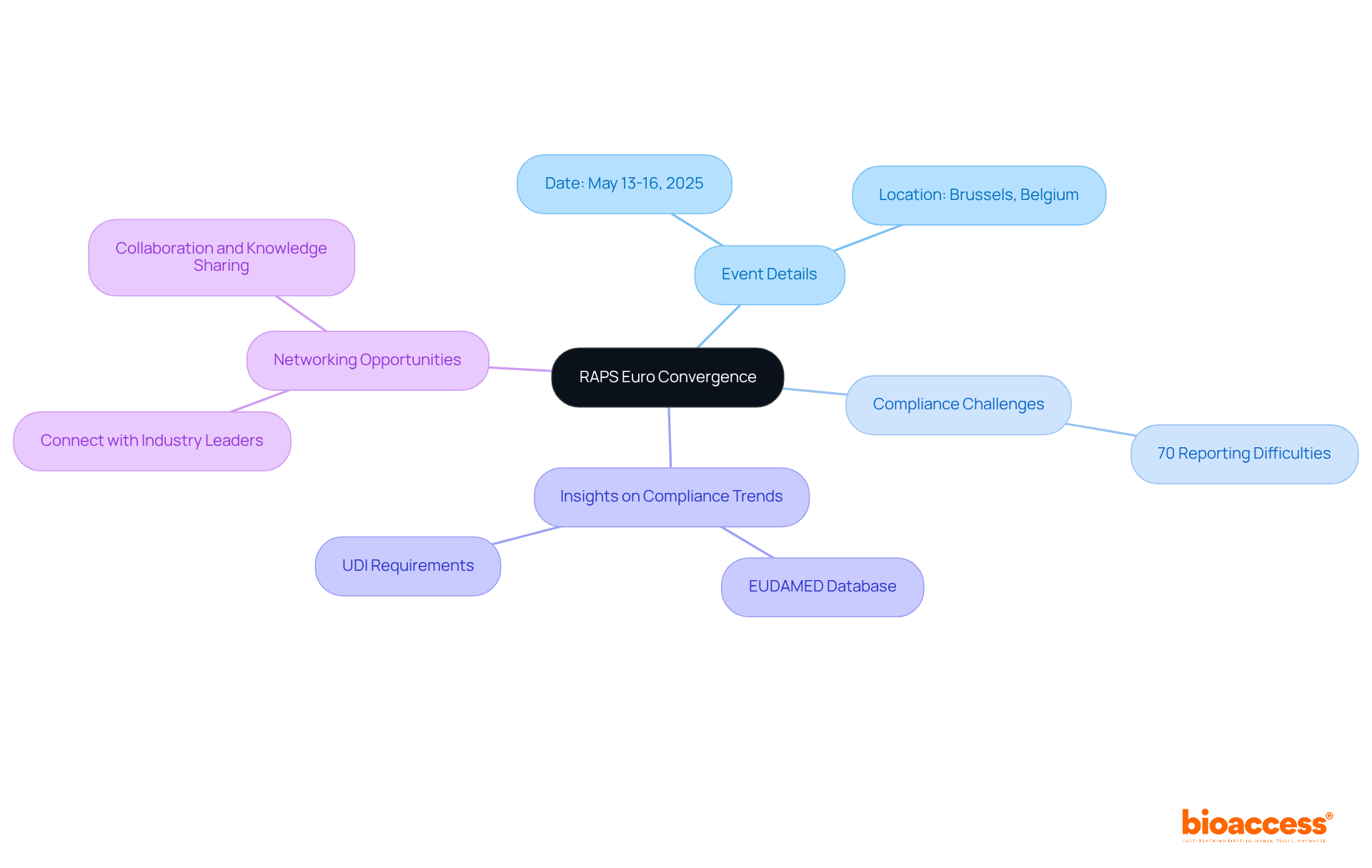
Scheduled for May 13-16, 2025, in Brussels, Belgium, RAPS Euro Convergence is a pivotal event among the medical device conferences 2025 for compliance professionals within the medical technology sector. This conference delves into pressing compliance challenges faced by Medtech firms across Europe, where approximately 70% report difficulties navigating the complex oversight landscape.
Attendees will gain insights into the latest compliance trends, including the implications of the EU's EUDAMED database and evolving requirements for Unique Device Identification (UDI). Expert-led sessions will focus on effective market access strategies, highlighting the critical role of robust clinical evidence in securing reimbursement and facilitating market entry.
Moreover, networking opportunities will allow participants to connect with industry leaders and compliance experts, fostering collaboration and knowledge sharing aimed at enhancing compliance strategies and driving innovation within the medical technology domain.
The European Implantable Device Conference, one of the key medical device conferences 2025, serves as a crucial gathering for Medtech companies dedicated to post-market compliance for implantable devices. This event will delve into the compliance landscape, providing attendees with vital knowledge on monitoring the safety and effectiveness of their products post-market entry.
Participants can expect to gain insights into the latest advancements in post-market surveillance, including effective risk management strategies that adhere to current legal standards. Industry leaders will present best practices, underscoring the necessity of a proactive approach to post-market surveillance that not only guarantees compliance but also encourages innovation and enhances product safety.
Engaging in these discussions will empower companies to maintain high standards in their product offerings, ultimately benefiting patient safety and satisfaction.
The MedTech Summit, one of the key medical device conferences 2025, represents a pivotal gathering for professionals in the medical technology sector. This summit will explore the latest trends, innovations, and challenges defining the industry landscape. Participants can expect to engage with influential figures who will share insights on urgent topics such as compliance, cybersecurity, and the integration of AI in healthcare. Notably, discussions will center on how AI is transforming patient care through predictive analytics and advanced imaging technologies.
A key highlight will be the examination of expedited approval processes, exemplified by bioaccess®'s innovative 6-8 week sprint approach for cardiology and neurology cohorts, which significantly reduces the typical approval timeline of 6-12 months observed in the US and EU. This method not only enhances patient enrollment but also addresses the regulatory challenges faced by startups in initial-phase trials.
Furthermore, the summit will tackle the latest challenges confronting the medical technology sector, including the pressing need for improved reimbursement processes, as illustrated by the recent launch of RAMPSociety's certification for reimbursement in the medical field. The collaboration between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier destination for clinical trials in Latin America, with support from Colombia's Minister of Health, will also be a focal point of discussion, underscoring the government's commitment to advancing clinical research in the region.
With over 1,800 companies from more than 40 nations participating, the MedTech Summit is expected to be an invaluable opportunity for networking, collaboration, and the exploration of innovative technologies, especially in the context of the medical device conferences 2025, that can propel businesses forward in a rapidly evolving environment.
The Generis European Medical Device Summit, one of the medical device conferences 2025, aims to examine access strategies for medical devices. This pivotal summit will convene industry leaders to discuss the challenges and opportunities inherent in navigating the European landscape. Attendees will gain valuable insights into regulatory requirements, reimbursement strategies, and best practices for successful product launches, equipping them with the essential knowledge needed to excel in a competitive environment.
Furthermore, bioaccess® will provide extensive trial management services—including:
This comprehensive combination of clinical research expertise will be crucial for biotech, biopharmaceutical, and radiopharmaceutical startups striving for success in Europe.
Scheduled for April 30-May 1, 2025, Device Talks Boston is a pivotal gathering among the medical device conferences 2025 for professionals in the field. This conference, which is one of the medical device conferences 2025, promises to unveil cutting-edge advancements in medical device technology, offering attendees a comprehensive view of innovative solutions and emerging trends that are shaping the industry.
With the US medical device market projected to reach $955.49 billion by 2030, this event is timely for those looking to capitalize on this growth. Participants will engage with industry leaders, fostering connections that can lead to collaborative opportunities and knowledge exchange.
Notably, the conference will spotlight successful innovations, including:
As the medical device industry is projected to grow at a CAGR of 6%, the medical device conferences 2025, including Device Talks Boston, will be an essential platform for examining approaches that foster innovation and enhance patient outcomes in the healthcare technology sector.
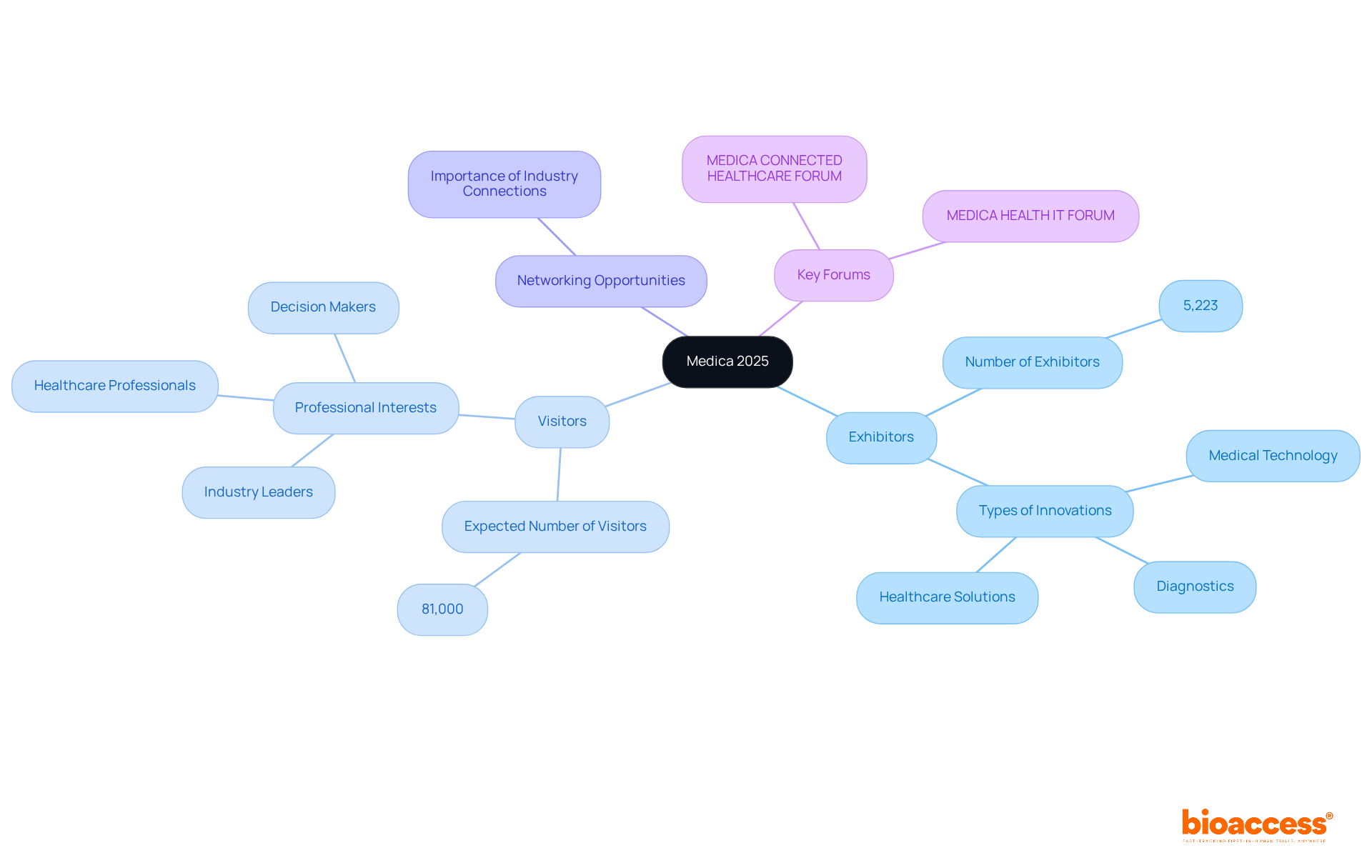
Medica 2025, scheduled for November 17-20, 2025, in Düsseldorf, Germany, represents the largest medical trade fair in the world and will feature numerous medical device conferences 2025, drawing over 5,223 exhibitors and an anticipated 81,000 visitors. This event serves as a crucial platform for the presentation of groundbreaking innovations in medical technology, diagnostics, and healthcare solutions, aligning seamlessly with bioaccess's commitment to advancing medical technologies through innovation and quality in healthcare.
Attendees will encounter unparalleled opportunities to network with industry leaders, explore cutting-edge products, and gain insights into emerging trends that are shaping the future of healthcare. With a strong emphasis on successful networking outcomes, Medica is indispensable for professionals eager to remain at the forefront of the Medtech industry.
Key forums, such as the MEDICA CONNECTED HEALTHCARE FORUM and MEDICA HEALTH IT FORUM, will facilitate discussions on the latest healthcare innovations, underscoring the significance of adaptability and technology in enhancing patient care.
Exhibitors are expected to share insights on how consumer health technologies can fortify the patient-physician connection, reflecting a burgeoning trend towards personalized and technology-driven healthcare solutions.
The medical device industry stands on the cusp of transformative advancements, with the upcoming conferences in 2025 serving as pivotal platforms for innovators and professionals to connect, collaborate, and propel their ideas forward. From the rapid regulatory processes offered by bioaccess® in Latin America to the cutting-edge innovations showcased at events like MD&M West and Device Talks Boston, these gatherings promise to tackle the pressing challenges and opportunities within the sector.
Key events such as the following have been underscored for their potential to foster networking, share insights on regulatory compliance, and unveil the latest technological advancements:
Each conference not only underscores the significance of collaboration but also illustrates how the medical device landscape is evolving, particularly with the integration of AI and the emphasis on patient-centric solutions.
As the medical technology field continues to expand and evolve, participation in these conferences becomes essential for anyone involved in the industry. Engaging with peers, learning from leaders, and exploring innovative solutions at these events can markedly enhance one's ability to navigate the complexities of this dynamic sector. Seize the opportunity to partake in these critical discussions in 2025 and contribute to shaping the future of healthcare technology.
What is bioaccess® and what role does it play in clinical research for medical devices in Latin America?
bioaccess® accelerates clinical research for medical devices in Latin America, particularly in Colombia, by leveraging the region's regulatory efficiency to secure ethical approvals in 4-6 weeks and offering cost savings of over 30% compared to North America and Western Europe.
What are the advantages of conducting clinical trials in Colombia?
Colombia offers significant advantages for clinical trials, including a diverse patient population exceeding 50 million, rapid ethical approval processes, cost savings, a robust healthcare system, and R&D tax incentives.
How does bioaccess® support Biopharma and Radiopharma firms?
With over 15 years of experience, bioaccess® provides top-notch research services that help Biopharma and Radiopharma firms expedite their innovations to market, enhancing the overall success of clinical trials.
What is the J.P. Morgan Healthcare Conference and when will it take place?
The J.P. Morgan Healthcare Conference is a premier networking event for leaders in medical technology, scheduled for January 13-16, 2025, in San Francisco.
What opportunities does the J.P. Morgan Healthcare Conference provide for participants?
The conference offers a platform for networking, collaboration, discussions on the latest trends, investment opportunities, and strategic partnerships, with over 500 companies represented and nearly $11 trillion in market capitalization.
What topics will be discussed at the J.P. Morgan Healthcare Conference?
Topics will include advancements in cell and gene therapy, trial management services, and the importance of global cooperation and innovation in the medical technology field.
What is MD&M West and when is it scheduled?
MD&M West is a major event for medical device manufacturers, scheduled for February 4-6, 2025, in Anaheim, CA, showcasing innovations in manufacturing technologies, materials, and processes.
What advancements can attendees expect to learn about at MD&M West?
Attendees can gain insights into AI technologies that enhance production efficiency, reduce costs, improve product quality, and facilitate predictive maintenance solutions.
Why is networking important at MD&M West?
Networking is crucial for sharing knowledge and strategies, with many companies reporting successful collaborations and recognizing AI as key for growth and innovation in manufacturing.
What is the projected market value for AI in manufacturing by 2034?
The AI in manufacturing market is projected to reach approximately USD 230.95 billion by 2034, emphasizing the significance of advancements discussed at MD&M West.