


The article presents an overview of the '10 Must-Attend Medical Devices Conferences for Industry Leaders,' underscoring pivotal events that provide invaluable networking opportunities, showcase groundbreaking innovations, and tackle regulatory challenges within the medical technology sector.
Each conference, including the J.P. Morgan Health Care Conference and MD&M West, is highlighted for its distinct contributions to industry collaboration, insights into emerging trends, and the facilitation of strategic partnerships.
These gatherings are essential for professionals striving to excel in the medical device field, reinforcing the necessity of engagement and collaboration in advancing the industry.
The landscape of medical technology is rapidly evolving, and conferences stand as vital hubs for innovation and collaboration. Industry leaders understand that attending these events is not merely beneficial; it is essential for maintaining a competitive edge. Yet, with numerous options available, how can professionals discern which conferences will deliver the most value and insights tailored to their specific needs? This article delves into ten must-attend medical devices conferences for 2025, highlighting opportunities for networking, learning, and showcasing groundbreaking advancements in the field.
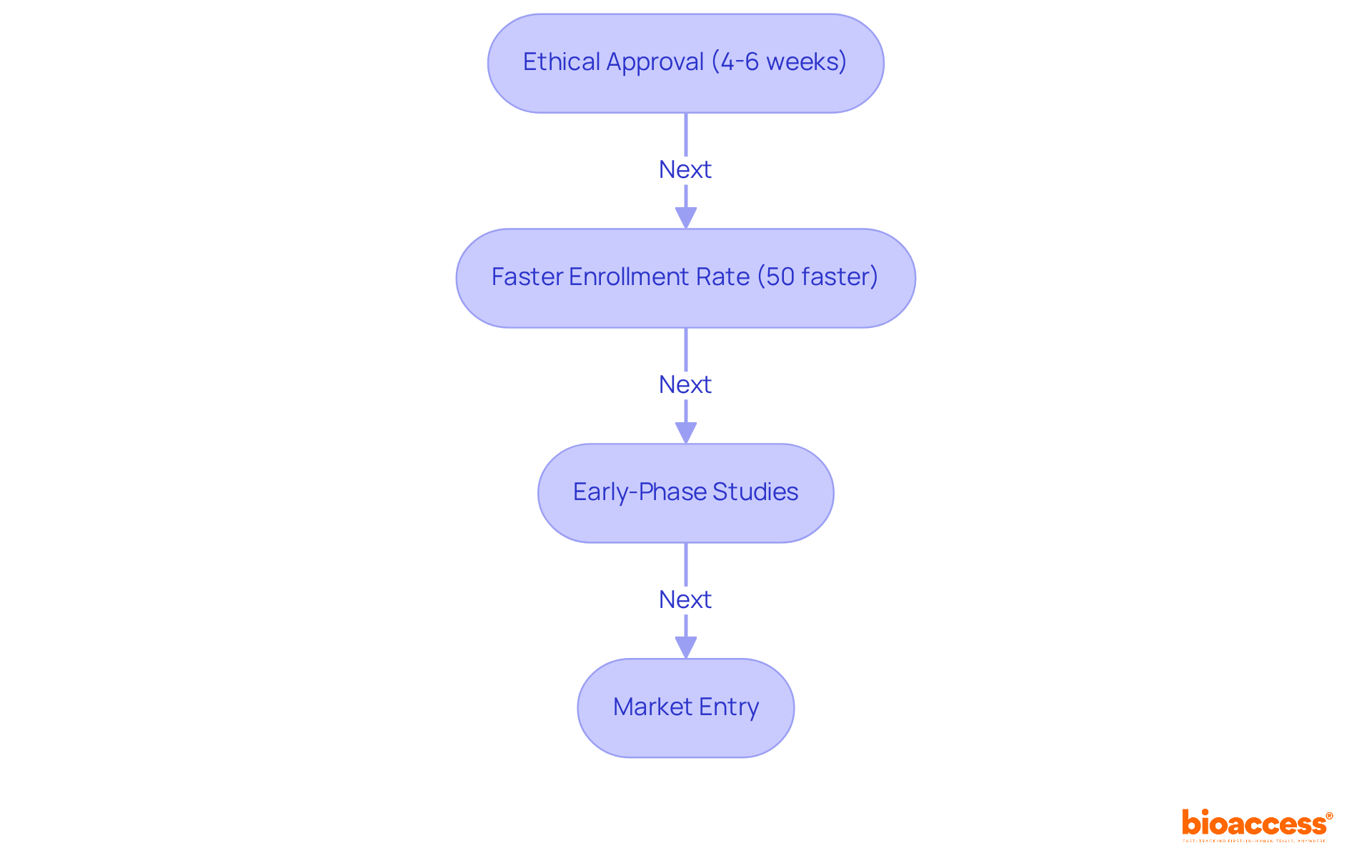
bioaccess® is leading clinical research for medical devices, particularly at medical devices conferences throughout Latin America. By leveraging the region's regulatory efficiency, bioaccess® secures ethical approvals in an impressive timeframe of just 4-6 weeks. This rapid process is complemented by a 50% faster enrollment rate compared to conventional sectors, establishing bioaccess® as the preferred partner for companies eager to accelerate their clinical studies.
With over 15 years of specialized expertise, bioaccess® delivers tailored solutions for early-phase studies, ensuring that groundbreaking medical devices can be showcased at medical devices conferences and reach the market more swiftly and effectively. Industry leaders acknowledge the critical importance of this speed; Julio G. Martinez-Clark, CEO of bioaccess®, refers to Latin America as a 'hidden gem' for first-in-human trials, underscoring the region's capacity to streamline the journey from innovation to commercialization.
As the medical technology landscape in Latin America continues to evolve, regulatory updates in 2025 are set to sustain this momentum, creating an ideal opportunity for medical technology firms to showcase their innovations at medical devices conferences and embark on successful early-phase clinical trials.
The J.P. Morgan Health Care Conference stands out as a pivotal event in the healthcare landscape, attracting leading executives and investors from the medical technology sector. This conference is not merely a gathering; it serves as a vital platform for networking, enabling leaders to forge connections with potential partners, investors, and industry thought leaders.
Attendees gain valuable insights into emerging trends and innovations, strategically positioning themselves in a competitive market. Success stories abound, with numerous healthcare technology partnerships emerging from interactions at this conference, underscoring its significance for those aiming to enhance their initiatives and collaborations within the industry.
Expert insights consistently highlight that the networking possibilities at the J.P. Morgan Health Care Conference can profoundly influence the trajectory of medical technology partnerships, rendering it an essential gathering for industry leaders in 2025.
MD&M West is known as the premier event among medical devices conferences, showcasing cutting-edge technologies and innovations in medical device manufacturing. This trade show features a diverse array of exhibitors, ranging from startups to established companies, each presenting the latest advancements in materials, manufacturing processes, and design.
At the medical devices conferences, participants will discover innovative solutions capable of enhancing product development efforts and optimizing manufacturing processes, thus creating an invaluable experience for professionals in the medical technology field.
By engaging in MD&M West, you collaborate with bioaccess™ to promote medical technologies through innovation and quality in healthcare, while also contributing to job creation and economic development in local communities. This reflects the broader impact of clinical studies within the medical technology sector.
The MedTech Forum serves as a pivotal gathering for industry leaders to confront compliance challenges and explore innovations within the Medtech sector. This forum offers essential insights into the latest compliance updates, such as the modification to the electronic Instructions for Use regulation, which is critical for manufacturers navigating adherence complexities. Participants engage in meaningful discussions with regulators and industry experts, acquiring valuable knowledge that shapes their strategies for successful product development and market entry.
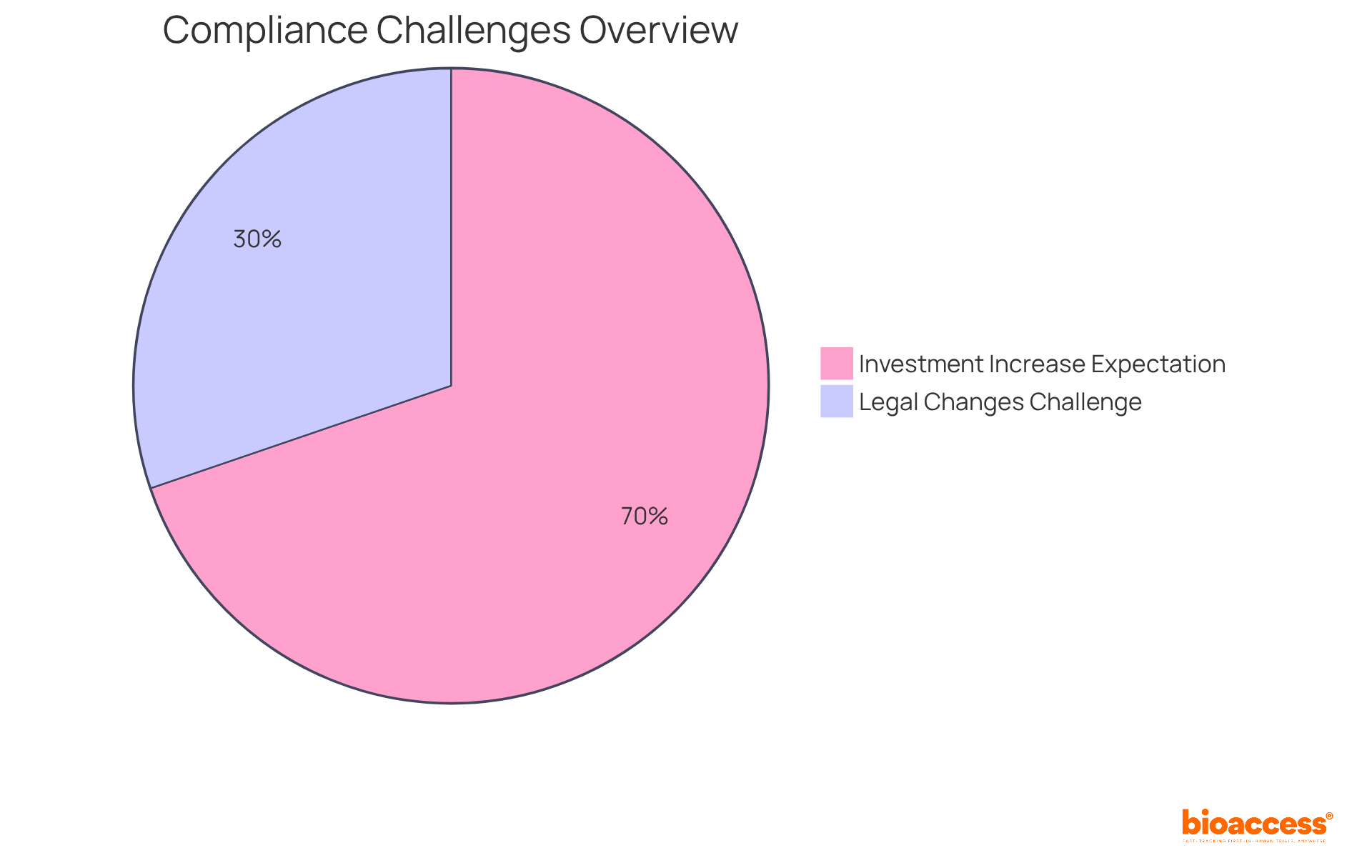
Notably, 39% of compliance teams cite legal and political changes as a significant challenge, making the forum an invaluable platform for exchanging effective compliance strategies. Moreover, insights from industry leaders underscore the necessity of proactive compliance measures, with 90% of compliance officers anticipating increased investment in compliance processes in the coming years.
By attending the MedTech Forum, companies can deepen their understanding of compliance best practices and strategically position themselves for success in an ever-evolving landscape.
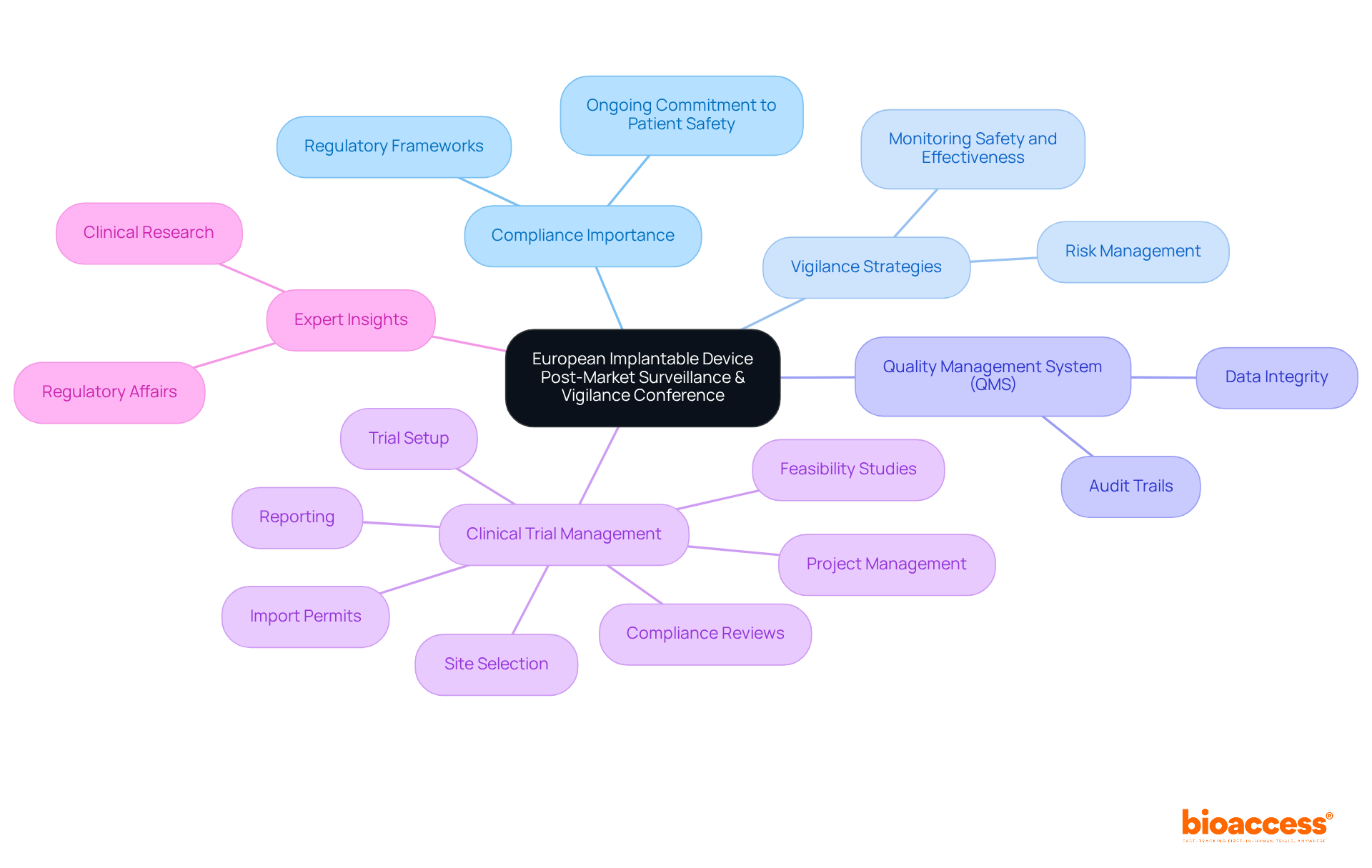
The European Implantable Device Post-Market Surveillance & Vigilance Conference is a pivotal platform for ensuring compliance in the post-market phase of medical devices, highlighting the importance of medical devices conferences. This event explores the latest regulations and best practices essential for monitoring the safety and effectiveness of implantable devices. Attendees will acquire insights into successful vigilance strategies that are crucial for maintaining compliance and safeguarding patient safety.
As Brittani Smith aptly notes, "Postmarket monitoring is essential to proving that your device remains safe and effective after it has received official approval."
With a focus on the evolving landscape of compliance demands, including the necessity for a robust Quality Management System (QMS) to guarantee data integrity, this medical devices conferences serves as an invaluable resource for Medtech professionals committed to excellence in post-market surveillance.
Furthermore, the conference will underscore the significance of comprehensive clinical trial management services, encompassing:
Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO, will also be crucial for attendees navigating the compliance landscape in Latin America.
Experts such as Ana Criado, Director of Regulatory Affairs at bioaccess, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, will impart valuable insights into the intersection of regulatory affairs and clinical research, particularly within the context of medical devices and in vitro diagnostics.
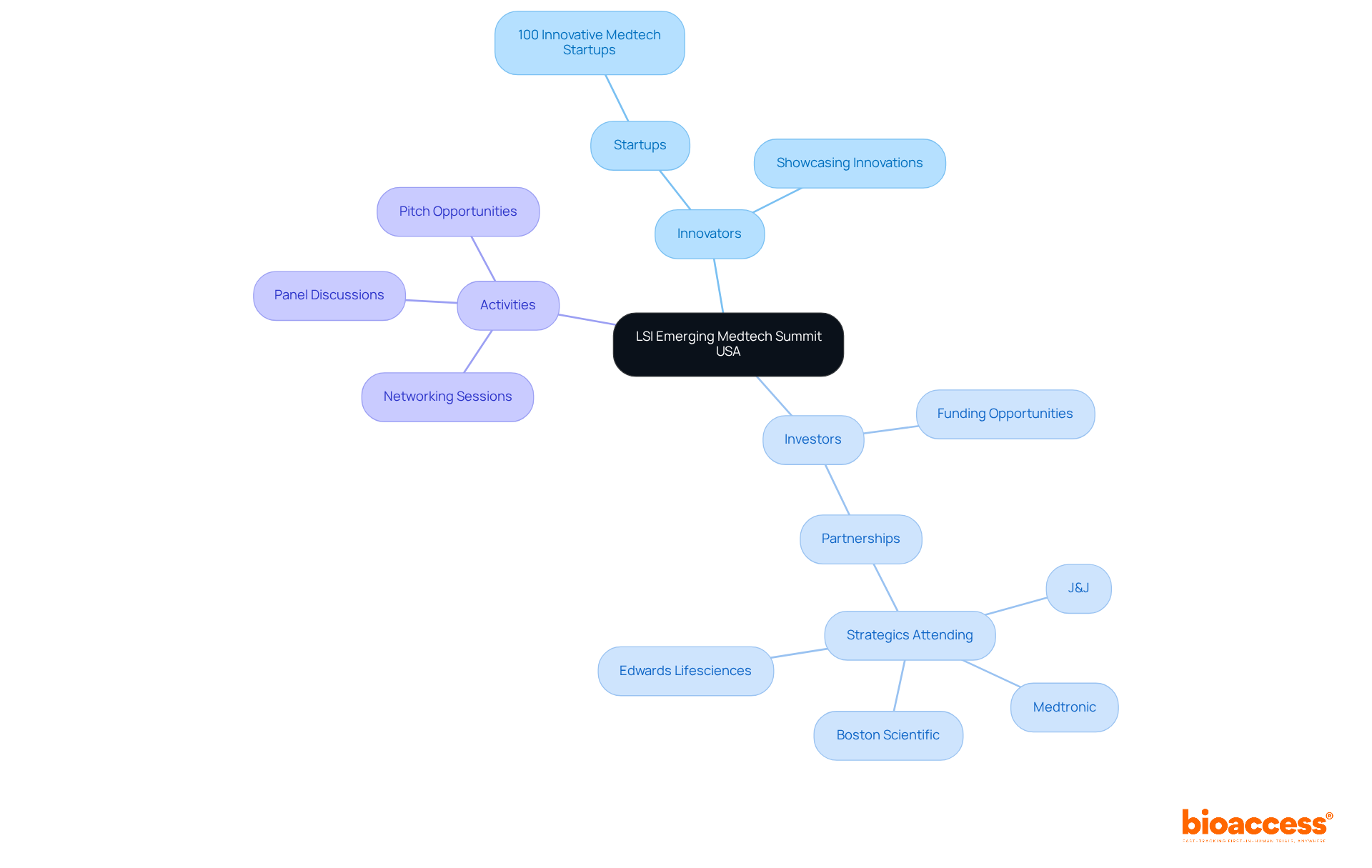
The LSI Emerging Medtech Summit USA stands out as a premier event designed to connect medical technology innovators with potential investors. This summit provides startups with a vital platform to showcase their groundbreaking innovations, thereby facilitating access to essential funding opportunities. Participants benefit from a rich array of networking sessions, interactive panel discussions, and competitive pitch opportunities, all meticulously crafted to foster meaningful connections.
Success stories from previous summits illustrate how partnerships formed here have propelled healthcare technology initiatives forward, underscoring the importance of this gathering for those eager to enhance their industry presence and drive innovation.
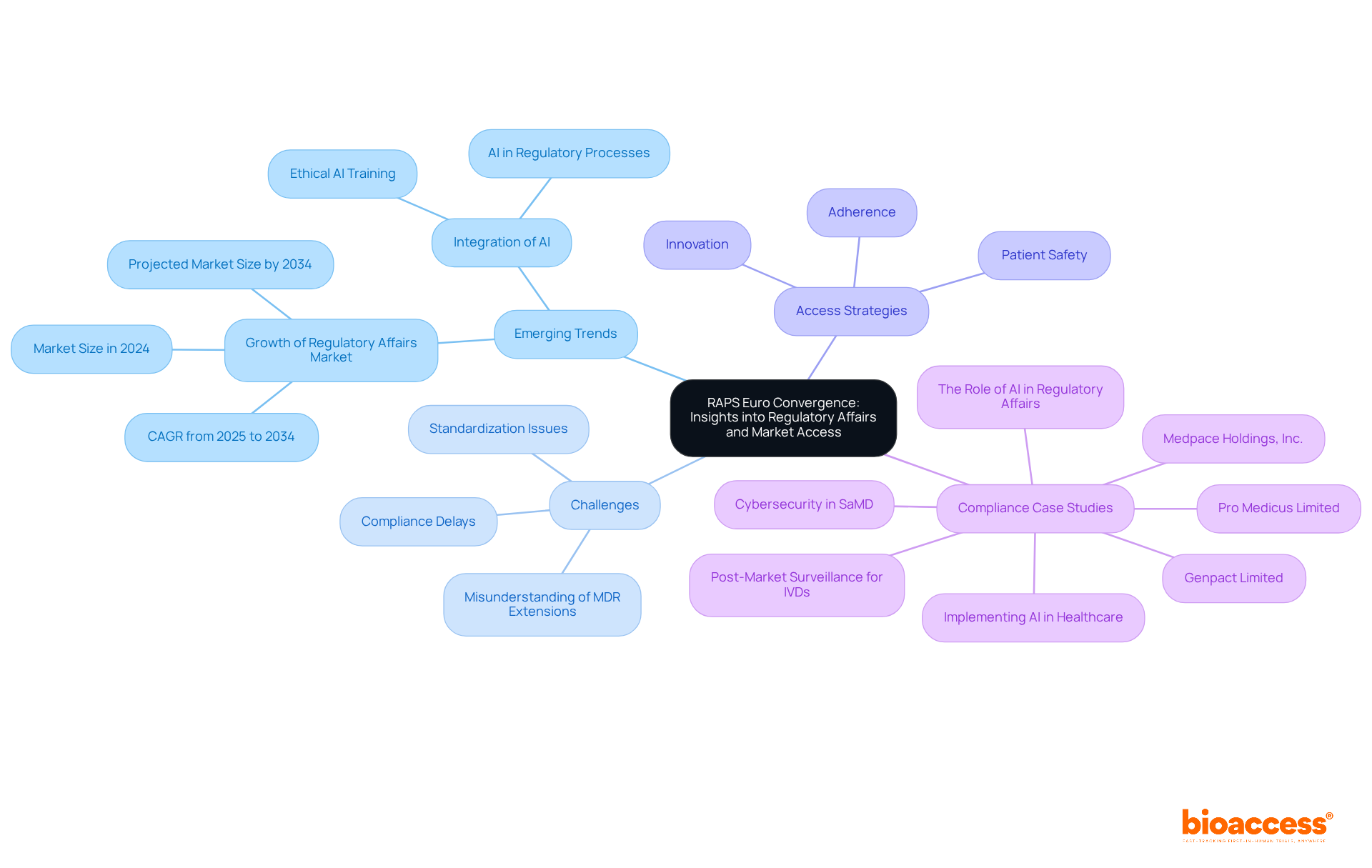
RAPS Euro Convergence emerges as a pivotal event focused on compliance matters and access strategies within the Medtech sector. This conference convenes compliance professionals to scrutinize emerging trends, challenges, and innovative solutions essential for navigating the complex regulatory landscape. Participants will gain invaluable insights into effective access strategies, which are increasingly critical as the global compliance sector is projected to grow from approximately USD 15,990 million in 2024 to around USD 36,490 million by 2034, reflecting a compound annual growth rate (CAGR) of 8.6% from 2025 to 2034.
The event also showcases successful compliance case studies from leading Medtech firms, illustrating how strategic planning and adherence to guidelines can facilitate industry entry. Notably, compliance experts emphasize that a robust access strategy must prioritize patient safety while balancing adherence and innovation. This aligns with the insights presented at RAPS Euro Convergence, where discussions consistently highlight the necessity of integrating real-world evidence and effective risk management into access frameworks.
As the medical technology landscape evolves, medical devices conferences like RAPS Euro Convergence provide essential resources for companies aiming to enhance their compliance strategies and achieve timely market access.
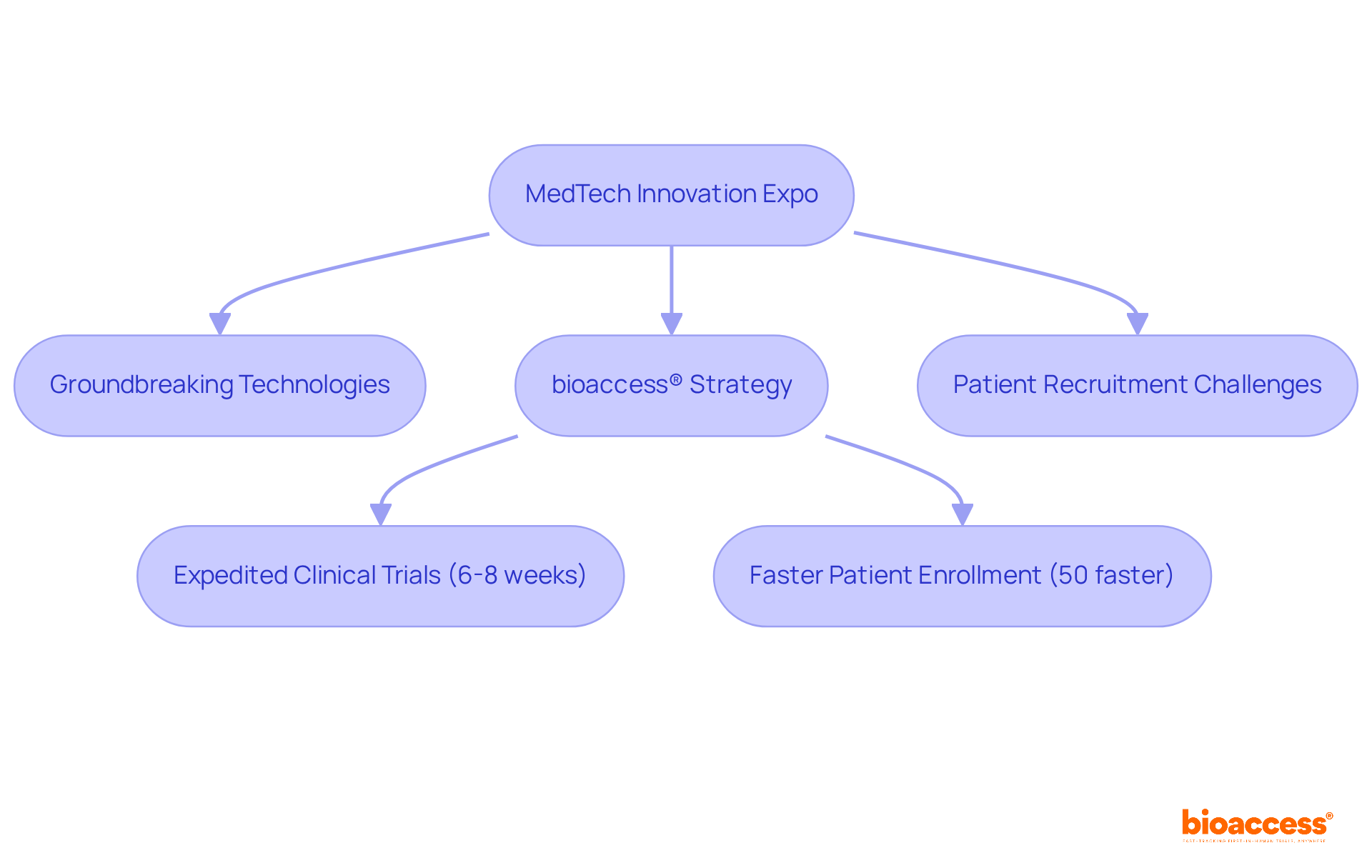
The MedTech Innovation Expo serves as a pivotal event within the medical technology landscape, particularly in the context of medical devices conferences, where groundbreaking technologies and innovations are spotlighted to revolutionize patient care. This expo draws a diverse range of exhibitors, from emerging startups to established industry leaders, all showcasing transformative solutions aimed at enhancing healthcare delivery.
Notably, bioaccess® will demonstrate its innovative strategy for expediting clinical trials, achieving regulatory approval in a mere 6-8 weeks, a significant reduction compared to the usual 6-12 months in the US/EU. Attendees will discover how bioaccess® enrolls treatment-naive cardiology and neurology cohorts 50% faster than Western sites, effectively tackling prevalent patient recruitment challenges encountered in early-phase clinical trials.
This positions the expo as an essential forum for those committed to staying at the forefront of medical technology innovation, especially at medical devices conferences, while exploring solutions that enhance efficiency in clinical research.
The Generis European Medical Device and Diagnostic Summit is a pivotal event among medical devices conferences for medical technology professionals seeking strategic insights and opportunities to expand their networks. This summit features a distinguished lineup of expert speakers who delve into pressing industry trends and regulatory challenges, offering innovative solutions tailored to the evolving landscape of the sector.
Attendees will encounter numerous networking sessions, facilitating connections that can foster fruitful collaborations and partnerships. Consequently, this summit, along with other medical devices conferences, represents an invaluable experience for industry leaders aiming to navigate the complexities inherent in the medical technology sector.
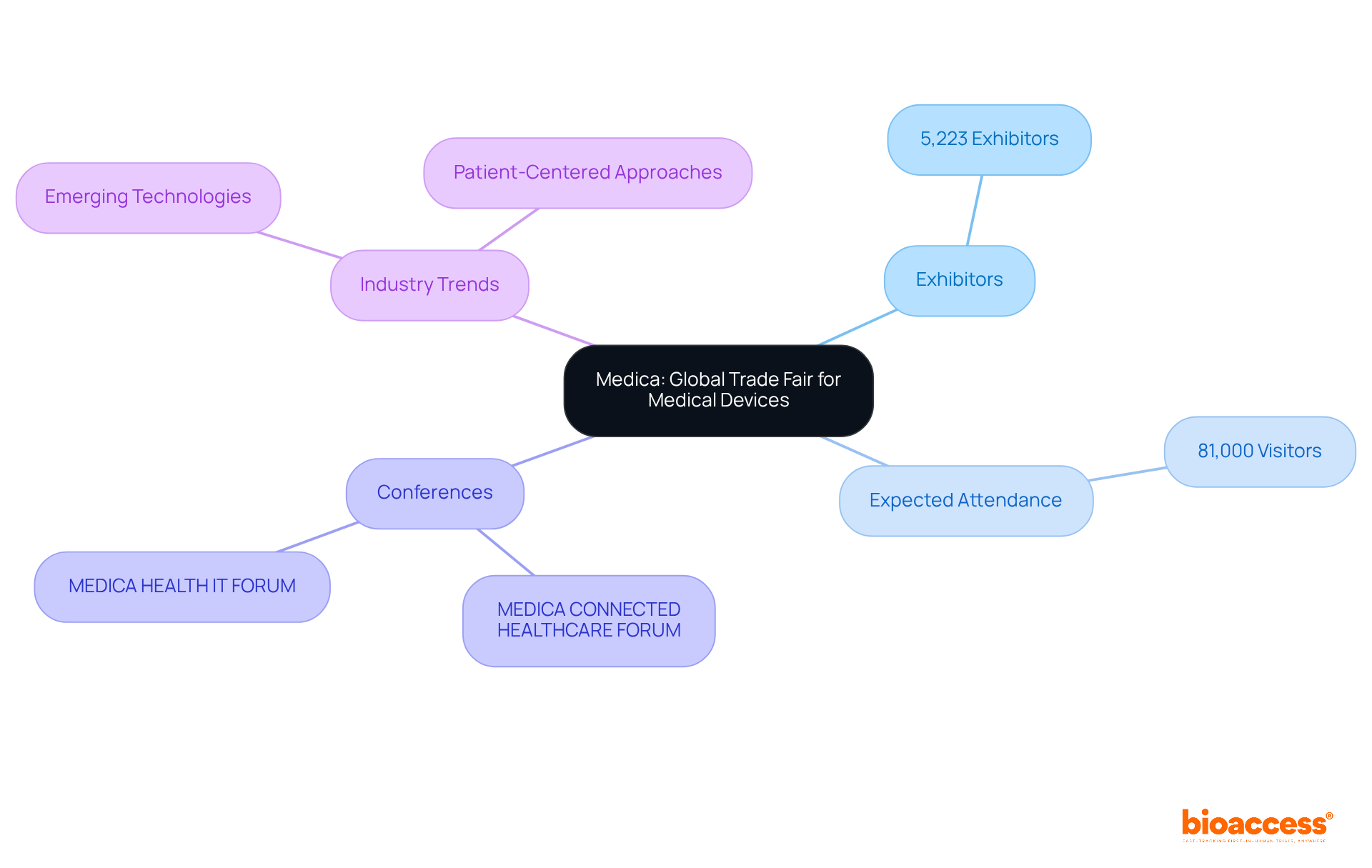
Medica stands as the world's largest trade fair for medical devices and technologies, including numerous medical devices conferences, drawing exhibitors and visitors from across the globe. This esteemed occasion not only highlights state-of-the-art advancements in the medical technology sector but also provides essential perspectives on emerging worldwide industry trends that will be a focal point at the medical devices conferences for 2025. With over 5,223 exhibitors and an expected attendance of 81,000 visitors, Medica serves as a vital platform for networking and collaboration at medical devices conferences, enabling industry leaders to forge successful global partnerships.
Attendees at medical devices conferences can explore the latest advancements in imaging, diagnostics, and medical services, making Medica an essential destination for those aiming to enhance their influence and reach within the Medtech market. The event's comprehensive program includes specialized forums and medical devices conferences, including the MEDICA CONNECTED HEALTHCARE FORUM and the MEDICA HEALTH IT FORUM, which delve into the transformative technologies shaping the future of healthcare.
As the industry evolves, Medica remains at the forefront, showcasing innovations that promise to redefine patient care and operational efficiency.
Attending medical devices conferences is essential for industry leaders striving to maintain a competitive edge in a rapidly evolving landscape. These events not only facilitate invaluable networking opportunities but also provide critical insights into emerging trends, regulatory challenges, and innovative solutions that can shape the future of medical technology. By engaging with peers and experts at these conferences, professionals can deepen their understanding and propel their initiatives forward, ensuring they remain at the forefront of the market.
Throughout this article, various conferences have been highlighted, each presenting distinct advantages. From the J.P. Morgan Health Care Conference's unparalleled networking potential to the MedTech Forum's emphasis on compliance and innovation, these events are meticulously designed to address the specific needs of medical technology professionals. The focus on rapid clinical research processes in Latin America, exemplified by bioaccess®, further underscores the significance of geographical insights within the global medical device sector. Each conference serves as a platform for collaboration, knowledge exchange, and the acceleration of groundbreaking technologies.
As the medical technology industry continues to advance, the importance of participating in these conferences cannot be overstated. Engaging with industry leaders, sharing insights, and exploring innovative solutions are vital steps for professionals seeking to navigate the complexities of the sector. Embrace the opportunity to attend these pivotal events and position yourself at the forefront of the medical devices industry, driving advancements that will ultimately enhance patient care and operational efficiency.
What is bioaccess® and what role does it play in clinical research for medical devices in Latin America?
bioaccess® is a leader in clinical research for medical devices, particularly at medical devices conferences in Latin America. It leverages the region's regulatory efficiency to secure ethical approvals in just 4-6 weeks and has a 50% faster enrollment rate compared to conventional sectors.
How does bioaccess® support early-phase studies for medical devices?
With over 15 years of specialized expertise, bioaccess® provides tailored solutions for early-phase studies, helping to showcase groundbreaking medical devices at conferences and facilitating their quicker market entry.
Why is Latin America considered a valuable region for first-in-human trials?
Latin America is referred to as a 'hidden gem' for first-in-human trials due to its capacity to streamline the journey from innovation to commercialization, as highlighted by Julio G. Martinez-Clark, CEO of bioaccess®.
What upcoming regulatory updates are expected in 2025 for medical technology firms in Latin America?
Regulatory updates in 2025 are anticipated to sustain the momentum of clinical studies, creating ideal opportunities for medical technology firms to showcase innovations and conduct successful early-phase clinical trials.
What is the significance of the J.P. Morgan Health Care Conference for medtech leaders?
The J.P. Morgan Health Care Conference is a pivotal networking event that attracts executives and investors from the medical technology sector, serving as a vital platform for forging connections and gaining insights into emerging trends.
How can attending the J.P. Morgan Health Care Conference benefit participants?
Attendees can gain valuable insights into market trends and innovations, and the networking opportunities can significantly influence the trajectory of medical technology partnerships.
What is MD&M West and what does it showcase?
MD&M West is a premier event among medical devices conferences that showcases cutting-edge technologies and innovations in medical device manufacturing, featuring a diverse array of exhibitors from startups to established companies.
How does MD&M West contribute to the medical technology field?
MD&M West provides professionals with innovative solutions to enhance product development and optimize manufacturing processes, while also collaborating with bioaccess™ to promote medical technologies and contribute to local economic development.