


The article titled "10 Strategies for Effective Drug Formulation in Clinical Research" serves to identify essential methodologies aimed at enhancing drug formulation processes within the clinical research environment. It delineates various strategies, such as:
Collectively, these strategies are designed to improve efficiency, compliance, and therapeutic outcomes in drug development, underscoring their significance in the Medtech landscape.
The pharmaceutical landscape is undergoing a rapid transformation, driven by the urgent need for innovative drug formulations that address the complexities of modern healthcare. As clinical research becomes increasingly competitive, navigating regulatory hurdles, leveraging cutting-edge technology, and adopting patient-centric approaches is paramount.
This article explores ten effective strategies designed to enhance drug formulation processes, empowering researchers to streamline development while simultaneously improving patient outcomes.
How can organizations effectively balance the urgency of market demands with the meticulous nature of drug formulation to ensure both efficacy and safety?
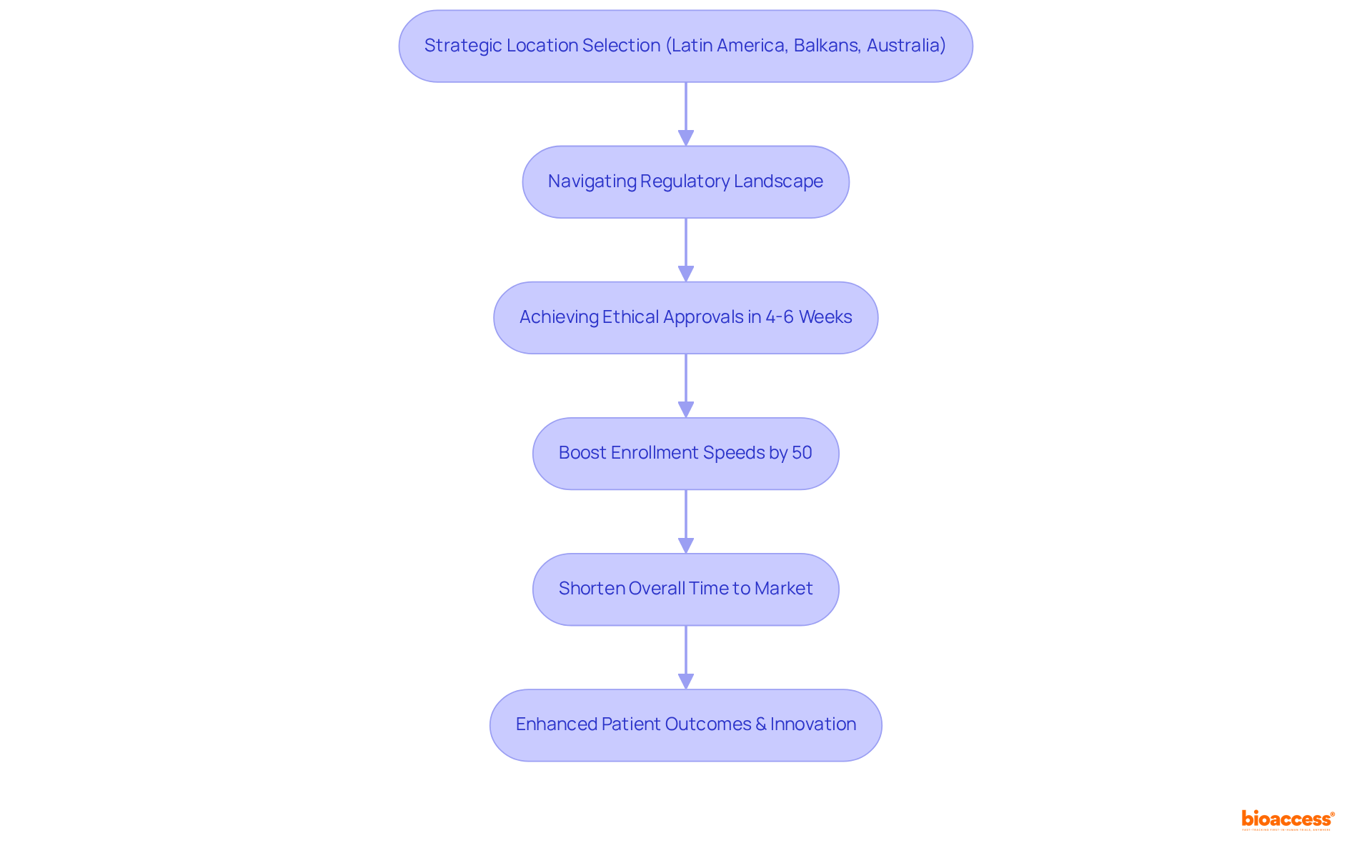
bioaccess® strategically leverages its locations in Latin America, the Balkans, and Australia to secure ethical approvals in an impressive 4-6 weeks. This expedited process is essential for Medtech, Biopharma, and Radiopharma innovators who are eager to accelerate their market entry. By adeptly navigating the regulatory landscape, bioaccess® minimizes delays, empowering clients to focus on their core research activities.
Rapid ethical approvals not only boost enrollment speeds by up to 50% but also significantly shorten the overall time to market—an imperative in a competitive industry where delays in clinical trials can cost sponsors between $600,000 and $8 million per day. Companies that have effectively harnessed this speed include those engaged in investigator-initiated studies (IIS), which have been shown to enhance patient outcomes and drive innovation.
For example, Sparta Biomedical reaped substantial benefits during its initial human study in Colombia, underscoring the advantages of collaboration with bioaccess®. As the MedTech industry has recorded at least 4% revenue growth annually over the past eight years, the ability to obtain swift regulatory approvals will continue to be a critical differentiator for success in clinical research.
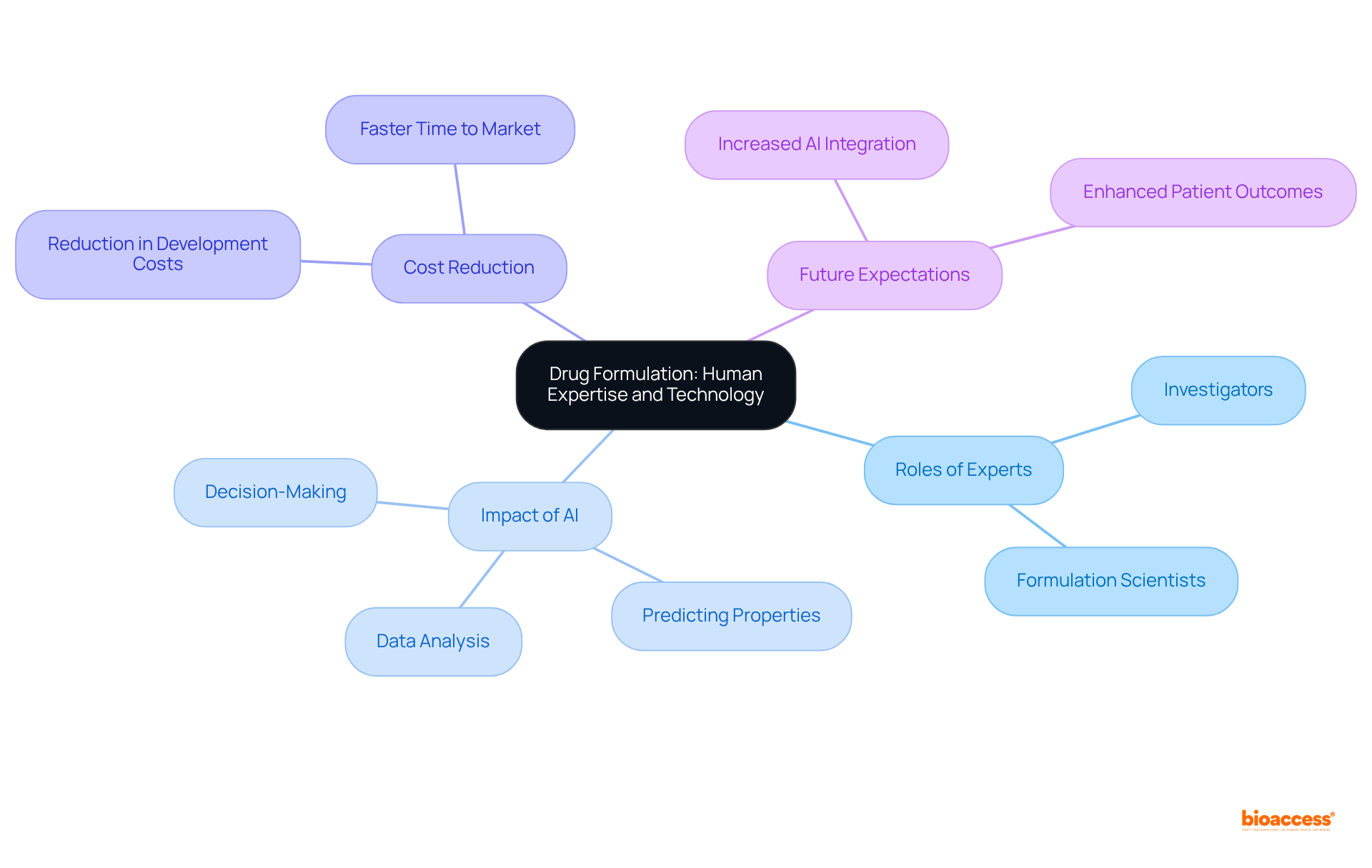
Successful medication development hinges on the collaboration of skilled experts and cutting-edge technology. Investigators, well-versed in the intricacies of scientific development, are pivotal in creating effective medications. The integration of advanced technologies, particularly artificial intelligence (AI) and machine learning, significantly enhances the development process. These technologies facilitate rapid iterations and improvements driven by real-time data analysis, optimizing pharmaceutical design and progression.
AI's impact on the efficiency of medicine development is profound. For example, AI systems can analyze extensive datasets to predict physicochemical properties, thereby enhancing solubility and stability while minimizing the risk of costly trial failures. This capability enables researchers to focus on innovative solutions, streamlining the timeline for progress. Moreover, AI-driven platforms have demonstrated the ability to reduce medication development costs by as much as 25%, underscoring their potential to transform traditional practices.
Formulation scientists are increasingly recognizing the critical role of technology in drug formulation and the development of medicine. Many assert that AI not only accelerates the identification of effective compounds but also enhances the accuracy of drug formulations, ultimately leading to improved patient outcomes. AI can aid in decision-making and facilitate logical medication design, further boosting efficiencies in the development process. As AI continues to evolve, its influence on medication development processes is expected to expand, fostering additional efficiencies and innovations within the pharmaceutical sector.
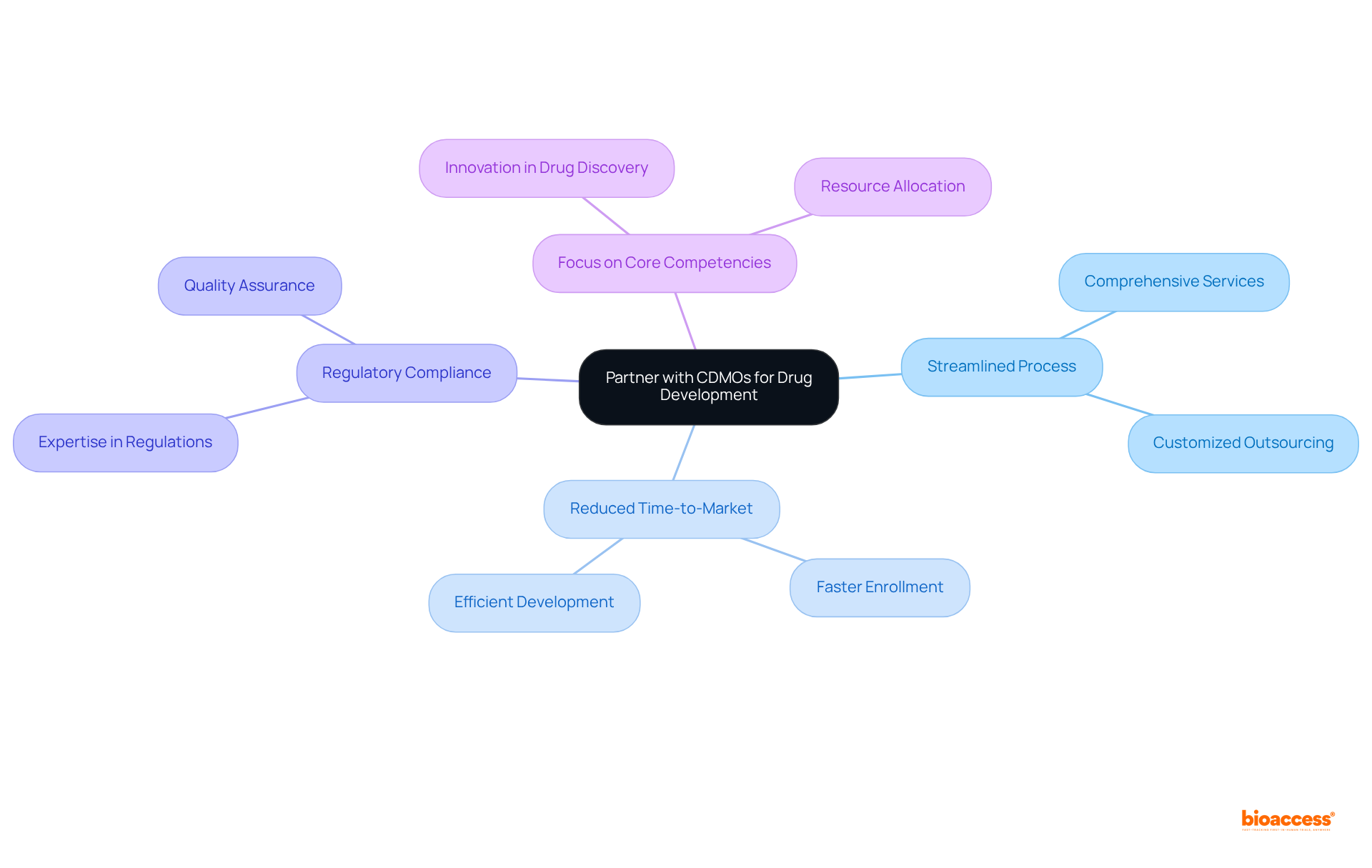
Collaborating with CDMOs significantly streamlines the pharmaceutical creation process. These organizations provide comprehensive services, encompassing everything from development to manufacturing. By harnessing their specialized expertise, companies can effectively reduce time-to-market and guarantee compliance with regulatory standards. This strategic partnership empowers innovators to concentrate on their core competencies while capitalizing on the CDMO's extensive knowledge and resources.
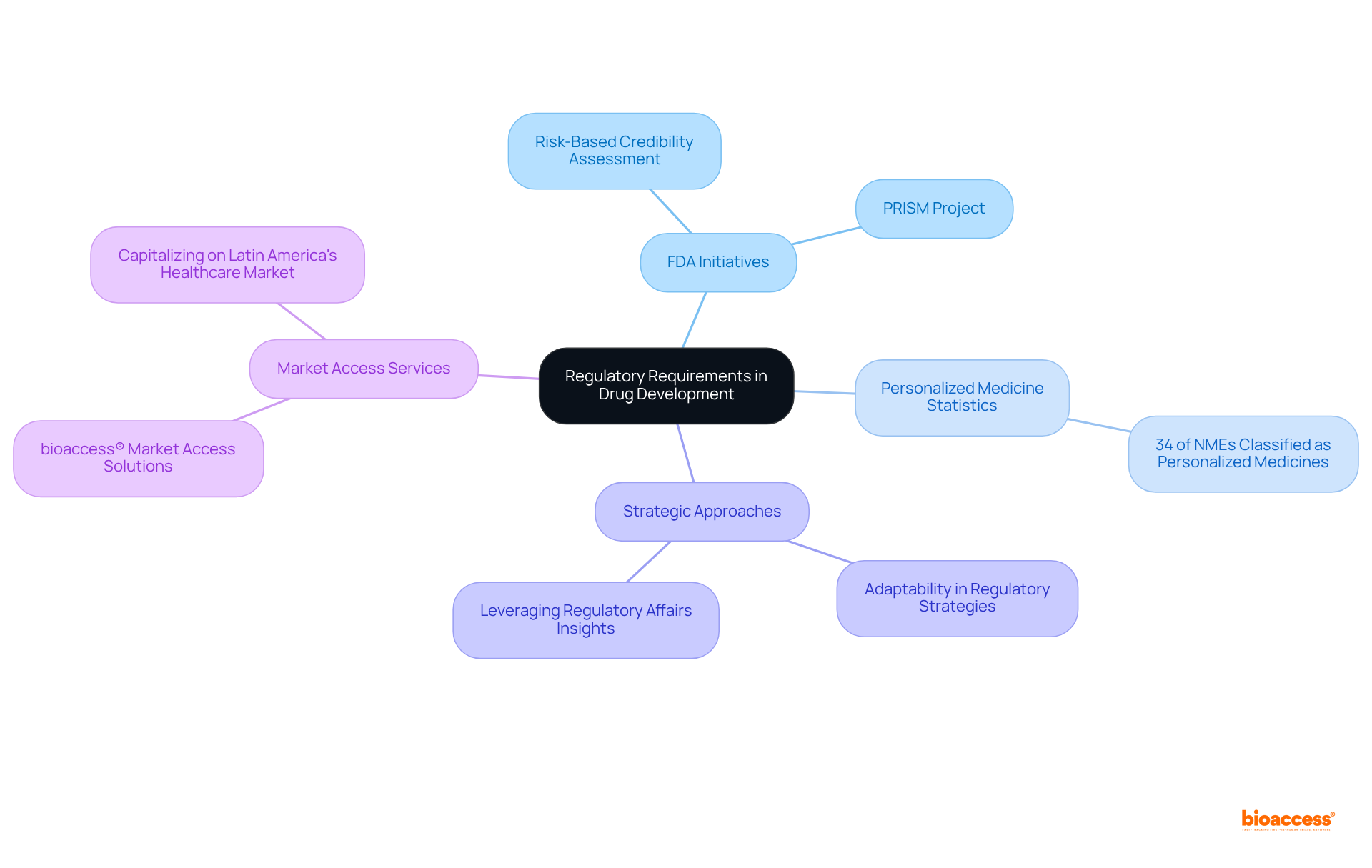
Navigating regulatory challenges in drug development necessitates a comprehensive understanding of the evolving requirements set by regulatory bodies. For companies aiming to ensure compliance and mitigate development challenges, staying informed about the latest guidelines is crucial. The FDA's recent initiatives, such as the introduction of a risk-based credibility assessment framework for AI models and the PRISM Project designed to streamline regulatory submissions via a cloud-based platform, underscore the importance of adaptability in regulatory strategies. Organizations that proactively adjust their production processes in response to these guidelines can significantly enhance their chances of securing smoother approvals and achieving quicker market entry.
Statistics reveal that approximately 34% of new molecular entities authorized by the FDA in 2022 were classified as personalized medicines, illustrating the growing complexity of compliance in development. This trend compels companies to not only grasp regulatory expectations but also to innovate in their strategic approaches to meet the demands of personalized medicine. By leveraging insights from regulatory affairs specialists, organizations can adeptly navigate compliance challenges and position themselves for success within the competitive landscape of clinical research. Furthermore, with the global pharmaceutical market projected to reach USD 2.8 trillion by 2035, effectively managing these regulatory hurdles is more critical than ever. bioaccess® offers market access services that empower clients to capitalize on the vast potential of Latin America's healthcare market, further exemplifying how companies can successfully navigate these complexities.
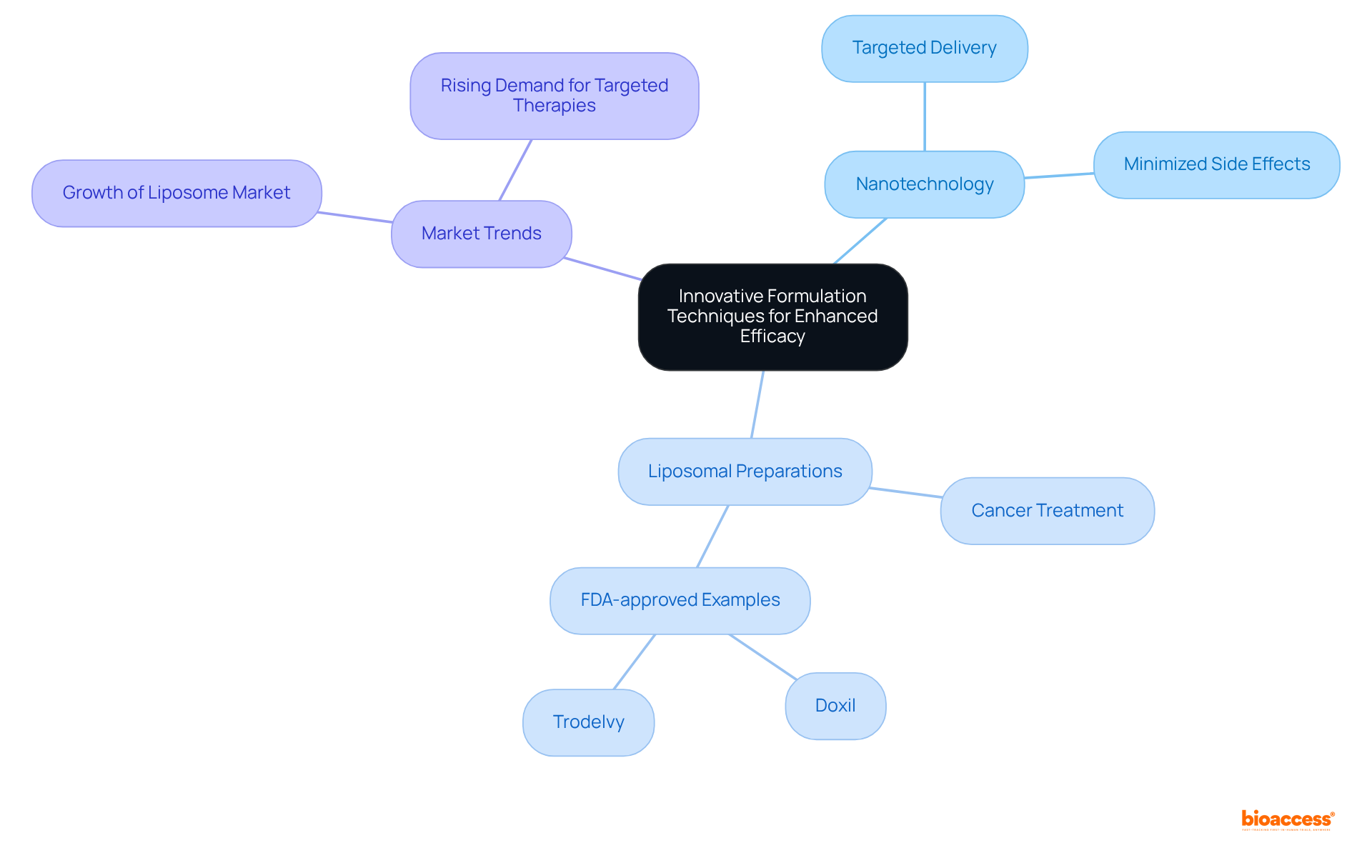
Integrating creative preparation methods is essential for enhancing the effectiveness of medicine. Techniques such as nanotechnology and liposomal preparations have shown significant improvements in bioavailability and therapeutic outcomes. For instance, lipid-based mixtures can enhance the solubility and uptake of poorly water-soluble medications, addressing a common challenge in pharmaceutical advancement. Notably, approximately 40% of medications in development are poorly water-soluble, making these innovative techniques crucial. The lipid-based preparation of cyclosporine (Neoral) improved bioavailability by 30-50% and reduced interpatient variability by 50-70%.
Recent advancements in nanotechnology have further revolutionized drug formulation. Nanoparticles and nanocarriers enable targeted medication delivery, maximizing therapeutic efficacy while minimizing adverse effects. The integration of these technologies into personalized medicine is gaining traction, facilitating tailored treatments that align with individual patient needs.
Current trends indicate an increasing reliance on liposomal formulations, particularly in cancer treatment, where they enhance medication delivery precision and mitigate side effects. The cancer therapy sector leads the liposome medication delivery market, driven by the rising incidence of cancer and the demand for targeted treatments. The liposome drug delivery market size in 2023 was estimated at USD 5.46 billion, underscoring its significance. Notable examples include FDA-approved liposomal preparations like Doxil and Trodelvy, which have demonstrated enhanced effectiveness in treating various cancers.
By remaining at the forefront of scientific development, companies can create more efficient therapies that meet the evolving needs of individuals, ultimately resulting in improved health outcomes.
Integrating a user-focused strategy in drug formulation is essential for aligning therapies with individual needs and preferences. This approach actively considers factors such as drug formulation, dosage form, administration route, and potential side effects during the development process. Engaging individuals in discussions about their experiences and preferences leads to the creation of more suitable and effective therapies.
Research indicates that:
Healthcare professionals recognize that individual preferences greatly influence treatment choices, with many advocating for shared decision-making to enhance adherence rates. As Brett Hauber observed, while existing studies have concentrated on appearance and swallowability, the importance of palatability and handling must not be overlooked. By prioritizing consumer feedback, medication creators can improve treatment outcomes and foster a more collaborative healthcare environment.
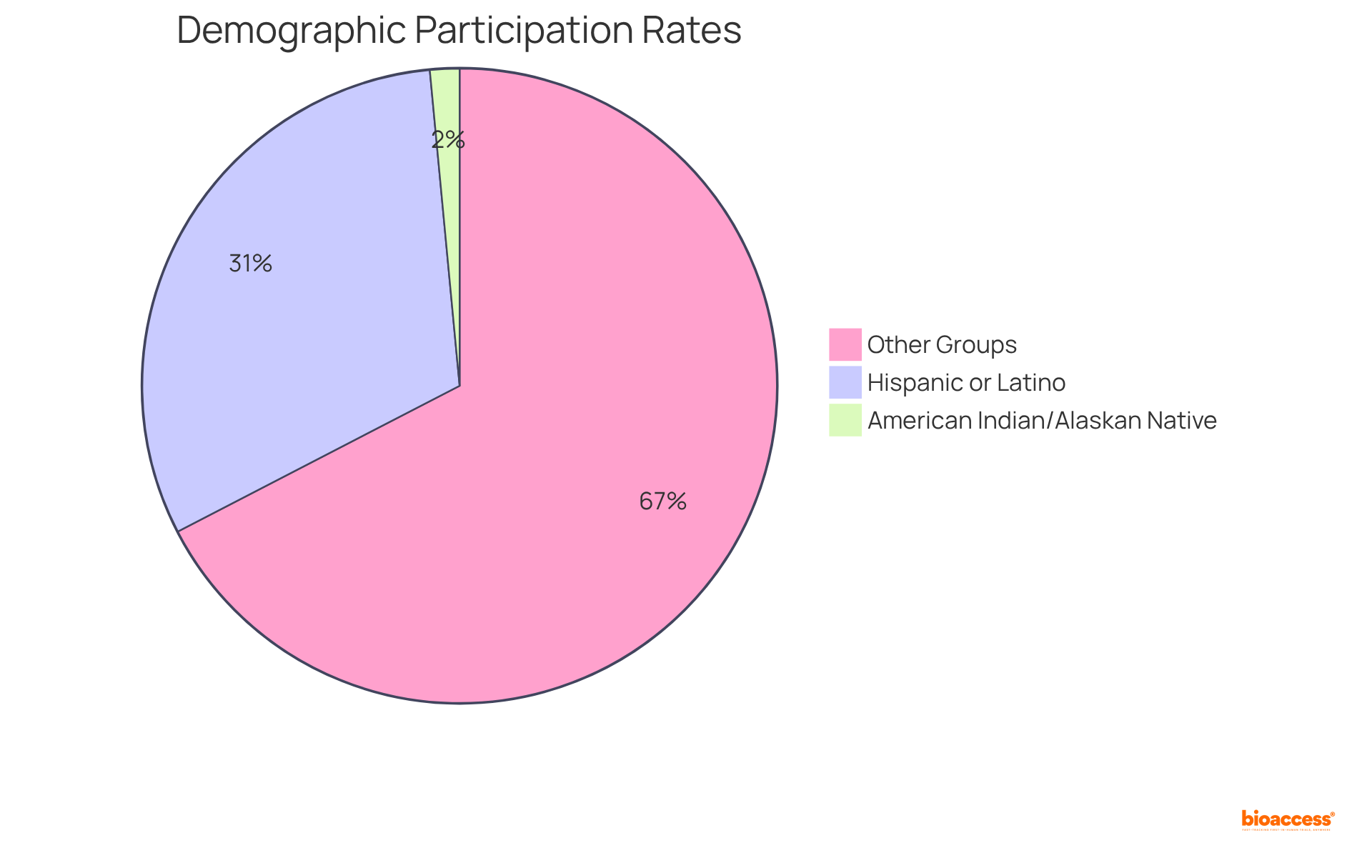
Including varied groups in clinical studies is crucial for obtaining a thorough understanding of how different demographics react to drug formulations. Studies have shown that variations in drug efficacy and safety can significantly differ across populations, underscoring the need for tailored treatments.
For instance, recent analyses indicate that minority groups often experience disparities in clinical trial representation, with Hispanic or Latino individuals participating at rates such as 31.1% in specific trials like Inpefa. Additionally, the participation of American Indian or Alaskan Native individuals has historically been under 1% to 2%.
By conducting research in diverse environments, researchers can ensure that their findings are pertinent to a wider array of individuals, ultimately enhancing the applicability of their results. This method not only promotes inclusivity but also stimulates innovation in drug formulation, as it enables the identification of unique responses and potential side effects that may not be clear in more homogeneous study groups.
As highlighted by Bibbins-Domingo K., a better understanding of trial enrollment and results for specific demographic populations is likely to accelerate scientific progress and lead to more effective treatment options for all patients. Moreover, with clinical trial failure rates varying between 80% and 90%, the importance of diverse participation becomes even more essential in enhancing pharmaceutical creation results.
Investing in ongoing education for research teams is essential for upholding high standards in medication development. Regular training sessions that focus on the latest techniques, regulatory updates, and industry trends equip team members with the critical knowledge and skills needed to excel. This commitment to professional growth not only enhances the quality of medication creation but also fosters a culture of excellence and innovation within the organization.
Notably, industry leaders acknowledge that organizations prioritizing employee development are:
Given that 90% of companies express concern about employee retention, the importance of investing in training becomes paramount for maintaining a competitive edge.
Utilizing data analytics is crucial for enhancing drug formulation processes. By analyzing data from earlier studies, researchers can uncover trends and correlations that inform decision-making. Predictive analytics not only helps in recognizing potential challenges but also enables proactive modifications, thereby improving the overall strategy.
For instance, advancements in machine learning, such as the ADA-DT model achieving an R² score of 0.9738 for drug solubility prediction, illustrate the significant improvements in accuracy that can be realized. Moreover, the methodical elimination of less significant features via Recursive Feature Elimination (RFE) can enhance model performance, resulting in more informed decision-making.
Data scientists, like Dr. Ashwin Kuchekar, emphasize that integrating predictive analytics with traditional methods can lead to better decision-making, ultimately reducing the time and costs associated with bringing new treatments to market.
To implement predictive analytics effectively, directors of clinical research should consider investing in training for their teams to utilize these advanced analytical tools, ensuring they can leverage data-driven insights in their strategy development.
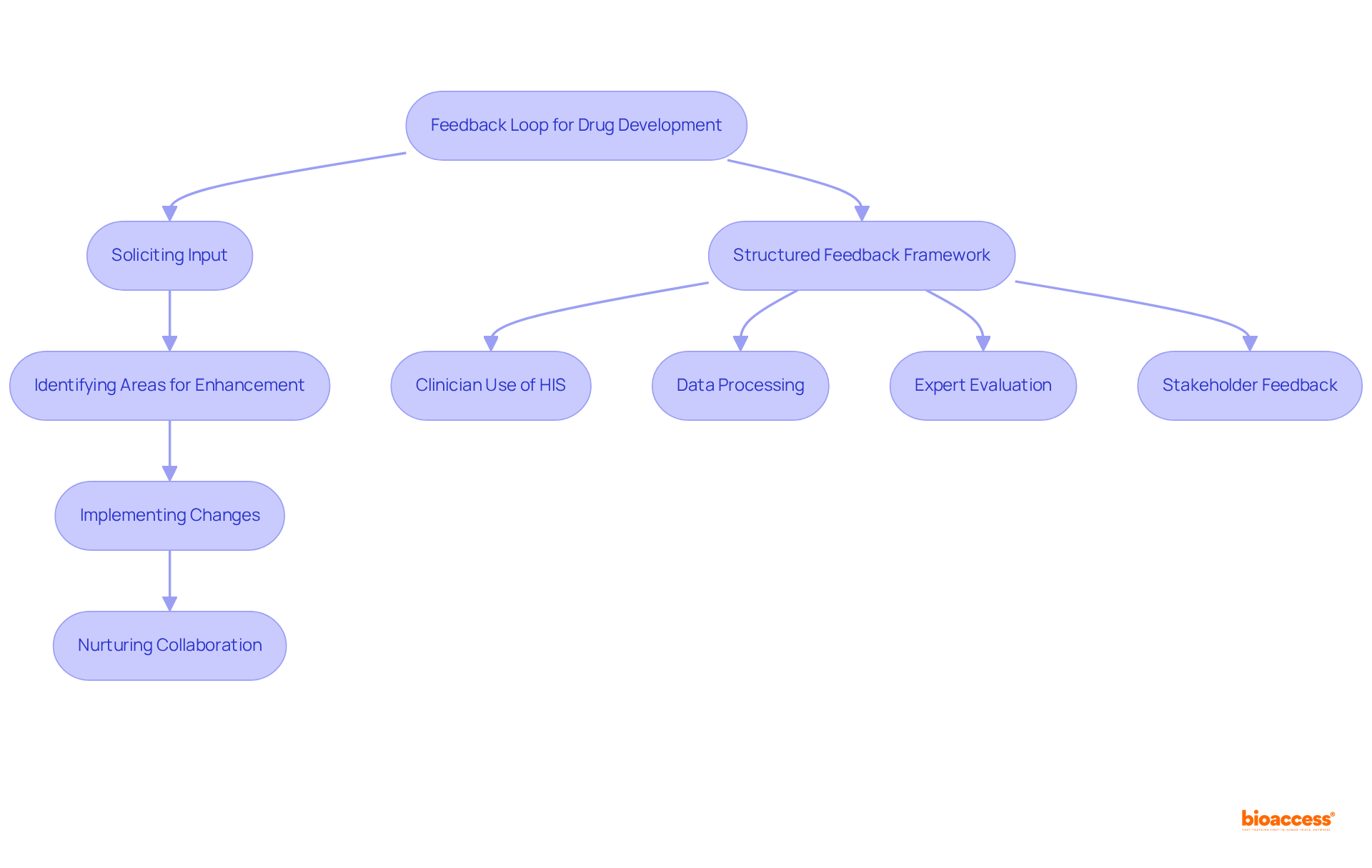
Creating feedback loops within the drug development process is essential for fostering continuous improvement. By actively soliciting input from team members, stakeholders, and clients, organizations can identify areas for enhancement and implement effective changes. This iterative approach not only improves drug formulation outcomes but also nurtures a collaborative environment that is conducive to innovation.
Notably, bioaccess® has shown that leveraging diverse patient pools and regulatory speed can significantly expedite ethical approvals and enrollment, achieving results 50% faster than traditional markets. Furthermore, industry leaders assert that feedback is critical for refining processes and enhancing drug development outcomes.
A structured feedback framework, which encompasses:
ensures that insights are systematically integrated into drug formulation strategies. Additionally, maintaining high data quality is vital for utilizing real-world data (RWD) in scientific contexts, ultimately leading to safer and more effective pharmaceutical products.
It is also imperative to recognize the limitations in analyzing free text not mapped to ATC codes, as this presents challenges within the feedback loop framework.
Effective drug formulation in clinical research stands as a multifaceted endeavor, necessitating a strategic approach to navigate the complexities of the pharmaceutical landscape. By implementing various strategies—such as expedited ethical approvals, leveraging human expertise and technology, and fostering partnerships with CDMOs—companies can significantly enhance their drug development processes. The focus on patient-centric approaches and the integration of diverse populations enriches the understanding of drug efficacy and safety, ensuring that therapies are tailored to meet the needs of all individuals.
Key insights from the article underscore the importance of:
Understanding regulatory requirements is essential for mitigating challenges, while innovative techniques like nanotechnology and liposomal preparations play a crucial role in enhancing drug efficacy. Additionally, the power of data analytics in optimizing drug formulation processes is paramount, as it empowers researchers to make informed decisions based on real-time insights.
Ultimately, continuous investment in training research teams and establishing feedback loops fosters a culture of improvement and innovation. By prioritizing these strategies, organizations can accelerate the development of effective medications and contribute to a more inclusive and responsive healthcare system. Embracing these practices is not merely a pathway to success in clinical research; it represents a commitment to improving patient outcomes and advancing the future of medicine.
What is bioaccess® and how does it benefit clinical research?
bioaccess® is a company that leverages its locations in Latin America, the Balkans, and Australia to secure ethical approvals for clinical research in an impressive 4-6 weeks. This expedited process helps Medtech, Biopharma, and Radiopharma innovators accelerate their market entry by minimizing delays and allowing clients to focus on their core research activities.
How do rapid ethical approvals impact clinical trials?
Rapid ethical approvals can boost enrollment speeds by up to 50% and significantly shorten the overall time to market. This is crucial in a competitive industry where delays in clinical trials can cost sponsors between $600,000 and $8 million per day.
Can you provide an example of a company that benefited from bioaccess®?
Sparta Biomedical experienced substantial benefits during its initial human study in Colombia by collaborating with bioaccess®, highlighting the advantages of their expedited approval process.
What role does AI and machine learning play in drug formulation?
AI and machine learning enhance the medication development process by facilitating rapid iterations and improvements through real-time data analysis. They help predict physicochemical properties, improve solubility and stability, and reduce the risk of costly trial failures.
How much can AI reduce medication development costs?
AI-driven platforms can reduce medication development costs by as much as 25%, transforming traditional practices in the pharmaceutical sector.
What is the role of formulation scientists in drug development?
Formulation scientists play a critical role in drug formulation and the development of medicine by integrating advanced technologies like AI, which aids in decision-making and enhances the accuracy of drug formulations, ultimately leading to improved patient outcomes.
What are the benefits of partnering with CDMOs in drug development?
Partnering with Contract Development and Manufacturing Organizations (CDMOs) streamlines the pharmaceutical creation process by providing comprehensive services that encompass development and manufacturing. This collaboration helps reduce time-to-market and ensures compliance with regulatory standards, allowing companies to focus on their core competencies.