


The primary focus of this article is to delineate effective strategies for achieving success in preclinical development. It underscores the significance of:
These crucial strategies, when effectively implemented, can markedly enhance the efficiency and outcomes of preclinical research. By adopting these practices, stakeholders can navigate the complexities of the Medtech landscape, addressing key challenges with confidence and expertise.
In the rapidly evolving landscape of medical research, the success of preclinical development is more crucial than ever. With the potential to accelerate innovations in Medtech, Biopharma, and Radiopharma, understanding and implementing effective strategies can significantly enhance the efficiency and outcomes of research efforts. However, navigating the complexities of regulatory requirements, participant recruitment, and data management poses substantial challenges.
What are the key strategies that can transform these hurdles into stepping stones for success in preclinical development?
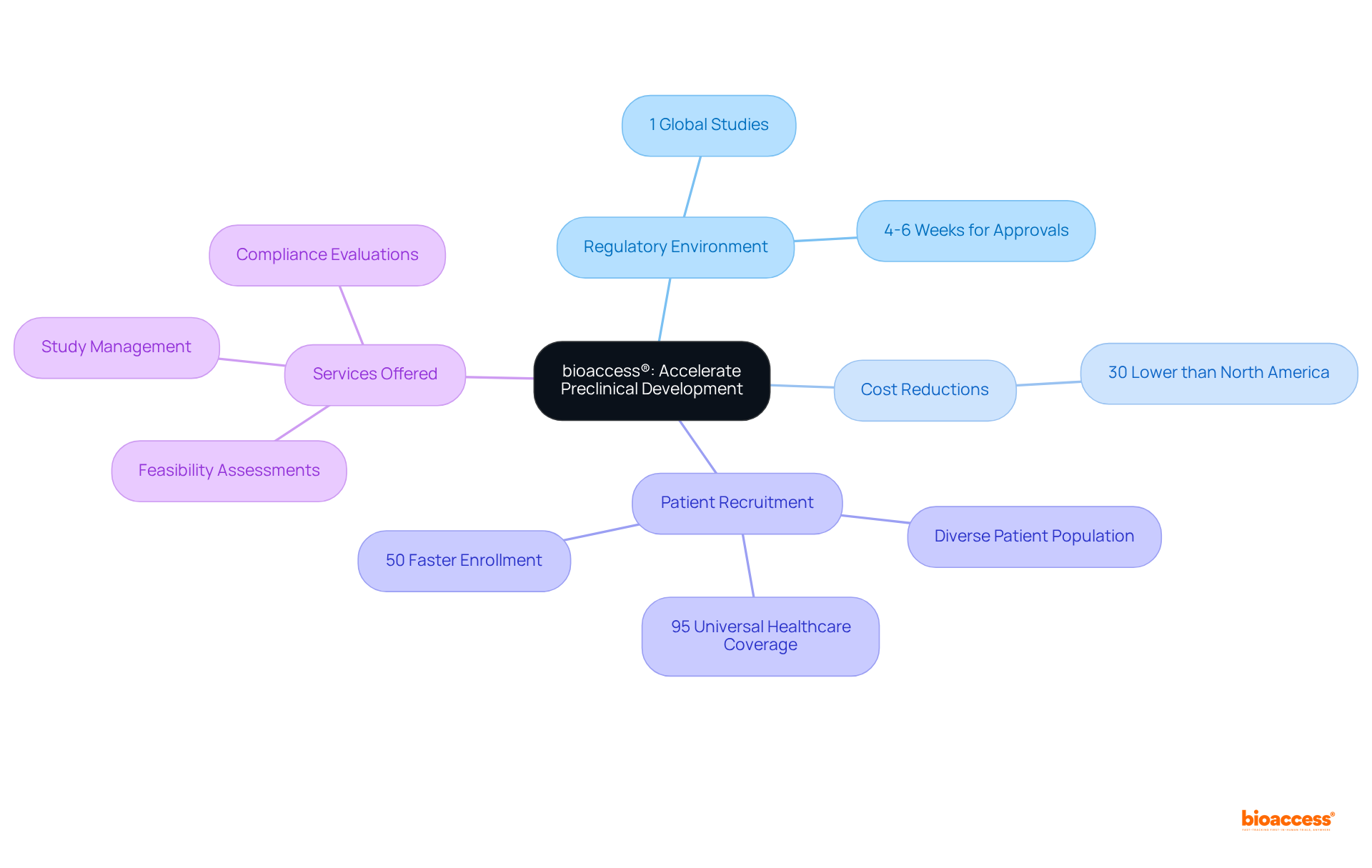
bioaccess® strategically capitalizes on the regulatory environment in Latin America, where merely 1 percent of global medical device studies are conducted. Colombia stands out with significant advantages, including cost reductions surpassing 30% compared to North America and Western Europe, alongside a healthcare system recognized as one of the finest in Latin America.
With a diverse patient population exceeding 50 million and approximately 95% of individuals covered by universal healthcare, bioaccess® guarantees accelerated patient recruitment and streamlined pathways for ethical approvals, achieving these in merely 4-6 weeks. This strategic combination empowers Medtech, Biopharma, and Radiopharma innovators to substantially expedite their processes in preclinical development, boasting enrollment rates that are 50% faster than traditional markets.
Notably, 35% of delays in medical studies stem from insufficient participant recruitment, underscoring the critical nature of recruitment efficiency. By leveraging these regional advantages, including R&D tax incentives that offer considerable financial benefits, bioaccess® enables clients to bring their innovations to market with greater speed and efficiency.
Our comprehensive services encompass:
establishing a robust support system for medical advancements. As Julio G. Martinez-Clark, CEO of bioaccess®, articulates, 'Latin America has become one of the most attractive locations for international clinical trials,' further accentuating the vital role of bioaccess® in this evolving landscape.
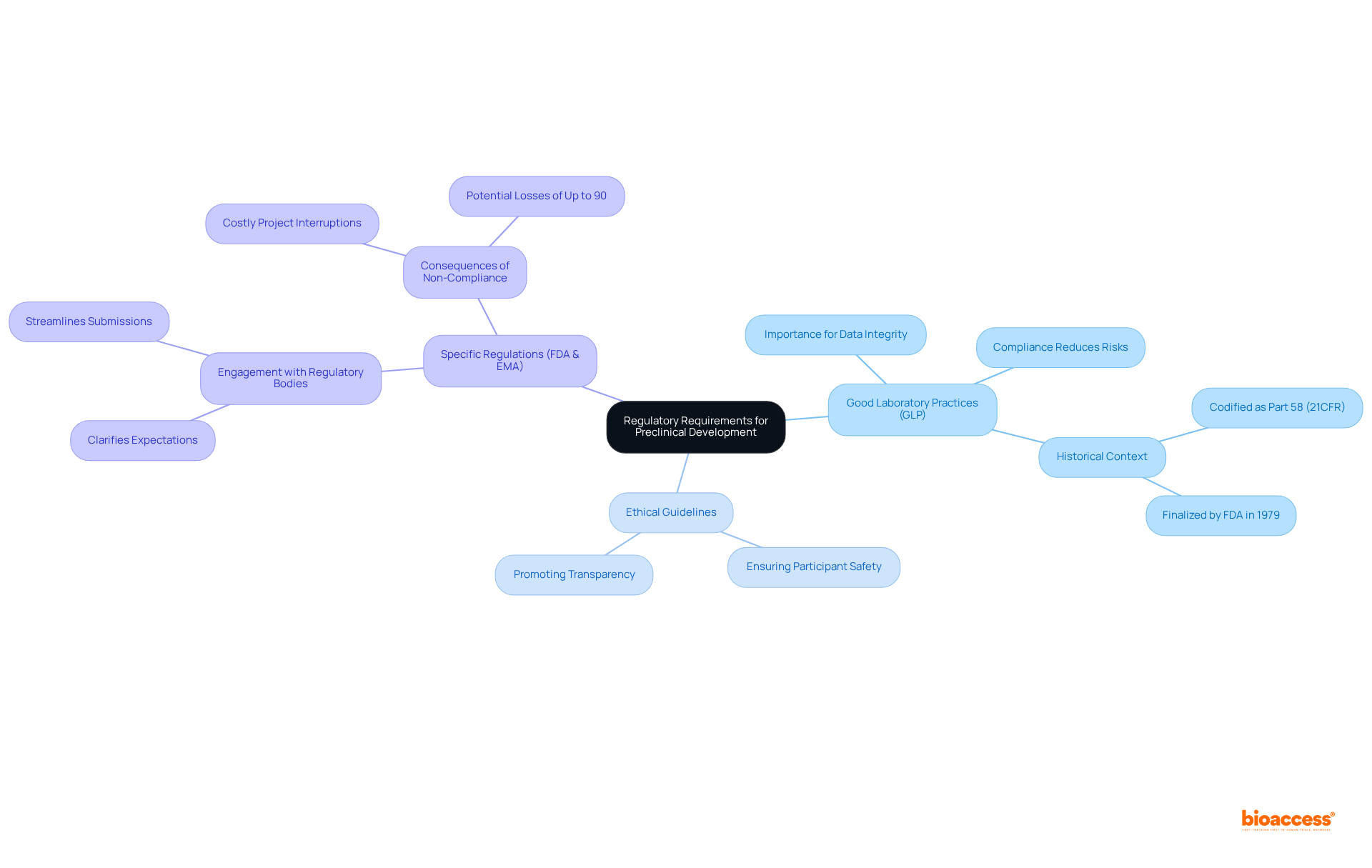
A thorough understanding of regulatory requirements is essential for successful preclinical development. Companies must be well-versed in:
Engaging with regulatory bodies early in the preclinical development process clarifies expectations and streamlines submissions, significantly reducing the risk of delays in the overall development timeline. For instance, adherence to GLP principles ensures the integrity and reliability of nonclinical safety data, a critical factor for preclinical development and regulatory approval.
Regular training and updates on regulatory changes are vital for maintaining compliance throughout the initial phase; non-compliance can lead to costly project interruptions, potentially resulting in losses of up to 90% of total investment. By prioritizing these strategies, companies can navigate the complexities of early-stage development more effectively.
Selecting appropriate animal models is crucial for achieving reliable results in preclinical development research. These models must closely replicate human physiology and the specific disease states under investigation. Factors such as genetic background, age, and sex of the animals significantly influence study outcomes.
For instance, validated models have demonstrated their capacity to efficiently forecast human reactions, with research indicating that:
Emerging alternatives, such as organ-on-chip technologies, are gaining traction in preclinical development research. These systems mimic in vivo conditions by combining organ-specific cell types and tissue-specific extracellular matrices, demonstrating remarkable predictive capabilities.
A notable study utilizing the Liver-Chip exhibited:
The potential of the Liver-Chip to save lives and enhance drug development productivity by an estimated $3 billion annually further underscores the economic benefits of adopting these innovative approaches. By integrating these technologies, researchers can improve the predictive validity of their studies while reducing reliance on conventional animal testing, ultimately enhancing drug development success rates.
Moreover, the ethical concerns surrounding animal research must be acknowledged, as the high failure rates and the push for alternatives highlight the need for more humane and effective research methodologies.
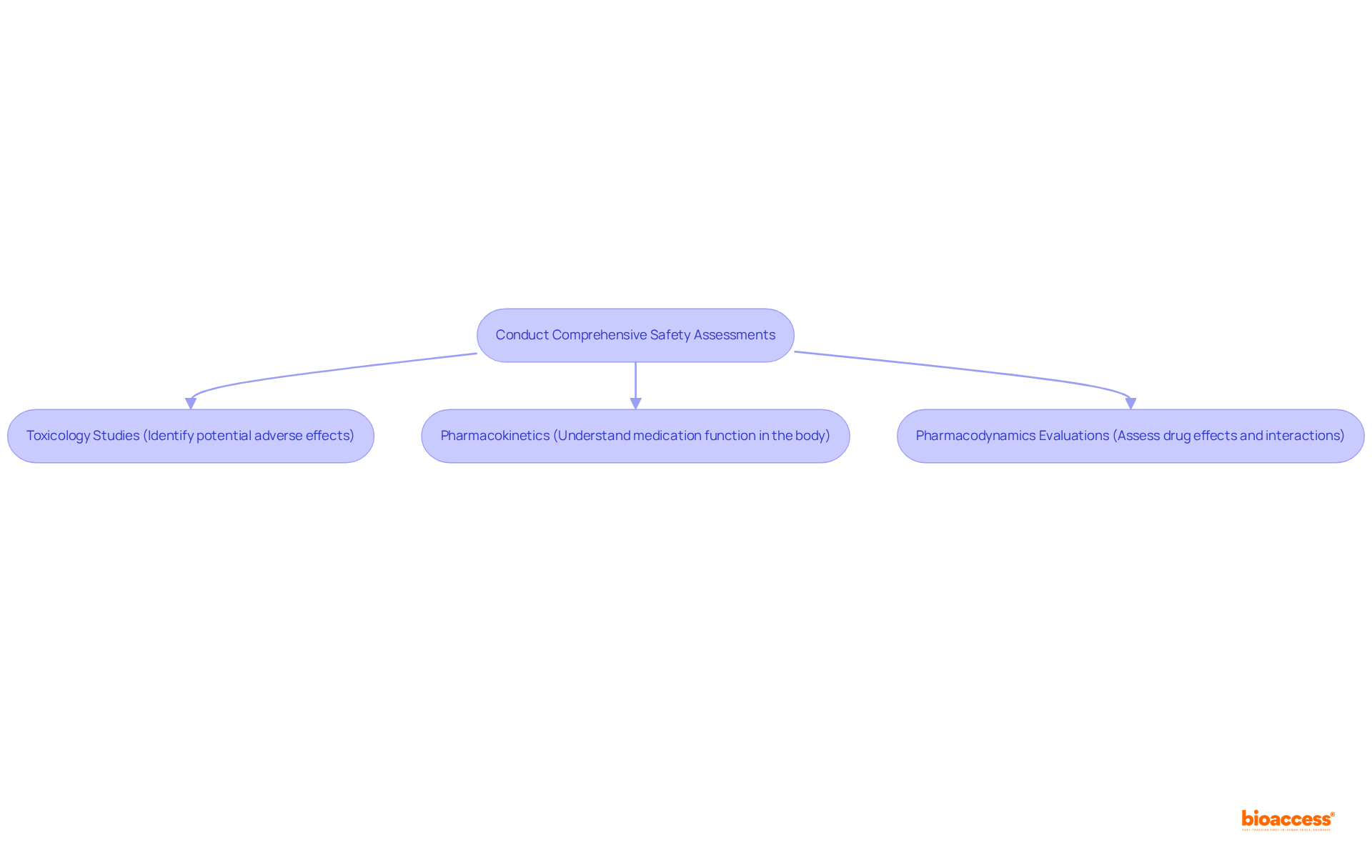
Thorough safety evaluations are paramount before initiating any medical study. These assessments must encompass:
to effectively identify potential adverse effects of the investigational product. Utilizing GLP-compliant facilities is essential, as it guarantees that the generated data is reliable and adheres to regulatory standards. Research has shown that early recognition of safety issues in preclinical development can significantly reduce the likelihood of costly failures in subsequent development phases, ultimately safeguarding patient safety during clinical evaluations. Experts emphasize that pharmacokinetic assessments are crucial for understanding how medications function within the body, guiding improved decision-making in study design. Current standards for pharmacokinetics and pharmacodynamics evaluations in earlier studies highlight the necessity for rigorous methodologies to ensure precise assessments. By prioritizing these comprehensive safety evaluations, organizations can enhance the likelihood of successful testing outcomes and contribute to the advancement of medical innovations.
Establishing clear endpoints is crucial for evaluating the success of preclinical development efforts. These endpoints must be specific, measurable, and directly aligned with the therapeutic goals of the research. Commonly defined endpoints include:
By establishing these parameters early in the study design, researchers can ensure that the data gathered is significant and relevant to later medical trials. The Medical Device Regulation (MDR) emphasizes that endpoints must address intended therapeutic benefits and be assessed using scientifically valid methodologies. Furthermore, incorporating patient-reported outcomes can enhance the relevance of endpoints, providing insights into the treatment's impact on quality of life.
As the terrain of medical research changes, utilizing best practices in endpoint selection will not only facilitate the shift from preclinical development to trial phases but also enhance the overall success rates of drug development, which currently stands at approximately 10.8%. This strategic method is crucial for navigating the complexities of regulatory requirements and ensuring that investigations can effectively demonstrate safety and efficacy. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, along with services such as regulatory approval, patient recruitment, and trial information management in regions like Latin America, Eastern Europe, and Australia, researchers can confidently navigate these challenges and expedite their clinical trial processes.
Implementing robust information management practices is vital for the success of preclinical development research. Establishing standardized protocols for information collection, storage, and analysis is critical. Electronic lab notebooks (ELNs) and laboratory information management systems (LIMS) are instrumental in streamlining information handling and fostering collaboration among research teams.
For instance, ELNs enhance information integrity by providing secure, tamper-proof documentation, which is essential for compliance with regulatory standards. Routine evaluations and information integrity assessments are necessary to ensure adherence to these standards, thus preserving the reliability of research results. The integration of ELNs with LIMS further fortifies data management practices, facilitating seamless data flow and improving accuracy in research documentation.
As the demand for digital solutions in preclinical development escalates, leveraging these technologies can significantly impact research outcomes, leading to more efficient and effective studies in the early stages. Notably, the ELN market is projected to reach $3.5 billion by 2033, growing at a CAGR of 12.5% from 2026 to 2033, with the pharmaceutical sector accounting for 38.5% of this market. This ongoing digital transformation in research and development underscores the critical role of ELNs and LIMS in contemporary research.
Collaborating with seasoned CROs like bioaccess significantly enhances the processes of preclinical development, particularly for first-in-human medical device studies in Colombia. Bioaccess provides a range of comprehensive services, including:
All of which improve study execution and efficiency. Their expertise in navigating local regulations and facilitating site activation ensures that research trials are conducted seamlessly and in compliance with INVIMA and other regulatory agencies. Notably, Dr. John B. Simpson, CEO of Avinger, Inc., has commended bioaccess for their exceptional experience conducting OCT-guided atherectomy clinical research in Cali, Colombia, highlighting the advantages of partnering with LATAM CRO experts.
When selecting a CRO, evaluating their track record, expertise in the relevant therapeutic area, and capacity to meet project timelines is essential.
Creating a comprehensive project timeline is essential for successful preclinical development efforts. This timeline must clearly delineate all key milestones, including:
The integration of project management tools is vital for tracking progress and identifying potential bottlenecks early in the process. Studies indicate that recruitment bottlenecks can postpone trials by as much as 30%, underscoring the necessity for effective management strategies. Regularly reviewing and updating the timeline ensures that all stakeholders remain aligned and that the project stays on track to meet its objectives. By leveraging these tools, teams can enhance efficiency and mitigate risks associated with delays, ultimately leading to faster and more successful outcomes in clinical research.
Monitoring and adjusting strategies in preclinical development based on emerging information is essential for enhancing research outcomes. A feedback loop facilitates real-time information analysis, enabling researchers to modify study protocols with agility. This adaptive approach not only improves the relevance of discoveries but also significantly increases the likelihood of success in subsequent research studies.
Research indicates that integrating real-time information analysis can yield a 15%-20% improvement in decision-making efficiency, ultimately reducing the time and costs associated with drug development. With bioaccess®'s FDA-prepared information, clinical trials can recruit treatment-naive cardiology or neurology groups 50% faster than in Western locations, resulting in $25K savings for each patient.
Regular team meetings focused on information trends and potential adjustments foster continuous improvement, ensuring that all team members are aligned and receptive to new insights. This mindset is critical in preclinical development and early-stage research, where the ability to adapt based on information can be the decisive factor between success and failure.
Given that the average cost of developing a medication is estimated at $1.5-$2.6 billion, enhancing research outcomes through real-time information analysis and leveraging bioaccess's capabilities is not merely advantageous; it is essential.
Initiating regulatory submissions promptly during the initial phase is crucial for a seamless transition to research studies. Organizations should commence the collection of essential documentation—such as study protocols, safety information, and compliance records—immediately upon the start of early research studies. Engaging with regulatory agencies throughout this process provides valuable insights and assists in identifying potential issues before they escalate. This proactive approach significantly enhances the likelihood of expedited approvals and streamlines the overall development timeline. Historical data reveal that early documentation and regulatory engagement correlate with improved success rates in clinical trials, highlighting the critical need for thorough preparation during the preclinical stage.
The journey of preclinical development is undeniably complex and multifaceted, necessitating strategic approaches to ensure success. By implementing the ten strategies highlighted, organizations can significantly enhance their efficiency and effectiveness in navigating this critical phase of research. Leveraging regional advantages, such as those offered by bioaccess®, alongside a thorough understanding of regulatory requirements, plays a vital role in streamlining the path to clinical trials.
Key insights, including the following:
These insights reinforce the necessity of meticulous planning and execution. Moreover, the integration of robust data management practices and collaboration with experienced CROs can markedly improve project timelines and outcomes. The emphasis on monitoring and adapting strategies based on emerging data underscores the need for agility in the ever-evolving landscape of preclinical research.
Ultimately, the significance of these strategies cannot be overstated. As the landscape of medical innovation continues to evolve, adopting best practices in preclinical development becomes essential for researchers aiming to bring effective treatments to market. Embracing these strategies not only enhances the likelihood of success but also contributes to the advancement of healthcare solutions that can improve lives globally.
What is bioaccess® and its role in preclinical development?
bioaccess® is a strategic platform that leverages the regulatory environment in Latin America to accelerate preclinical development for Medtech, Biopharma, and Radiopharma innovators, offering significant advantages such as cost reductions and faster patient recruitment.
Why is Colombia an attractive location for clinical trials?
Colombia offers over 30% cost reductions compared to North America and Western Europe, a diverse patient population of over 50 million, and a healthcare system recognized as one of the best in Latin America, making it an appealing site for clinical trials.
How does bioaccess® improve patient recruitment and ethical approvals?
bioaccess® ensures accelerated patient recruitment and streamlined pathways for ethical approvals, achieving these in just 4-6 weeks, which is significantly faster than traditional markets.
What are the main services provided by bioaccess®?
bioaccess® offers comprehensive services including feasibility assessments, study management, and compliance evaluations to support medical advancements.
What are the regulatory requirements important for preclinical development?
Key regulatory requirements include understanding Good Laboratory Practices (GLP), ethical guidelines, and specific regulations set by agencies like the FDA and EMA.
Why is engaging with regulatory bodies early in the development process important?
Early engagement with regulatory bodies helps clarify expectations and streamline submissions, significantly reducing the risk of delays in the overall development timeline.
What are the challenges associated with selecting animal models in preclinical research?
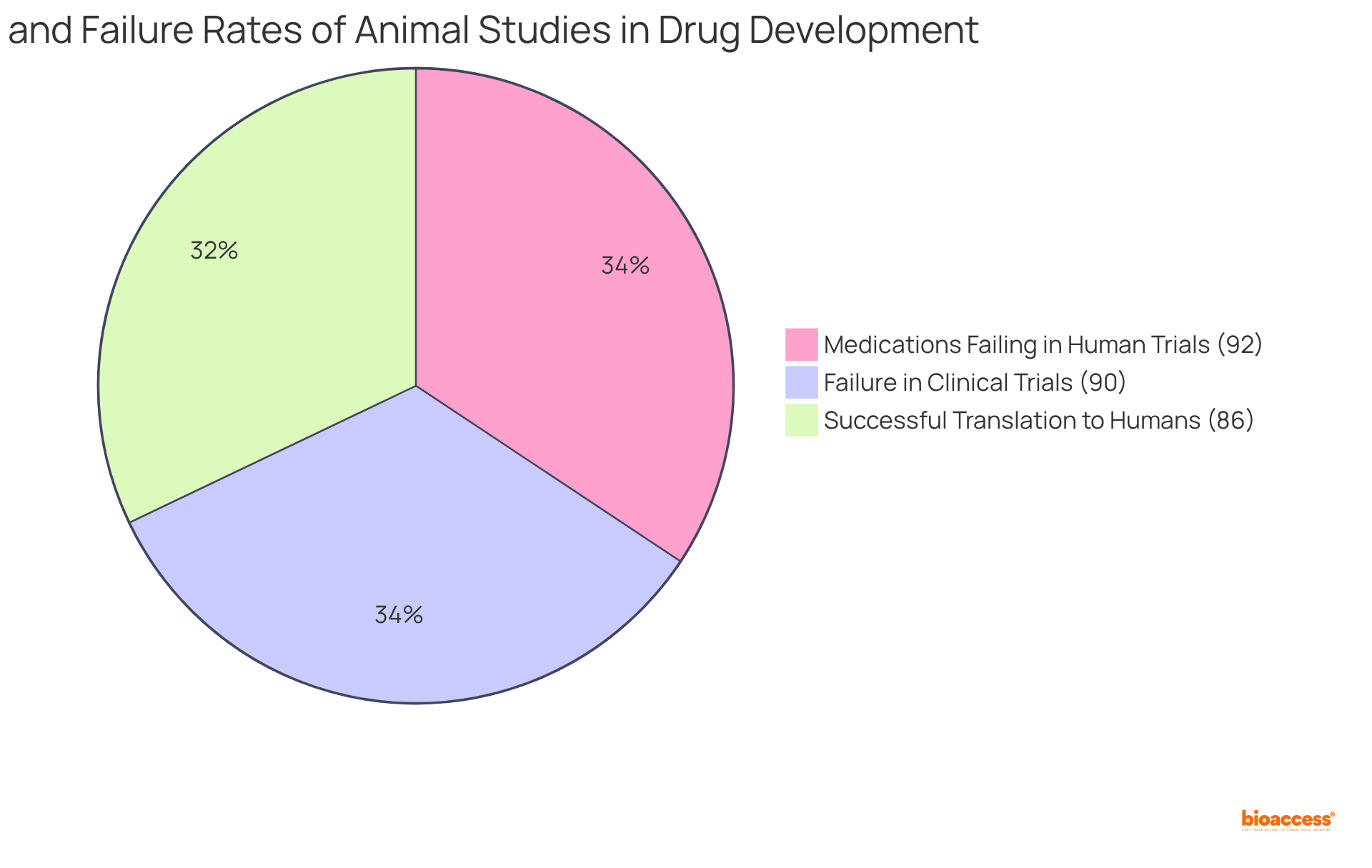
Selecting appropriate animal models is crucial as factors like genetic background, age, and sex can influence study outcomes, and a high failure rate in translating results from animal studies to human trials highlights the need for careful model selection.
What is the success rate of animal studies translating to human experiments?
Research indicates that 86% of favorable results in animal studies translate into positive outcomes in human experiments, but 90% of drugs tested in animals fail in clinical trials.
What alternatives to animal models are emerging in preclinical development?
Alternatives like organ-on-chip technologies are gaining traction, as they can replicate in vivo conditions and demonstrate improved predictive capabilities for drug testing.
How effective is the Liver-Chip technology in identifying toxic drugs?
The Liver-Chip has shown 87% sensitivity and 100% specificity in identifying toxic drugs, significantly outperforming traditional models.
What are the economic benefits of adopting innovative preclinical technologies?
Integrating technologies like the Liver-Chip can enhance drug development productivity, potentially saving an estimated $3 billion annually while improving the predictive validity of studies.
Why are ethical concerns important in animal research?
The high failure rates of animal studies and the growing push for alternatives highlight the need for more humane and effective research methodologies in drug development.