


The landscape of cancer research is experiencing a profound transformation, driven by the innovative implementation of virtual trials that promise to enhance both efficiency and accessibility. By leveraging advanced digital technologies, these trials streamline participant recruitment and facilitate real-time data collection, significantly improving the overall research process. However, as these virtual methodologies gain traction, critical questions emerge:
Exploring the transformative potential of virtual trials unveils not only exciting opportunities but also essential challenges that must be navigated to revolutionize cancer treatment. The integration of these methodologies into clinical research is not merely a trend; it represents a pivotal shift that could redefine how we approach patient engagement and data management in the fight against cancer.
bioaccess® leverages its extensive experience in early-phase clinical research to implement virtual trials that significantly enhance the efficiency of cancer research. By utilizing advanced digital technologies and optimized processes, bioaccess® enables swift participant recruitment and effective data collection. This agility is particularly crucial in oncology, where timely results can expedite treatment advancements and greatly improve outcomes for individuals.
As we approach 2025, the integration of virtual assessments is set to transform oncology clinical studies, enhancing their responsiveness and effectiveness. The execution of these studies not only addresses recruitment challenges but also boosts overall research efficiency, paving the way for innovative treatment approaches that can be delivered to individuals more swiftly than ever before.
In this evolving Medtech landscape, bioaccess® stands at the forefront, tackling key challenges in clinical research. The importance of collaboration cannot be overstated, as it is essential for driving these advancements forward. As we look to the future, the role of virtual trials in oncology will be pivotal in shaping the next generation of cancer treatments.
Virtualtrials significantly enhance participant recruitment by eliminating geographical barriers, allowing individuals to join from the comfort of their homes. This flexibility not only expands the potential participant pool but also contributes to higher retention rates. With nearly 85% of clinical studies struggling to recruit enough participants and 80% facing delays due to recruitment challenges, the ability to engage diverse groups through digital platforms is crucial. Moreover, there is a growing global emphasis on recruiting outside the United States, which is vital for diversifying participant pools.
By leveraging these tools, bioaccess® effectively connects with various demographic groups, ensuring that studies are both representative and inclusive. The integration of real-time monitoring and user-friendly digital interactions, supported by digital health technologies (DHTs) that gather valuable patient data, creates a more engaging experience, ultimately boosting patient satisfaction and commitment to the study. Additionally, bioaccess® offers comprehensive management services for studies, including feasibility assessments and site selection, which are essential for overcoming the recruitment hurdles faced by Medtech and Biopharma startups.
Consequently, virtualtrials are revolutionizing oncology studies, enhancing both accessibility and efficiency. To further improve recruitment efforts, clinical research directors should consider specific digital marketing strategies that effectively target diverse population groups.

Harness Real-Time Data Collection in Virtual Trials
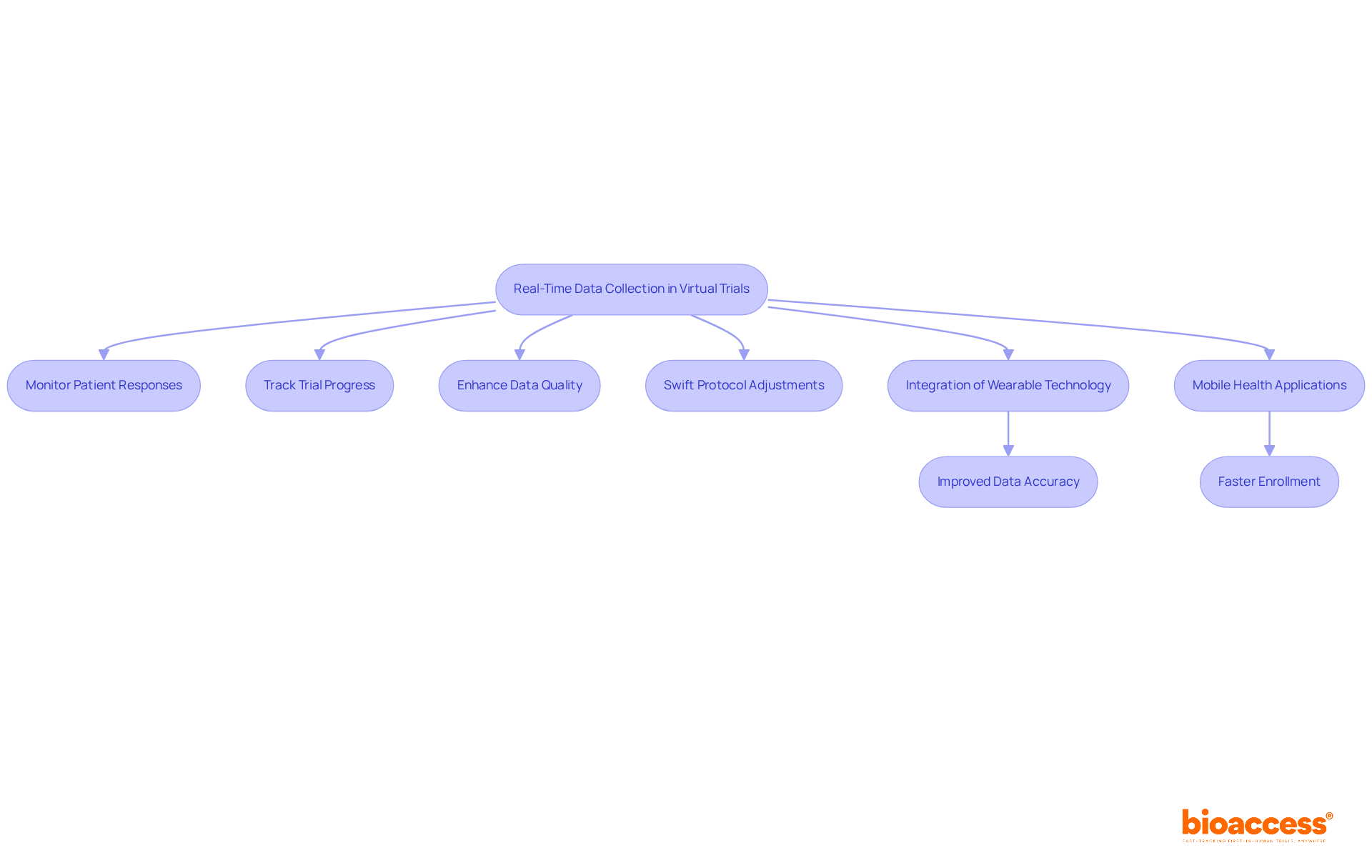
Real-time data collection in virtual trials is revolutionizing clinical research by empowering researchers to continuously monitor patient responses and track trial progress. This immediacy significantly enhances data quality, enabling swift protocol adjustments when necessary. By integrating wearable technology and mobile health applications, bioaccess® ensures accurate and efficient data capture, leading to more dependable study outcomes.
For instance, wearable devices provide objective assessments of physical activity and health metrics, which are essential for understanding individual experiences in oncology studies. Studies indicate that utilizing wearables can improve data accuracy by minimizing human error and enhancing compliance with Good Clinical Practice (GCP) standards. Moreover, the adoption of virtual trials in conjunction with wearables has been linked to achieving enrollment goals 50% faster, thus optimizing the entire research process.
As the landscape of cancer research evolves, the integration of these technologies is becoming increasingly vital for driving innovation and enhancing outcomes for individuals, particularly in alignment with FDA regulatory approaches. How can your organization leverage these advancements to improve trial efficiency and patient engagement? The time to act is now.
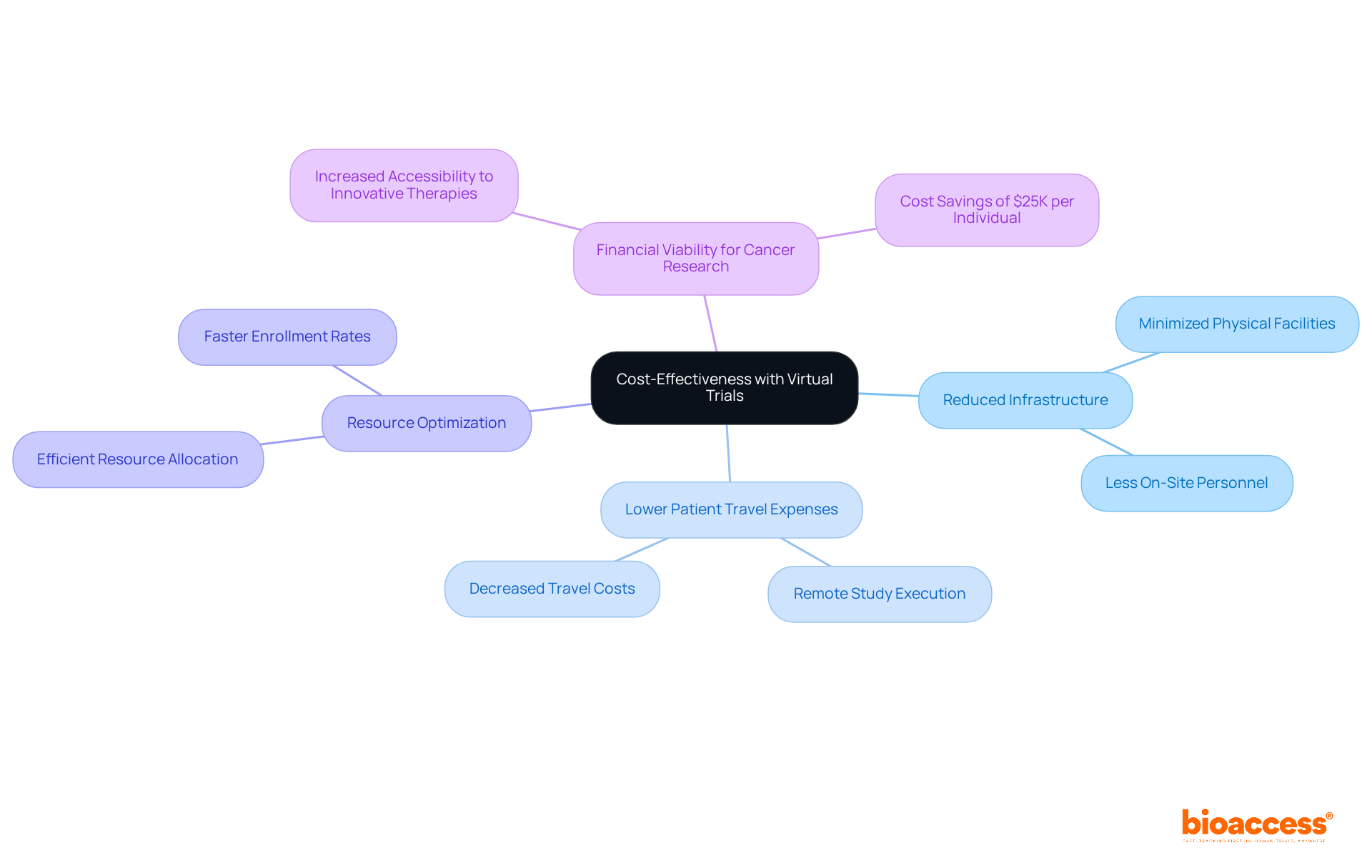
Virtual trials represent a groundbreaking approach to reducing costs associated with traditional clinical evaluations. By removing the necessity for extensive physical infrastructure and on-site personnel, organizations can effectively optimize their resource allocation. This shift not only cuts down on patient travel expenses but also enables remote study execution, leading to substantial overall savings. Such advancements enhance the financial viability of cancer research for sponsors and align with the increasing adoption of adaptive study designs, which have gained traction in recent years. As industry leaders assert, the financial benefits of virtual trials are pivotal in making innovative therapies more accessible, ultimately propelling progress in cancer treatment.
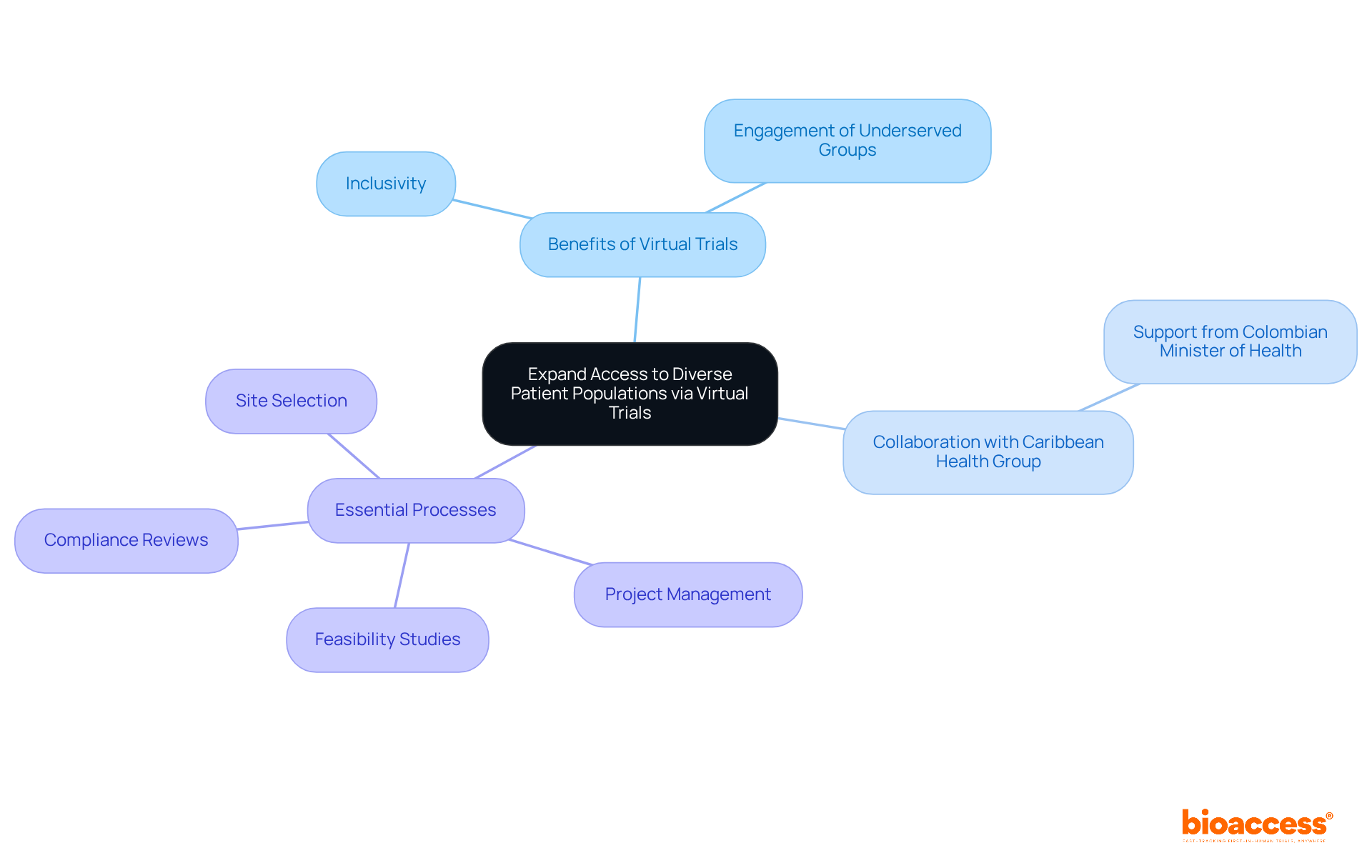
Virtualtrials inherently promote inclusivity by allowing individuals from diverse backgrounds to participate without the constraints of travel. This approach is particularly beneficial for engaging underserved groups who may face barriers to traditional study participation. By leveraging digital outreach and community engagement strategies, bioaccess™ ensures that diverse patient voices are integral to cancer research.
Furthermore, through its collaboration with Caribbean Health Group, bioaccess™ is poised to enhance clinical research services in Barranquilla, with backing from the Colombian Minister of Health. This partnership aims to streamline essential processes such as:
Ultimately, these efforts are geared towards driving global health improvement and fostering innovation in medical technologies.
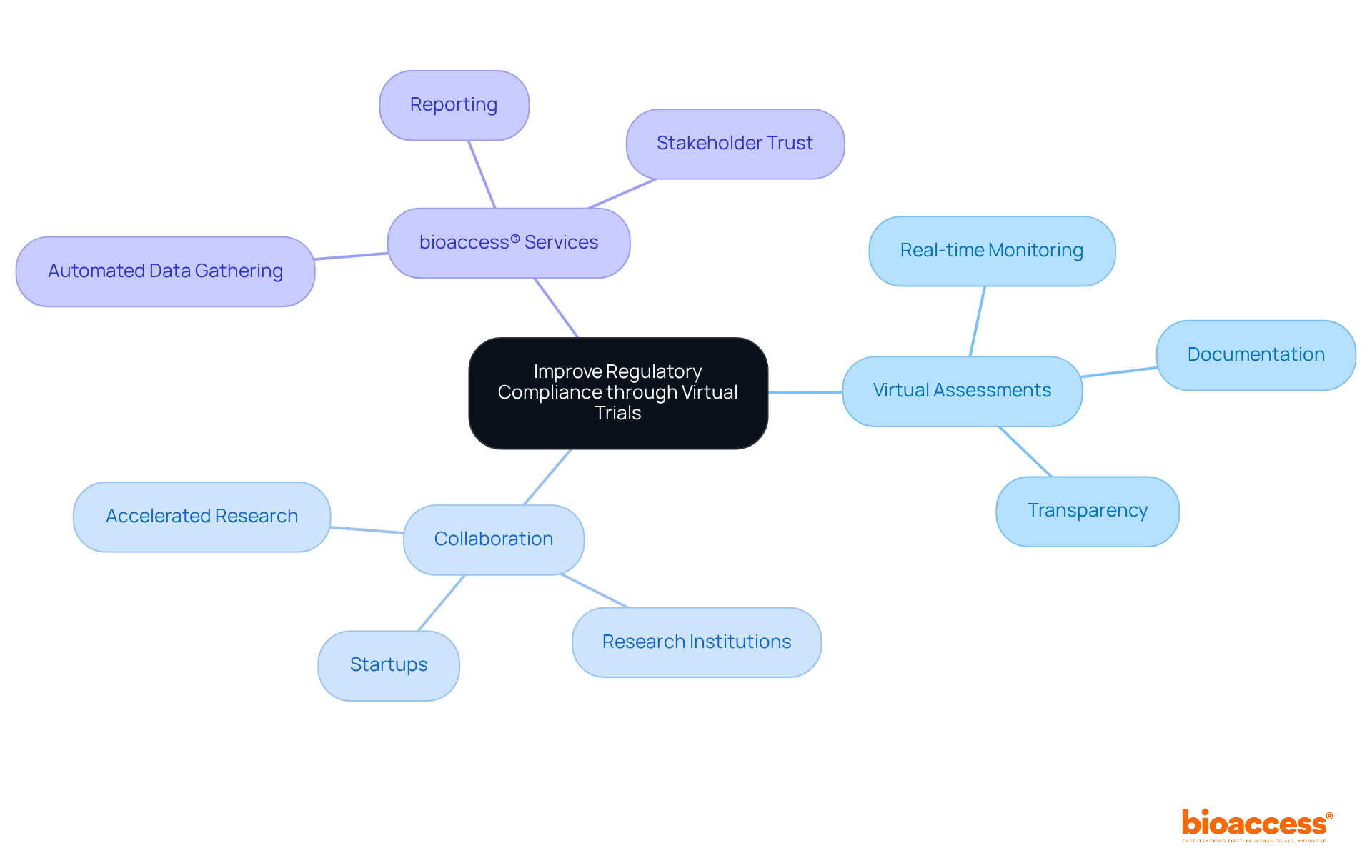
Virtual assessments play a crucial role in enhancing regulatory compliance, leveraging digital tools for real-time monitoring and documentation. With bioaccess®'s expert services, we automate data gathering and reporting, ensuring transparency and traceability in all activities. This proactive strategy not only meets regulatory requirements efficiently but also fosters trust with regulatory bodies and stakeholders.
In the evolving Medtech landscape, bioaccess® connects innovative startups in Medtech, Biopharma, and Radiopharma with leading research facilities across Latin America. This collaboration accelerates the research process, facilitating a quicker transition to pivotal studies and regulatory approvals. By addressing key challenges in clinical research, we position ourselves as a vital partner in driving innovation and compliance.
The importance of collaboration in this field cannot be overstated. As we continue to bridge gaps between startups and research institutions, we invite you to consider how bioaccess® can support your journey towards successful regulatory outcomes.
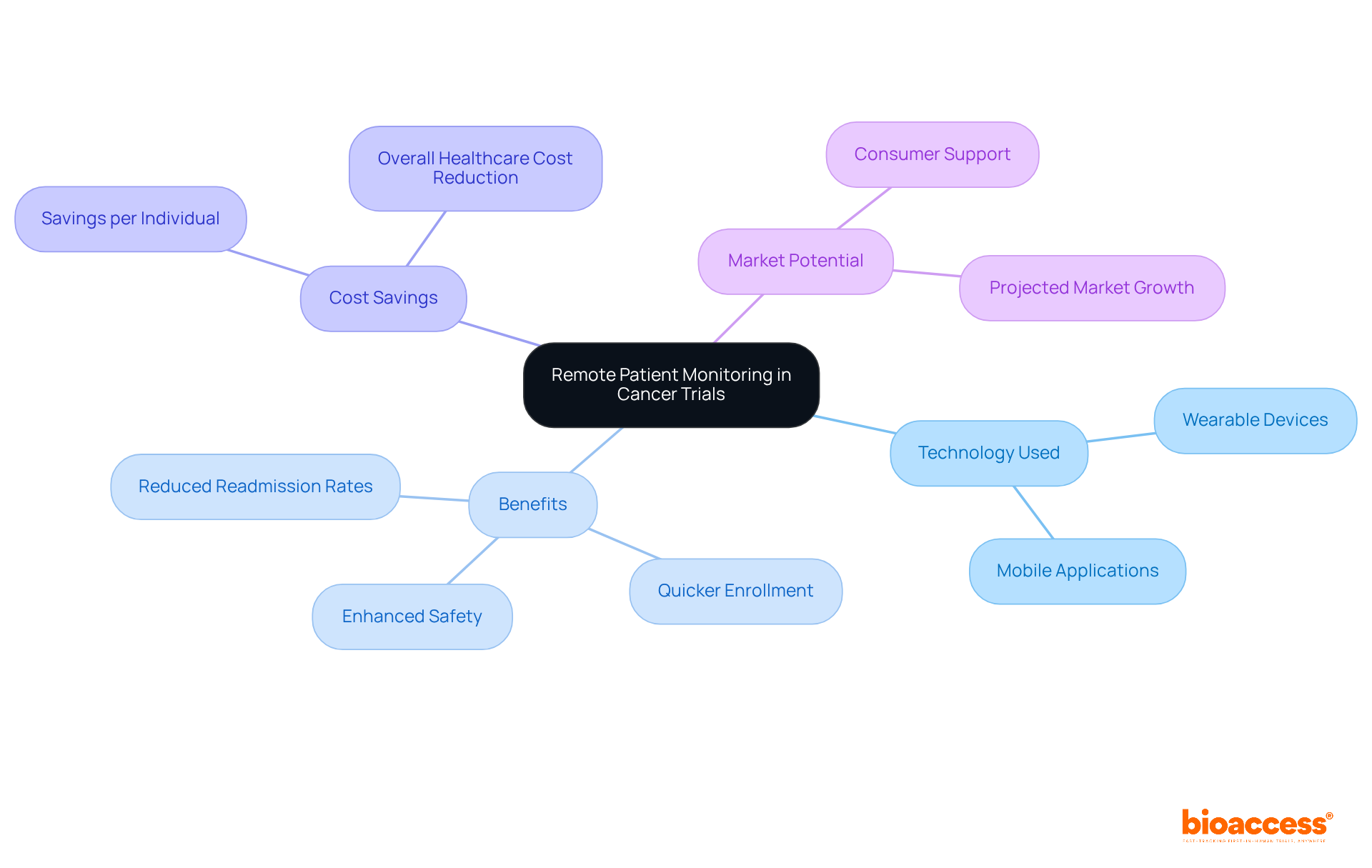
Remote participant monitoring (RPM) significantly enhances the continuous evaluation of individuals' health status throughout clinical trials. By leveraging wearable devices such as smartwatches and biosensors, along with mobile applications, bioaccess® can monitor vital signs and symptoms in real-time, facilitating timely interventions when necessary. This capability not only enhances safety for individuals but also guarantees that the data gathered truly represents participants' genuine experiences.
Notably, bioaccess® allows treatment-naive cardiology or neurology groups to be enrolled 50% quicker than conventional Western sites, resulting in $25K savings per individual with FDA-ready data-no rework, no delays. This swift enrollment directly addresses the typical challenges of participant recruitment in early-phase clinical trials. For instance, research from the University of Pittsburgh Medical Center has indicated that RPM can lead to a 76% decrease in hospital readmission rates, showcasing its effectiveness in improving outcomes for individuals.
Moreover, wearable gadgets have proven essential in cancer studies, enabling accurate monitoring of health metrics, which is vital for modifying treatment plans. As the RPM market is projected to reach around $42 billion by 2028, the incorporation of these technologies in cancer studies is becoming increasingly crucial for enhancing care and boosting research efficiency. As highlighted by industry specialists:
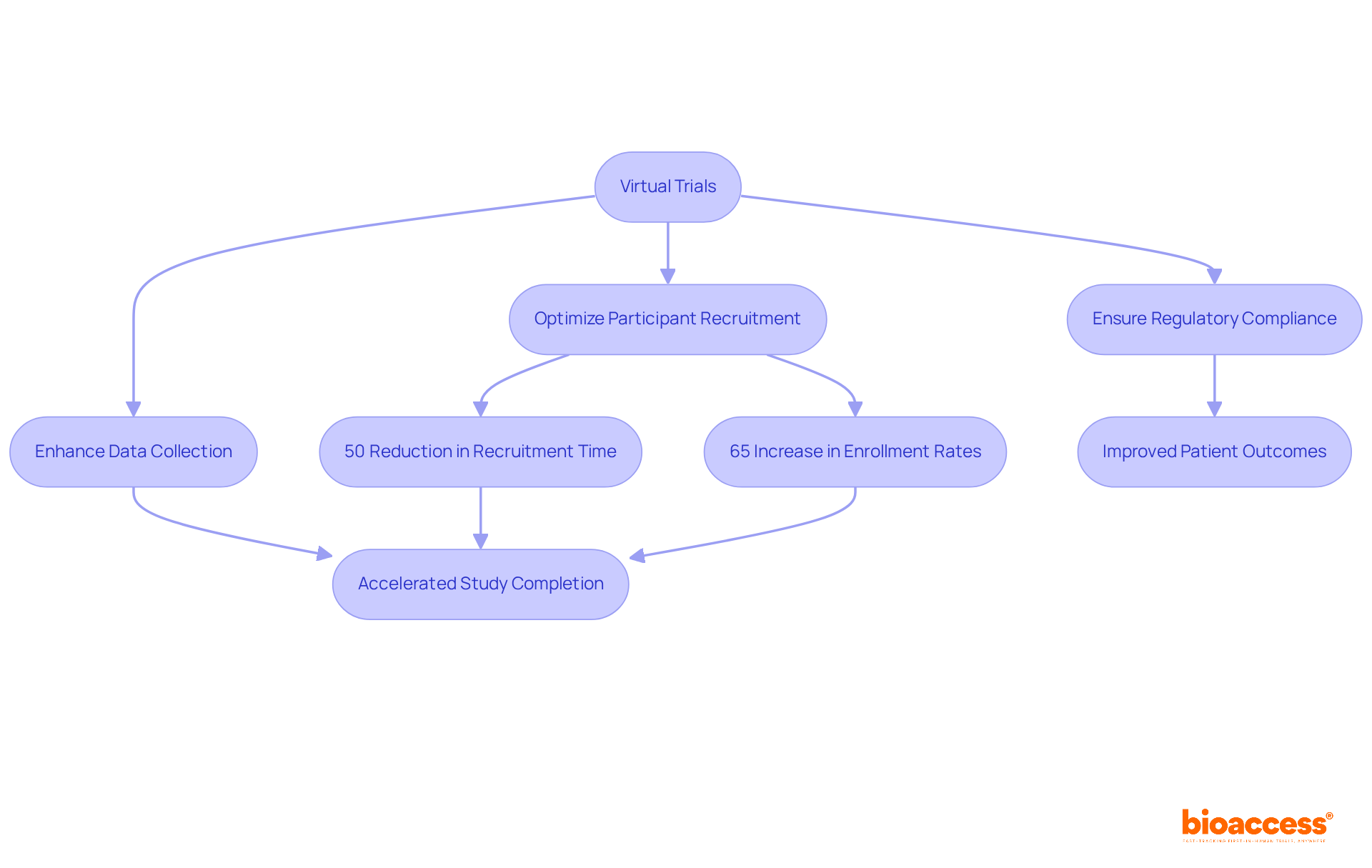
Virtual assessments are revolutionizing the speed at which new cancer therapies reach the market. By optimizing participant recruitment, enhancing data collection, and ensuring regulatory compliance, bioaccess® significantly accelerates study completion times. This rapid advancement is vital in oncology, where timely access to innovative therapies can dramatically improve outcomes and survival rates.
For instance, Verily's Project Baseline study demonstrated a reduction in recruitment timelines by over 50% through remote technologies. This highlights the potential of virtualtrials to expedite access to essential treatments. Furthermore, AI-driven participant recruitment tools have shown the capability to boost enrollment rates by as much as 65%, underscoring how technology can streamline processes in clinical studies.
As the demand for effective cancer treatment options grows, the integration of virtualtrials emerges as a critical strategy for enhancing market access and ultimately supporting patients in need. The collaboration between technology and clinical research is not just beneficial; it is essential for advancing healthcare solutions.
Virtual experiments significantly enhance collaboration among research teams by leveraging digital communication tools and platforms. This connectivity facilitates real-time sharing of data and insights, creating a more effective environment for teamwork. By dismantling geographical barriers, bioaccess® integrates expertise from diverse locations into the experimentation process.
With a commitment to expedited site activation in under eight weeks and the provision of FDA/EMA/MDR-ready datasets, bioaccess® streamlines the research process across various regions, including LATAM, the Balkans, and Australia. This efficient approach not only boosts collaboration but also ensures regulatory compliance, empowering Medtech and Biopharma companies to navigate the complexities of early-phase studies with confidence.
The skilled leadership group at bioaccess®, with extensive experience in implementing successful clinical studies, plays a crucial role in fostering innovation and efficiency within the clinical landscape. As the Medtech sector continues to evolve, the importance of collaboration cannot be overstated. Companies must consider how they can leverage such partnerships to overcome challenges and drive progress in clinical research.

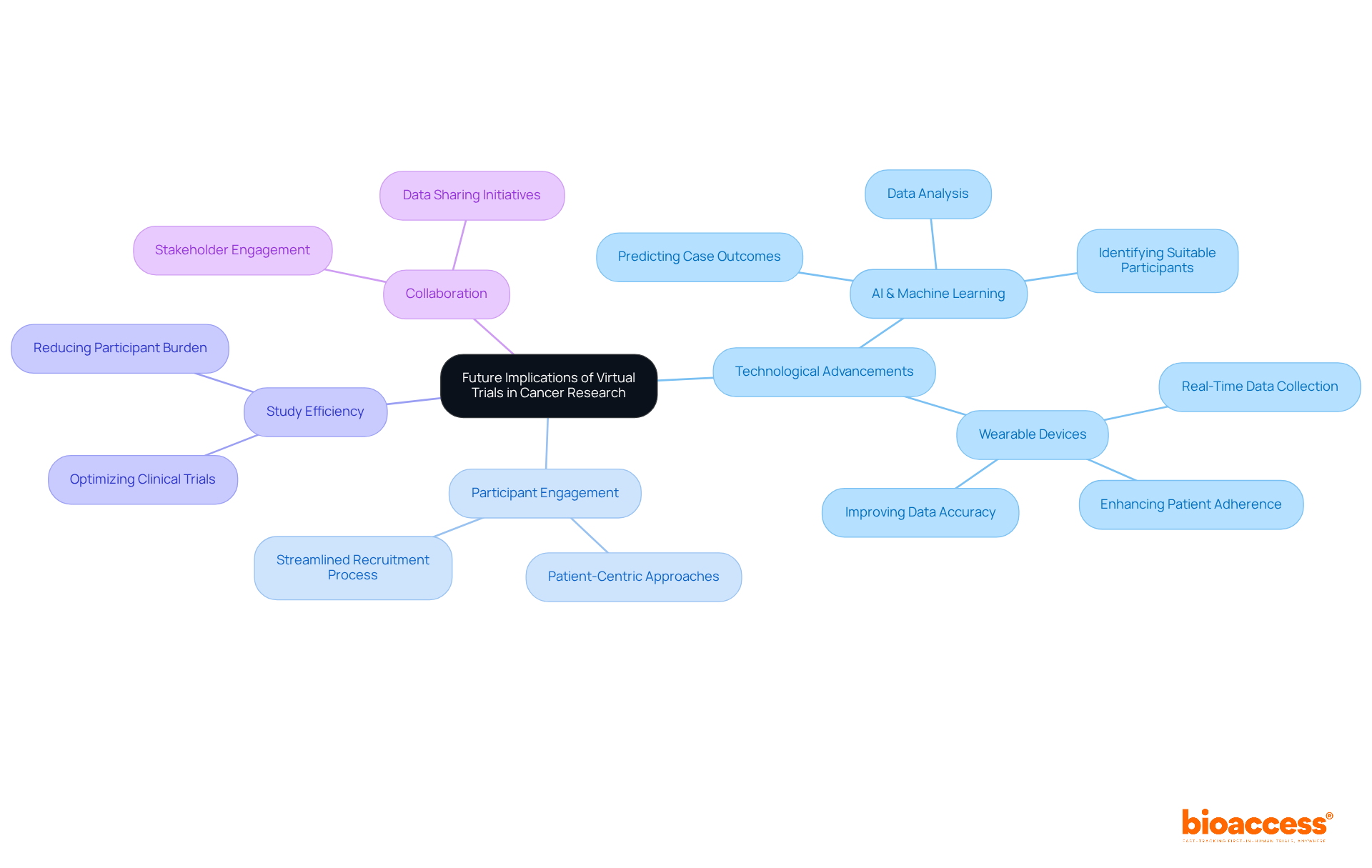
The outlook for virtual trials in cancer research is exceptionally promising, driven by rapid advancements in technology and data analysis aimed at enhancing study efficiency and participant engagement. As regulatory bodies evolve to embrace the digital landscape, bioaccess® is strategically positioned to spearhead the implementation of innovative study designs that prioritize patient-centric approaches.
The integration of artificial intelligence and machine learning is set to revolutionize testing procedures, significantly improving participant recruitment, data evaluation, and overall outcomes. For instance, AI can predict case outcomes and identify suitable participants, streamlining the recruitment process considerably. Furthermore, wearable devices facilitate real-time data collection, which not only boosts patient adherence but also enhances the accuracy of study data.
These technological advancements, such as virtual trials, not only optimize the efficiency of clinical trials but also lay the groundwork for more effective cancer treatments, ultimately reshaping the landscape of oncology research. As we move forward, collaboration among stakeholders will be crucial in harnessing these innovations to address the pressing challenges in clinical research.
The power of virtual trials in cancer research is undeniable. By harnessing advanced digital technologies and innovative processes, these trials not only enhance efficiency but also streamline patient recruitment, ultimately leading to quicker access to groundbreaking treatments. This shift towards virtual assessments marks a significant transformation in oncology studies, effectively addressing traditional challenges and paving the way for more effective and inclusive research.
Key insights from the exploration of virtual trials reveal their substantial impact:
As the landscape of cancer research evolves, embracing virtual trials is not merely an option but a necessity. Stakeholders must leverage these advancements to optimize their clinical studies and ensure that innovative therapies reach those who need them most. The future of oncology research hinges on the successful integration of technology and collaboration, ultimately leading to improved outcomes and a more responsive healthcare system.
What is bioaccess® and how does it contribute to cancer research?
bioaccess® is a company that leverages its experience in early-phase clinical research to implement virtual trials, significantly enhancing the efficiency of cancer research through advanced digital technologies and optimized processes.
How do virtual trials improve participant recruitment in oncology studies?
Virtual trials eliminate geographical barriers, allowing individuals to participate from home, which expands the potential participant pool and increases retention rates. This is crucial as many clinical studies struggle with recruitment challenges.
What percentage of clinical studies face difficulties in recruiting enough participants?
Nearly 85% of clinical studies struggle to recruit enough participants, and 80% experience delays due to recruitment challenges.
How does bioaccess® ensure diverse participant engagement in studies?
bioaccess® utilizes digital platforms to connect with various demographic groups, ensuring studies are representative and inclusive, which is essential for diversifying participant pools.
What technologies does bioaccess® use for real-time data collection in virtual trials?
bioaccess® integrates wearable technology and mobile health applications for real-time data collection, enabling continuous monitoring of patient responses and trial progress.
How does real-time data collection enhance the quality of clinical research?
Real-time data collection allows for swift protocol adjustments and improves data quality by minimizing human error, leading to more dependable study outcomes.
What benefits do wearable devices provide in oncology studies?
Wearable devices offer objective assessments of physical activity and health metrics, improving data accuracy and compliance with Good Clinical Practice (GCP) standards.
How much faster can enrollment goals be achieved by adopting virtual trials with wearables?
The adoption of virtual trials in conjunction with wearables has been linked to achieving enrollment goals 50% faster.
What is the future outlook for virtual trials in oncology?
The integration of virtual trials is expected to transform oncology clinical studies by enhancing responsiveness and effectiveness, paving the way for innovative treatment approaches that can be delivered more swiftly.