


The three best practices for risk management in medical devices are:
These practices are essential for ensuring patient safety and compliance with regulatory standards throughout the device lifecycle. Supported by methodologies such as FMEA and adherence to ISO 14971, they create a proactive safety framework that significantly enhances the overall effectiveness of risk management in the Medtech landscape.
In an industry where patient safety is paramount, managing the risks associated with medical devices transcends mere regulatory compliance; it is a critical component of healthcare innovation. This article explores best practices for risk management, underscoring the necessity of a structured approach that encompasses the entire lifecycle of medical devices. As healthcare technology continues to evolve in complexity, organizations must consider how to not only meet standards like ISO 14971 but also effectively protect patients from potential hazards. What strategies can be employed to ensure both compliance and safety in this dynamic landscape?
Hazard control in healthcare instruments is a structured and continuous procedure that encompasses the recognition of potential dangers, assessment of related threats, and the application of methods to mitigate those threats. It is essential to understand that hazard control must be integrated throughout the entire lifecycle of a medical instrument, from design and development to post-market monitoring. Key components of this process include:
Creating a robust safety framework not only enhances product security but also ensures compliance with regulatory standards. Statistics indicate that efficient hazard control can significantly reduce the likelihood of equipment failures, thereby improving patient outcomes. As specialists emphasize, incorporating hazard oversight into a comprehensive quality control system (QMS) fosters a holistic approach to safety and quality throughout the product lifecycle. This proactive strategy is vital in navigating the complexities of healthcare technology development, ensuring that patient safety remains paramount.

ISO 14971 is the cornerstone of risk management for medical devices, providing a structured framework that emphasizes several key components.
Documentation is essential; comprehensive records of all risk management activities must be maintained, including detailed risk assessments and the implementation of control measures. This documentation not only supports compliance but also serves as a reference for future evaluations.
A robust Risk Control Strategy is crucial. This plan should clearly outline the threat management process, define responsibilities, and establish timelines for each phase of the threat management lifecycle.
Moreover, Post-Market Surveillance is vital. Continuous observation of the product's performance in the market is necessary for recognizing any new or emerging hazards. This proactive approach ensures that any potential issues are addressed swiftly, preserving the safety and effectiveness of the equipment.
Adhering to ISO 14971 supports effective risk management for medical devices, fulfilling regulatory obligations and building trust among stakeholders, including patients, healthcare providers, and regulatory authorities. Frequent assessments and revisions of management procedures are essential to ensure conformity with the latest standards, thereby enhancing the overall safety and efficiency of healthcare instruments.

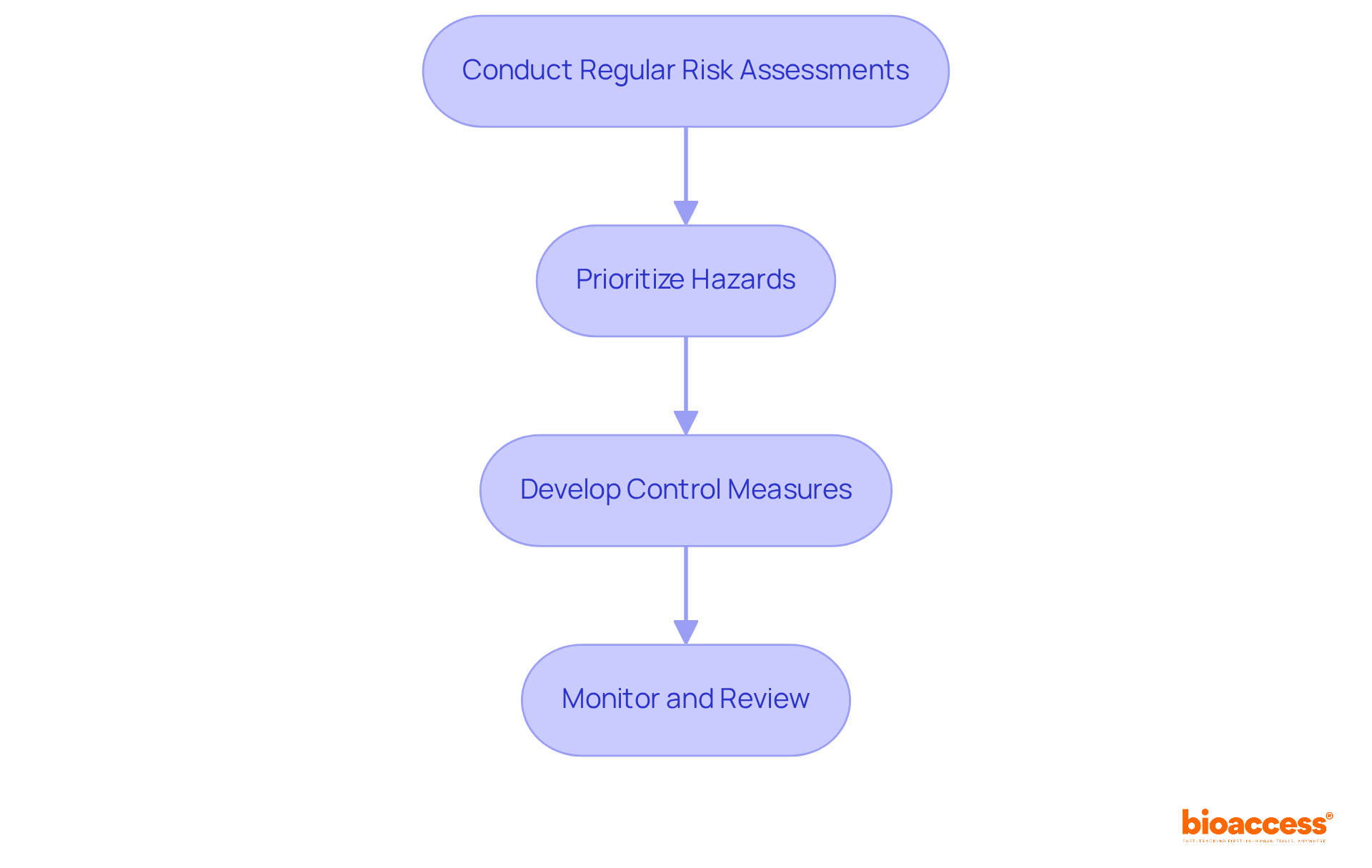
To implement effective risk management for medical devices, organizations must follow a systematic approach that addresses potential risks associated with these devices.
Conduct Regular Risk Assessments: Employ methodologies such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) to systematically identify and evaluate potential risks. FMEA enables teams to analyze failure modes and prioritize safety measures effectively, ensuring a comprehensive understanding of the risks involved.
Prioritize Hazards: Utilize a matrix to categorize identified threats based on their severity and likelihood. This prioritization allows teams to concentrate on the most critical issues initially, thereby ensuring that resources are allocated effectively to mitigate major challenges.
Develop Control Measures: Identify and implement suitable control measures tailored to the specific challenges identified. These measures may encompass design modifications, user training programs, or enhanced labeling to inform users about potential hazards, thereby enhancing safety and usability.
Monitor and Review: Establish a robust process for ongoing observation of threats and the effectiveness of implemented control measures. This continuous supervision guarantees that emerging threats are swiftly recognized and managed, preserving the safety and effectiveness of health instruments throughout their lifespan.
By adopting a proactive approach to risk management for medical devices, organizations can significantly reduce the likelihood of adverse events and enhance the overall safety profile of their products.
Understanding and implementing effective risk management practices in the medical device sector is crucial for ensuring patient safety and compliance with regulatory standards. This article highlights the necessity of a structured approach to hazard control, emphasizing that risk management must be an integral part of the entire lifecycle of a medical device, from its design to post-market surveillance.
Key practices include:
By maintaining comprehensive documentation and conducting regular risk assessments, organizations can prioritize hazards and implement tailored control measures that enhance the safety and effectiveness of their products. Continuous monitoring and review of these processes further ensure that emerging threats are addressed promptly.
Ultimately, adopting these best practices not only mitigates risks associated with medical devices but also fosters trust among stakeholders, including patients and healthcare providers. As the landscape of healthcare technology evolves, organizations must remain vigilant and proactive in their risk management strategies to safeguard patient health and maintain compliance with ever-changing regulatory requirements. Embracing these principles will lead to improved outcomes and a more secure healthcare environment.
What is hazard control in healthcare instruments?
Hazard control in healthcare instruments is a structured and continuous procedure that involves recognizing potential dangers, assessing related threats, and applying methods to mitigate those threats throughout the entire lifecycle of a medical instrument.
What are the key components of risk management in medical devices?
The key components include hazard identification, risk analysis, threat assessment, and risk management for medical devices.
What is the purpose of hazard identification?
Hazard identification aims to recognize potential sources of harm associated with the medical device, which is essential for effective risk management.
How is risk analysis conducted?
Risk analysis evaluates the likelihood and severity of harm resulting from identified hazards, providing a quantitative basis for decision-making.
What does threat assessment involve?
Threat assessment determines the acceptability of dangers based on predefined criteria, ensuring that only acceptable threats are carried forward in the risk management process.
What measures can be taken in risk management for medical devices?
Measures may include design modifications, warnings, or user training to minimize uncertainties to an acceptable level.
Why is creating a robust safety framework important?
A robust safety framework enhances product security and ensures compliance with regulatory standards, ultimately improving patient outcomes.
How does efficient hazard control impact equipment failures?
Efficient hazard control can significantly reduce the likelihood of equipment failures, thereby improving patient outcomes.
What role does hazard oversight play in quality control systems?
Incorporating hazard oversight into a comprehensive quality control system fosters a holistic approach to safety and quality throughout the product lifecycle.
Why is a proactive strategy vital in healthcare technology development?
A proactive strategy is vital to navigate the complexities of healthcare technology development, ensuring that patient safety remains paramount.